Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ojrmi.2021.110015Article Views : 159Article Downloads : 89
Clinical relevance of combining Thermography with Nailfold Capillaroscopy to improve differentiation between primary and secondary Raynaud’s Phenomenon
Yumiko Vreeburg MD1, Laura van Vugt2, Sabrina Simonnet MSc2, Daphne Valk3, Gus Schardijn MD PhD2, Willem Lems MD PhD1 and Richard van Vugt MD PhD1,2*
1Department of Rheumatology, Amsterdam University Medical Centre, Amsterdam, The Netherlands
2Specialisten Polikliniek Buitenveldert, Amsterdam, The Netherlands
3Veterinary Department, Artis Natura Magistral Royal Zoo, The Netherlands
*Corresponding Author: Dr. Richard van Vugt MD PhD, Amsterdam University Medical Centre-Department of Rheumatology, De Boelelaan 1117, 1081HV Amsterdam, The Netherlands, Tel: +31-(0)20-444-4444; Email: r.vanvugt@amsterdamumc.nl
Article Information
Aritcle Type: Research Article
Citation: Yumiko Vreeburg, Laura van Vugt, Sabrina Simon, et al. 2021. Clinical relevance of combining Thermography with Nailfold Capillaroscopy to improve differentiation between primary and secondary Raynaud’s Phenomenon. O J Radio Med Img. 4: 39-48.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2021; Yumiko Vreeburg
Publication history:
Received date: 22 March, 2021Accepted date: 31 March, 2021
Published date: 02 April, 2021
Abstract
Objectives: Early microvascular damage and dysfunction are clinically mirrored in Raynaud’s phenomenon (RP). Currently, nailfold capillaroscopy (NC) is applied to differentiate between primary RP (PRP) and secondary RP (PRP), associated with connective tissue disease. However, abnormal morphology can also be caused due to age-related changes and cardiovascular disease. Thermography (TG) is a non-invasive technique which enables quantification of cutaneous vascular function. An approach using both NC and TG could improve the differentiation between PRP and SRP.
Methods: Thirty RP patients (PRP, n = 21; SRP, n = 9) underwent nailfold capillaroscopy and thermography. Morphologic features were scored and patients were categorized according to the guidelines of EULAR Study group on Microcirculation in Rheumatic Diseases. TG of the hand was performed before, directly and ten minutes after a cold challenge test. Baseline images and rewarming curves were analyzed.
Results: Capillary abnormalities with NC were found in all SRP patients (9/9) and in 48% (10/21) of PRP patients. Out of 10 PRP patients with altered capillary morphology, 9 (90%) had a cardiovascular disease. For all patients mean temperature was significantly higher 10 minutes after cold induction than before (p < 0,01). The gradient of the rewarming curve was significantly lower in patients with SRP compared to PRP patients (p = 0.015).
Conclusions: Nailfold capillaroscopy and thermography can reliably be used to measure microvascular damage and dysfunction. Additional thermography can assist in differentiating between PRP and SRP, especially in elderly patients or in presence of a cardiovascular disease.
Keywords: Raynaud’s phenomenon; Nailfold capillaroscopy; Thermography
Key messages
• Currently, nailfold capillaroscopy is applied to differentiate between primary Raynaud’s phenomenon and secondary Raynaud’s phenomenon, associated with connective tissue disease.
• However, abnormal morphology can also be caused due to age-related changes and cardiovascular disease.
• Thermography is a non-invasive technique which enables quantification of cutaneous vascular function.
• The approach using both nailfold capillaroscopy and thermography can improve the differentiation between primary and secondary Raynaud’s phenomenon.
• Prospective studies using thermography can be conducted to investigate the change in vascular function over time to explore the possibility of predicting diagnosis (primary or secondary Raynaud’s phenomenon) and evaluating therapeutic efficacy.
