Indexing & Abstracting
Full Text
Review ArticleDOI Number : 10.36811/rjstt.2019.110001Article Views : 1475Article Downloads : 33
The relation of vitamin D level to adverse prognostic factors in Non Hodgkin lymphoma: A single center analysis
Ghada ElGohary GM1,3*, Moustafa NN1, Abdelbary HM1, Fekry GH1, Bakr SI2 and Elkourashy SA1
1Clinical Hematology and Bone Marrow Transplantation Unit, Division of Internal Medicine, Ain Shams University, Cairo, Egypt
2Clincal Pathology Department, Ain Shams University, Cairo, Egypt
3Department of Adult Hematology/Oncology, King Khaled University Hospitals, College of Medicine, King Saud University, Riyadh, Saudi
*Corresponding author: Ghada ElGohary GM, Clinical Hematology and Bone Marrow Transplantation Unit, Division of Internal Medicine, Ain Shams University, Cairo, Egypt, Email: ghelgohary@gmail.com
Article Information
Aritcle Type: Review Article
Citation: Ghada ElGohary GM, Moustafa NN, Abdelbary HM, et al. 2019. The relation of vitamin D level to adverse prognostic factors in Non Hodgkin lymphoma: A single center analysis. Res J Stem Cell Ther Transplant. 1: 01-08.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Ghada ElGohary GM
Publication history:
Received date: 22 February, 2019Accepted date: 09 March, 2019
Published date: 11 March, 2019
Abstract
Vitamin D insufficiency has been found to be associated with higher incidence of NHL, besides the lower levels are linked with poor prognosis. The current study was conducted to assess vitamin D level in newly diagnosed chronic lymphocytic leukemia (CLL) and diffuse large B- cell lymphoma (DLBC) patients. In addition, grade of lymphoma was evaluated with the relation of hypo-vitaminosis D and adverse prognostic parameters. 74 patients (50% CLL and 50% DLBCL) were enrolled in this study. All patients with CLL had vitamin D insufficiency which correlated with advanced Rai and Binet stage. About 91.8% of DLBCL patients had vitamin D insufficiency which correlated with bad ECOG performance, advanced Ann-Arbor staging, high LDH and extranodal involvement. Vitamin D levels in the CLL group were significantly lower than DLBCL group. However, we could not detect an impact of the severity of deficiency on the lymphoma grade.
Keywords: Vitamin D Insufficiency; Dlbcl; Cll
Introduction
Vitamin D is known to play a major role in maintaining calcium level and skeletal homeostasis, besides its well-known pleiotropic effect on the cellular differentiation, proliferation , apoptosis and angiogenesis [1].One of the most potent effect of extra-renal 25(OH) vitamin D is the regulation of cell proliferation which occurs through the increased transcription of two cell cycle negative regulators which is known as (p21 and p27), induction of apoptosis and increased cell proliferation signals [2,3]. In lymphoma cells, 1,25(OH)?D has been observed to promote the cellular differentiation and the anti-proliferative effects in vitro [4]. While an inverse association has been reported between the circulating levels of vitamin D and prognosis in several types of malignancy, low vitamin D level may be associated with poorer prognosis in colorectal and breast cancer as well as multiple myeloma [5,6,7]. A combined analysis of 10 different studies reported higher levels of recreational sun exposure were associated with the lower risk of NHL [8]. Moreover, another study reported that 1,25(OH)?D can induce regression in follicular, small cleaved cell lymphoma [9]. Another evidence indicates that 25(OH)? D level impacts the prognosis of CLL and its insufficiency affects the disease outcome [10]. Besides, other studies have observed a spectacular 13month remission in CLL patients after administration of cholecalciferol in elder patients with vitamin D deficiency [10]. At the cellular level, the vitamin D effect may be related to the level of expression of vitamin D receptors on the lymphocytes surface [11], especially proliferating cells such as expanding B lymphocytes through increasing the level of p27 [12]. In fact, de novo patients DLBCL showed low/null p27 expression with impairment of cell cycle control [13].
Aim Of this Study
The purpose of this study is to find out the relation of hypovitaminosis D in 2 groups of lymphoproliferative disorders (CLL and DLBCL) to several adverse prognostic factors in both diseases as disease stage, CD38 expression, serum LDH level and extra-nodal involvement. The study also compares between the severity of hypovitaminosis in both disorders being representing indolent and aggressive lymphomas and its impact on the grade of lymphoma.
Study Design
Patients and Methods
This study has included newly diagnosed lymphoma patients who attended the clinic of Hematology-Ain Shams University Hospital-airo, over a period of 48 months from june 2013 till june 2015.