Introduction
Microvascular damage and dysfunction represent the earliest morphological and functional markers of a progressive connective tissue disease. These early microvascular changes are clinically mirrored in Raynaud’s phenomenon (RP) defined as episodic attacks of artery and arteriole vasoconstriction. This is characterized by three stages of discoloration of the digits, in response to stimuli such as stress, cold environment and certain medication [1]. RP can be divided into two groups: primary (PRP), usually benign, and secondary (SRP) which is often associated with connective tissue disease [1,2]. Due to the difference in prognosis and treatment, it is crucial to (timely) differentiate between these two forms [2]. A frequently encountered difference between these two patient groups is the altered capillary morphology in SRP patients [1,2]. Currently, nailfold capillaroscopy (NC) is being used to study the capillary morphology and facilitate the differentiation between the two forms of RP [2,3]. Due to the high accuracy in early detection of SRP, this technique has become an essential part of the clinical work-up in patients with RP [2]. However, a study in 3029 patients with a mean follow-up of 4.8 years described the progression of PRP into SRP in at least 1123 (37.1%) patients with a normal capillary morphology at baseline [4]. In addition, abnormal capillary morphology can also be caused due to age-related changes and has been described in patients with diabetes and cardiovascular disease [3,5,6]. These limitations can therefore lead to an increase in false positives as well as false negatives when only abnormal capillary morphology is considered in diagnosing SRP. This warrants the evaluation of other (functional) techniques to improve the diagnostic yield. Thermography (TG) is a non-invasive technique which enables quantification of cutaneous blood vessel function by measuring differences in skin temperature [7]. These physiological alterations precede anatomical abnormalities observed in NC [8]. Recent studies have reported the use of TG to detect joint inflammation, infections and peripheral vascular disease [7,9,10]. TG has also successfully been implemented to detect functional differences between RP patients and healthy subjects [11,12]. Furthermore, the additional value of TG in comparison to NC has been demonstrated in differentiating between patients with systemic sclerosis, PRP and healthy subjects [13]. However, to date there is no data with regard to the accuracy of TG in differentiating between PRP and SRP, as the previous reports in literature have focused on patients with systemic sclerosis. This study investigated the clinical relevance of a combined approach using thermography and nailfold capillaroscopy towards improving differentiation between primary- and secondary Raynaud's phenomenon.
Methods
This study was conducted in a tertiary institution in Amsterdam, the Netherlands. The study complied with the declaration of Helsinki and written informed consent was obtained from the study subjects.
Patients
The study population consisted of patients with known primary and secondary Raynaud’s phenomenon. PRP patients were defined as patients with Raynaud’s phenomenon, without any clinical suspicion or laboratory findings (such as antibodies) which supported an underlying systemic disease. Patients were excluded in case of an active arthritis of the hands.
Nailfold capillaroscopy
NC was performed by an experience rheumatologist using a Greenough Optical System (Olympus SZ51, Olympus Corporation). The subject was seated in a room with a temperature of 24°C and the hands were placed at heart level [14]. A clear image of the capillaries was obtained by using immersion oil in the nailfold and by enhancing the image with a 100x magnification. Nailfold images were taken in digit II-V of both hands. The nailfold morphologic features were analyzed manually by an experienced rheumatologist.
Morphologic features were defined as follows:
• Capillary density: Loss of capillary loops associated hypoxemia (≤ 7 over 1mm of the nail fold).
• Giant capillaries: giant capillaries (enlarged microvascular loops) are the earliest and most striking feature of secondary RP. An increased diameter above 50 ?m was considered as pathologically enlarged.
• Shape of individual capillaries: Capillaries with a “hairpin” shape, (once or twice) crossing shape or tortuous shape (the afferent and efferent limb bend [= undulate] but do not cross) are defined as being “normal”, on the condition that the tip of the capillary is convex. All other shapes are defined as being “abnormal” [15].
• Hemorrhages: Extra vasa ton of red blood cells due to capillary wall damage [16-19].
• Each finger was scored on the four morphologic features and categorized into one of two categories, following the fast-track algorithm of the EULAR Study Group on Microcirculation [3,14]. The fast-track protocol focusses mainly on giant capillaries; however, it has been reported earlier that a classification based on capillary density and hemorrhages in addition to giant capillaries might be more reliable [20]. Therefore, we combined these two classification methods to categorize the patients in a ‘normal NC’ (fast track category 1) and an ‘abnormal NC’ group (fast track category 2).