Exclusion Criteria
1. Patients who started chemotherapy.
2. Those who had transformed DLBCL and/or B-CLL.
3. Patient with HIV infection were excluded.
Informed consents were obtained from all participants. The study was conducted in accordance with the stipulations of the local ethical and scientific committees of Ain Shams University and the procedures respected the ethical standards in Helsinki declaration of 1964. Complete blood count (CBC) using coulter counter (Coulter LH 750 analyzer), erythrocyte sedimentation rate (ESR; in mm/hour) first hour by the Westergren method were determined. Liver functions, Kidney functions, Serum electrolytes, Serum LDH, and uric acid were done on a Synchron X9 (Beckman instrument Inc., USA). Bcl? expression was assessed in lymph node biopsies in patients with DLBCL. Bone marrow aspiration with Flow cytometry and/or biopsy with immune-histochemistry were done for diagnosis of lymphoproliferative disorders. 17 p deletion has been assessed in our CLL patients using FISH analysis. Assessment of 25 OH vitamin D level was done using DRG 25-OH Vitamin D total ELISA kit (DRG International Inc.USA).
Statistical analysis
Data were analyzed using statistical program of Social Science (SPSS) version 18.0. Quantitative data have been expressed as mean ±standard deviation (SD), Qualitative data have been expressed as frequency and percentage.The following tests were done: Independent–sample t-tests of significance, Chi-square (X²) test, Pearson's correlation coefficient (r) tests, Spearman's rank correlation coefficient and receiver operating characteristic (ROC ) curve analysis. ROC was used to find out the overall predictivity of the parameter and to find out the best cut-off value with detection of sensitivity and specificity at this cut-off value.
Results
Seventy-four patients of B-CLL and DLBCL (37 patients of each of them) were enrolled in this study. There were 48 male patients and 26 female patients, their ages ranged from 44-72 with mean ±SD (57.22±6.43). Thirty-seven age- and sex-matched healthy subjects were used as a control. The control group included 13 females and 24 males their ages ranged from 44-72 years. Level of 25-OH Vitamin D level in the studied groups was highly significantly reduced in all patients of both diseases compared to the control group (p value 0.000). Moreover, the level of 25-OH Vitamin D in CLL patients group showed a highly significant lower level when compared to DLBCL patients’ group (p value 0.000) (Table 1) and (Figure 2).25-OH Vitamin D level showed a marked significant decrease in DLBCL patients with advanced stage, extra-nodal involvement, bad ECOG performance and high LDH level (p value 0.02). However, it was not significantly correlated with bone marrow involvement in those patients (Table 2). 13 out of 37 patients (36%) had Bcl? expression. Low level of vitamin D was not correlated with Bcl? expression (p value 0.160) Correlation of 25-OH Vitamin D level in CLL patients with CD 38 expression and staging, revealed that 25-OH Vitamin level was highly significantly decreased in advanced disease stage (both Rai and Binet staging) p-value 0.000 and 0.008 respectively. CD38 was expressed only in 2 out of the 37 CLL patients (Table 3). 17 p deletion could not be detected in any of our CLL patients.Diagnostic performance study using Receiver-operating characteristic (ROC) curve analysis was used to define the best cut-off levels for 25-OH Vitamin D level for discriminating normal from diseased patients which was ≤10 with diagnostic sensitivity, specificity, predictive positive and negative values of 81.08%, 100%, 100%, 84.1% respectively (Figure 2).
|
Table 1: Comparison between patient groups and control group and between each other as regard 25-OH Vitamin D level. |
|||||||||
|
Groups
Parameter |
CLL group |
DLBCL group |
Control group |
CLL |
DLBCL vs Control |
CLL |
|||
|
N:37 |
N:37 |
N:37 |
vs |
vs |
|||||
|
Mean±SD |
Mean±SD |
Mean±SD |
Control |
DLBCL |
|||||
|
(Range) |
(Range) |
(Range) |
P |
Sig |
P |
Sig |
P |
Sig |
|
|
25 OH Vit D |
4.80±3.25 |
18.36±10.76 |
44.62±10.08 |
|
HS |
0 |
HS |
<0.001 |
HS |
|
(ng/mL) |
(0.2-10) |
(1-55) |
(30-71) |
<0.001 |
|||||
|
HS: Highly significant S: Significant. |
|||||||||
|
Table 2: Comparative statistics between 25-OH Vitamin D level in DLBCL patients and prognostic markers (extra-nodal affection, ECOG, BM infiltration and staging). |
|||||
|
Vit D3
Prognostic markers |
Mean±SD |
Range |
P-value |
Sig. |
|
|
Exta- nodal |
Negative: n=22 |
21±6 |
Jan-55 |
0.041 |
S |
|
Positive: n= 15 |
17±5 |
Feb-35 |
|||
|
ECOG |
1:n= 25 |
24±7 |
15-55 |
0.048 |
S |
|
2:n=7 |
19±6 |
Sep-30 |
|||
|
3:n=4 |
17±4 |
Jan-42 |
|||
|
4:n=1 |
11±0 |
11-Nov |
|||
|
BM infiltration |
Negative: n=33 |
18±10 |
Jan-55 |
0.513 |
NS |
|
Positive: n= 4 |
22±15 |
Sep-42 |
|||
|
Stage |
1:n= 14 |
24±13 |
15-55 |
0.043 |
S |
|
2:n= 9 |
21±6 |
Dec-35 |
|||
|
3:n= 6 |
14±14 |
Feb-42 |
|||
|
4:n= 8 |
11±7 |
Jan-21 |
|||
|
LDH |
|
380.38±184.01 |
160-901 |
0.02 |
s |
|
NS: Non-significant S: Significant. |
|||||
Figure 1: Diagnostic performance of serum 25-OH Vitamin D level in discrimination between patients and control.
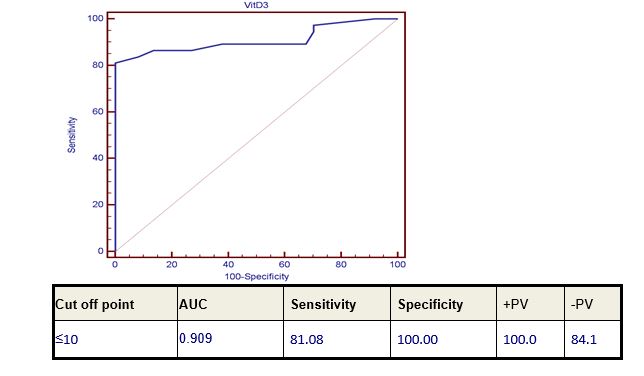
|
Table 3: Comparative statistics between 25-OH Vitamin D level in CLL patients and CD 38 expression and staging. |
|||||
|
Vit D3
Prognostic markers |
Mean±SD |
Range |
P-value |
Sig. |
|
|
CD 38 |
Negative: n= 35 |
5±3 |
0-10 |
0.895 |
NS |
|
Positive: n=2 |
5±1 |
04-May |
|||
|
Rai staging |
0: n= 11 |
8±1 |
06-Oct |
<0.001 |
HS |
|
1: n= 10 |
6±2 |
04-Aug |
|||
|
2: n= 6 |
4±2 |
01-Jun |
|||
|
3: n= 10 |
1±0 |
0-1 |
|||
|
Binet staging |
A: n= 11 |
8±1 |
06-Oct |
0.0008 |
HS |
|
B: n= 16 |
5±2 |
01-Aug |
|||
|
C: n= 9 |
1±0 |
0-1 |
|||
Figure 2: Comparison between vitamin D level in CLL and DLBCL.

Discussion
The current study has been conducted to assess the hypovitaminosis D in patients with newly diagnosed NHL conditions such as CLL and DLBCL. In addition, the evaluation of probable correlation between vitamin D levels and the various prognostic markers in both diseases has been performed. The study results indicated that the levels of vitamin D were significantly reduced in both CLL and DLBCL patients. Moreover, it has been found that CLL patients had significantly lower levels than DLBCL patients. In DLBCL patients, vitamin D levels significantly correlated with the adverse prognostic markers such as extranodal involvement, advanced disease stage, bad ECOG performance and high LDH levels. Besides, advanced Rai and Binet staging in CLL are also found to be significantly correlated with Vitamin D levels. In vitro, vitamin D has been shown to inhibit proliferation and induce differentiation of both lymphocytes and lymphoma cell lines [4,14]. There is associations between low levels of both 25(OH )D and 1,25(OH?) D and OS but not EFS in some NHL subtypes, however , the role of vitamin D supplementation to maintain 25 (OH)D levels for primary prevention of NHL is not yet known [15].Though , Raina et al. in their study of 34 patients with low-grade NHL treated with a synthetic analogue of 1,25 (OH) vitamin D reported a tumor regression in 24% patients (4 complete remissions; 4 partial remissions) [9].