Thermography
TG of the hand was performed using an infrared camera (Flir B620, FLIR Systems). The thermographic images were evaluated by two independent researchers. For the acquisition, the subjects were seated in a special room, with constant temperature of 24°C measured by three different thermometers for ten minutes for the hands to acclimatize to room temperature. The hands were then exposed to manchettes (long sleeved gloves) of 3-4°C for five minutes. Thermography was performed on the dorsal aspect of both hands and thermographic images were taken before (baseline), directly after (T0) and ten minutes (T10) after the cold challenge test. The temperature was measured at the base and distal phalanx of digit II-V using the infrared camera. The thermographic images were analyzed after scaling for room temperature and distance using a FLIR Quick Report 1.2 SP1 program. Rewarming curves were determined between T0 and T10.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 22.0. Continuous variables were expressed as mean ± SD. Mean and standard deviation of the temperatures were calculated per patient group (PRP/SRP) over different conditions (baseline, after immersion and 10 minutes after immersion) and analyzed using independent sample T-tests. At last, we calculated the recovery time of the temperature per minutes between ’after immersion’ and ’10 min after immersion’ for SRP and PRP groups. A p-value <0.05 was considered statistically significant.
Results
Thirty patients with Raynaud’s phenomenon (PRP, n = 21; SRP, n = 9) were included in this study. The study population was mostly female (n = 27; 90%). The mean age at inclusion was 68 years (range 56-85 years) in the PRP group, 71 years in SRP group (range 64-87). In total, 21 patients suffered from cardiovascular disease, which consisted of cerebrovascular attacks, transient ischemic attacks, myocardial infarction, hypertension, diabetes mellitus, hyperlipidemia, valvular heart disease and arrhythmias. The definitive diagnoses in the SRP group included sarcoidosis, rheumatoid arthritis, systemic sclerosis, Sjogren’s syndrome and psoriatic arthritis.
Additional patient characteristics are provided in table 1.
|
Table 1: Baseline Characteristics. |
||
|
|
Primary RP (N=21) |
Secondary RP (N=9) |
|
Age (years) |
68 (range 56-85) |
71 (range 64-87) |
|
Sex (M) |
3 (14%) |
0 |
|
CVD |
14 (19%) |
7 (44%) |
|
Smoking |
4 (19%) |
3 (33%) |
|
Medication |
|
|
|
- ASA |
7 (33%) |
4 (44%) |
|
- OAC |
2 (10%) |
0 |
|
Diagnosis |
|
|
|
- Systemic Sclerosis |
- |
3 (33%) |
|
- Other CTD |
- |
6 (67%) |
|
CVD, cardiovascular disease; ASA, acetylsalicylate acid; OAC, oral anticoagulation; CTD, connective tissue disease. |
||
Nail fold capillaroscopy
Half of the patients with PRP (n=11, 52%) had a normal NC. Capillary abnormalities were found in all patients with SRP (9/9) and in 48% (10/21) of patients with PRP. Nine out of the ten PRP patients with altered capillary morphology (90%) suffered from cardiovascular disease (hypertension, myocardial infarction, cerebrovascular event). In the PRP group with normal capillary morphology only five out of eleven patients (45%) had cardiovascular disease (hypertension of TIA).
Thermography
In patients with PRP, temperature significantly decreased after cold induction (-2.34 °C, p = 0,01), whereas in SRP patient temperature stayed consistent (+0.07 °C, p = 0,46) (figure 1, left column). For all patients the mean temperature was significantly higher 10 minutes after cold induction (30.38°C) than before cold induction (29.82°C), (+0,56°C, p <0,01). This significant increase in temperature 10 minutes after cold challenge test was also present in the SRP group (SRP +1.64 °C, p < 0,01) (figure 1, right column). The gradient of the rewarming curve was significantly lower in patients with SRP compared to the PRP group (figure 2, median 0.16 vs. 0.26°C/min; p = 0.015). The speed of recovery of the fifth digit was significantly faster in both groups compared to the other digits (0,52°C/min vs. 0.15°C/min for dig V and the average for dig II-IV respectively; p < 0.01). TG results are summarized in table 2.