In CLL
The association between vitamin D insufficiency and the clinical outcome was confirmed in an independent cohort study of CLL patients and several studies suggested that vitamin D insufficiency may be the first potentially modifiable host factor which is associated with the prognosis in the newly diagnosed CLL patients [10].Further confirmation has been obtained in a Chinese meta-analysis that has found a direct proportional relationship between the poor prognosis in lymphoma and leukemia patients with the low serum of 25(OH) D. Similarly,a multivariate analysis from an Egyptian study revealed that 25(OH) D insufficiency is an independent poor prognostic factor in both CLL and NHL the issue that agrees with our own results [17].
While the prevalence of vitamin D insufficiency in CLL patients in the current study was 100%, a rate of 56.7% and 82.2% was observed in CLL patients from Pakistan and Italy respectively [18,19]. These results revealed an important scientific data especially that the vitamin D insufficiency may be associated with inferior time to treatment (TTT) and could decrease overall survival (OS) [10]. Our cross-sectional study has demonstrated that the lower levels of vitamin D were observed in CLL patients with advanced Rai or Binet stage (P-value ?0.001 and ?0.0008 respectively). This is in contrast to the result of Shanafelt et al. who found that serum vitamin D levels neither correlated with Rai stage nor with absolute lymphocytic count [10]. In their study, Serum vitamin D levels were a predictor for time to treatment (TTT) among both Rai stage 0 patients and patients with Rai stage ?1 when these groups were evaluated separately and on multivariate analysis controlling for the disease staging [10].
In DLBCL
This study has revealed that 91.8% of the DLBCL patients had insufficient 25(OH) D levels. Moreover, these levels were significantly decreased in patients with bad ECOG performance status, advanced Ann-Arbor staging, high LDH and in patients with extranodal involvement. However, it was not significantly correlated with BM involvement and Bcl2 expression. Our results are correlated with the study that was held by Drake et al. who showed that over 40% of patients with NHL had insufficient 25(OH) D levels and low levels were associated with inferior event free survival (EFS) and overall survival (OS) for DLBCL. Therefore, the prognostic effect of vitamin D may be directly related to its impact on the lymphoma and it is not considered as simply as a general host effect [15].
Vitamin D is playing a fundamental role in the lymphoma genesis, this could be explained as vitamin D is capable of modulating several critical cellular process, including inhibition of carcinogenesis by induction of cellular differentiation, inhibition of proliferation, angiogenesis and promotion of. Apoptosis [1]. Also, 1,25 (OH)?D may induce apoptosis either indirectly through its effects on insulin-like growth factor receptors and tumor necrosis factor α or directly via Bcl 2 family system, the ceramide pathway, the death receptors (e.g.Fas) and the stress activated protein kinase pathways. Besides, the inhibition of the tumor invasion induced by vitamin D has been demonstrated to be related to the inhibition of serine proteinases, metalloproteinases and the angiogenesis [20].
B-CLL and DLBCL
Our study also detected that the level of 25(OH) vitamin D level in CLL patients was significantly lower compared to DLBCL patients (P-value ?0.001). This could be related to the advanced age of CLL patients who are usually presented in older age compared to DLBCL patients. The impact of pretreatment vitamin D level on follicular lymphoma as an example of low grade lymphoproliferative disorders was evaluated by Kelly et al. They found that after a median follow up of 5.4 years, the adjusted PFS and overall survival hazard ratios for the SWOG cohort were 1.97 and 4.16 respectively, for those patients with vitamin D deficient. After a median follow up of 6.6 years, the adjusted PFS and OS hazard ratios for the LYSA cohort were 1.50 and 1.92 respectively for those who were vitamin D deficient.
Although statistical significance was not reached in our LYSA cohort, the consistent estimates of the association between low vitamin D levels and Follicular Lymphoma (FL) outcomes in two independent cohorts, this is suggesting that the serum vitamin D might be the first potentially modifiable factor to be associated with FL survival [21]. Because in our study, it is not yet confirmed whether normalizing the vitamin D levels in CLL and other types of NHL patients with documented insufficiency would improve the patient, s outcome, a phase II study will follow to assess its therapeutic effect.
Conclusion
Vitamin D levels were significantly lower in patients with CLL and DLBCL. Moreover, these levels has been correlated with advanced stages in both diseases. However, we couldn’t find any impact of the severity of hypovitaminosis D on the grade of lymphoma. Further studies are warranted to explore this relationship.
Abbreviations
1- DLBCL
2- CLL
3- ESR
4- CBC
5- SD
6- TTT
7- OS
8- LDH
References
- Bikle D. 2009. Non classic actions of vitamin D.J Clin Endocrinol Metabo. 94: 26-34. [Ref.]
- Deluca MF. 2004. overview of general physiologic features and functions of vitamin D. Am J clin Nutr. 80: 1689-1696. [Ref.]
- Holick MF. 2004. Vitamin D importance in the prevention of cancers, type I diabetes, heart diseases and osteoporosis. Am J Clin Nutr. 79: 362-367. [Ref.]
- Hickish T, Cunningham D, Colston K, et al. 1993. The effect of 1,25-dihydroxyvitamin D3 on lymphoma cell lines and expression of vitamin D receptor in lymphoma. Br J Cancer. 68: 668-672. [Ref.]
- Ng K, Meyerhardt JA, Wu K, et al. 2008. Circulating 25 hydroxyvitamin D levels and survival in patients with colorectal cancer. J Clin Oncol. 26: 2984-2991. [Ref.]
- Goodwin PJ, Ennis M, Pritchard KI, et al. 2009. prognostic effects of 25 hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 27: 3757-3763. [Ref.]
- Ng AC, Kumar SK, Rajkumar SV, et al. 2009. Impact of vitamin D deficiency on clinical presentation and prognosis of patients with newly diagnosed multiple myeloma. Am J Hematol. 84: 397-400.[Ref.]
- Kricker A, Armstrong BK, Hughes AM, et al. 2008. Personal sun exposure and risk of non-Hodgkin lymphoma. A pooled analysis from the interlymph consortium.Int .J.Cancer. 122: 144-154. [Ref.]
- Raina V, Cunningham D, Gilchrist N, et al. 1991. Alfacalcidol is a nontoxic, effective treatment of follicular small-cleaved cell lymphoma. Br J Cancer.63: 463-465. [Ref.]
- Shanafelt TD, Drake MT, Maurer MJ. 2011. Vitamin D insufficiency and prognosis in chronic lymphocytic leukemia. Blood. 117: 1492-1498. [Ref.]
- JB Arlet, Celine Callens, Olivier Hermine, et al. 2011. CLL responsive to vitamin D administration, BJH. 156: 148-149. [Ref.]
- Bohnsack BL, Hirschi K. 2004. Nutrient regulation of cell cycle progression. Ann Rev Nutri. 24: 433-453. [Ref.]
- Bai M, Vlachonikolis J, Agnantis NJ, et al. 2001. Low expression of p27 protein combined with altered p53 and Rb/p16 expression status is associated with increased expression of cyclin A and cyclin B1 in diffuse large B-cell lymphomas. Mod Pathol. 14: 1105-1113. [Ref.]
- Provvedini DM, Tsoukas CD, Deftos LJ. et al. 1983. 1,25-dihydroxyvitamin D? receptors in human leucocytes.Science. 221: 1181-1183. [Ref.]
- Drake MT, Maurer MJ, Link BK, et al. 2010. Vitamin D insufficiency and prognosis in Non Hodgkin Lymphoma, J Clin Oncol. 28: 4191-4198. [Ref.]
- Wang W, Li G, He X, et al. 2015. Serum 25-hydroxyvitamin D levels and prognosis in hematological malignancies: a systematic review and meta-analysis. Cell Physiol Biochem. 35: 1999-2005. [Ref.]
- Aref S, Ibrahim L, Azmy E. 2013. Prognostic impact of serum 25-hydroxivitamin D [25(OH)D] concentrations in patients with lymphoid malignancies. Hematol. 18: 20-25. [Ref.]
- Parveen S, Zeeshan R, Sultan S, et al. 2015. Serum 25-hydroxyvitamin D Insufficiency in B-Chronic Lymphoid Leukemia at the Time of Disease Presentation in Pakistan. Asian Pacific Journal of Cancer Prevention. 16: 5983-5986. [Ref.]
- Molica S, Digiesi G, Antenucci A, et al. 2012. Vitamin D insufficiency predicts time to first treatment (TFT) in early chronic lymphocytic leukemia (CLL). Leuk Res. 36: 443-447. [Ref.]
- Seubwai W, Wongkhan C, Puapairoj, et al. 2007. Overexpression of vitamin D receptor indicates a good prognosis for cholangiocarcinoma ; implications for therapeutics ,Cancer. 109: 2497-2505. [Ref.]
- Kelly JL, Salles G, Goldman B, et al. 2015. Low Serum Vitamin D Levels Are Associated with Inferior Survival in Follicular Lymphoma: A Prospective Evaluation in SWOG and LYSA Studies. JCO. 33: 1482-1490. [Ref.]




