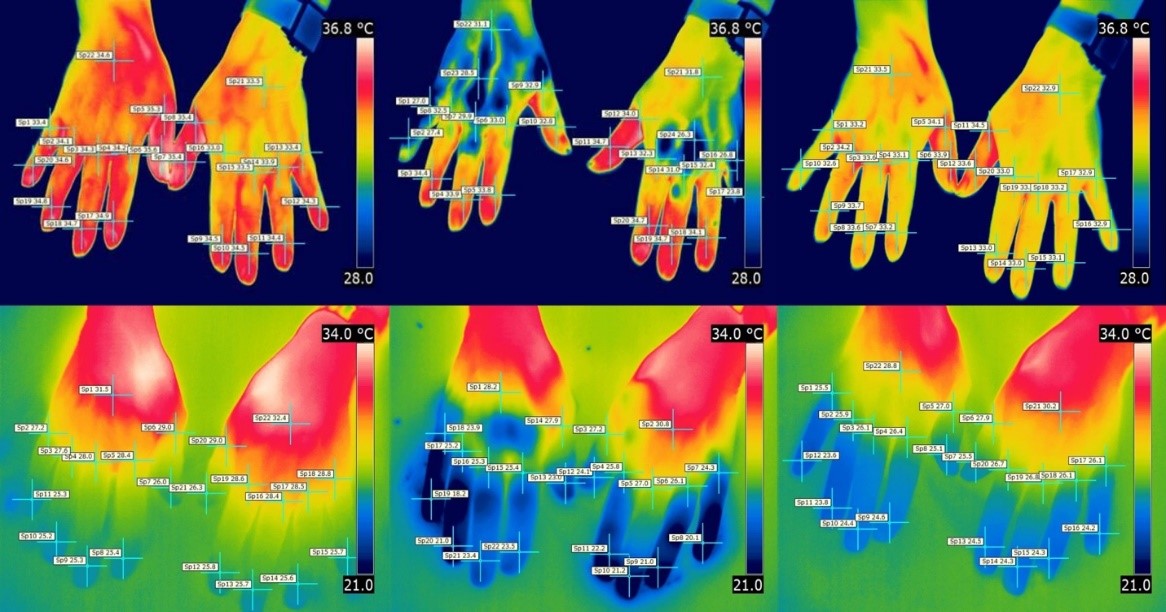
Figure 1: Thermographic images at baseline, directly after (T=0) and 10 min after cold challenge test (T=10).
Left column image depicts the TG images at baseline for the PRP group (top row) and SRP group (bottom row). A lower average temperature was found directly after the cold challenge test in the PRP group compared to the SRP group (middle column). Right column shows the increase in temperature ten minutes after cold induction for both groups.
|
Table 2: Average temperature and recovery speed between PRP and SRP groups measured with thermography. |
||||
|
|
Baseline |
T = 0 min (SD) |
T = 10 min (SD) |
DTemp (°C/min) |
|
Primary Raynaud |
29.49 °C |
27.15 °C (1.65)* |
29.77 °C (0.20) |
0.26 |
|
Secondary Raynaud |
31.17 °C |
31.24 °C (0.05) |
32.81 °C (1.16)* |
0.16 |
|
All patients |
29.82 °C |
27.97 °C (1.31)* |
30.74 °C (0.39)* |
0.24 |
|
* p<0.05 compared to baseline temperature. |
||||
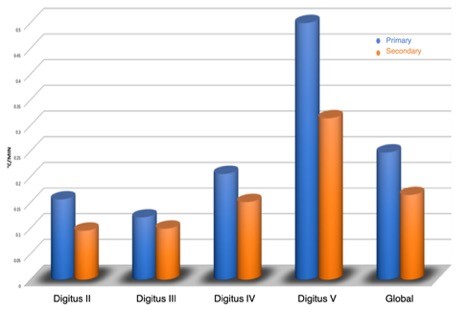
Figure 2: Temperature recovery speed for primary and secondary Raynaud.
The gradient of the rewarming curve was significantly lower in patients with SRP (orange bars) compared to the PRP group (blue bars) (median 0.16 vs. 0.26 °C/min; p = 0.015).
|
Table 3: Recovery speed per digit for all patients (PRP and SRP) with Thermography. |
|
|
|
DTemp (°C/min) |
|
Digit II |
0,15* |
|
Digit III |
0,10* |
|
Digit IV |
0,19* |
|
Digit V |
0,52* |
|
* p<0.05 |
|
Discussion
This study investigated the clinical relevance of combining thermography and nailfold capillaroscopy in differentiating between primary and secondary Raynaud’s phenomenon in elderly patients. To our knowledge, this is the first study to report the use of NC and TG to differentiate between PRP and SRP in the general population. In addition, this is also the first study to expose extremities to an extreme temperature of three degrees Celsius. Cold challenge tests have been described earlier up to temperatures of nine degrees Celsius with a duration of maximum four minutes [11,12,21,22]. The main findings are as follows: (1) a part of the patients with PRP have capillaroscopic abnormalities; (2) temperature of the fingers in SRP patients remains constant after cold induction; and (3) rewarming of the fingers in SRP patients is slower than in PRP patients.
Capillaroscopic abnormalities
The benign primary Raynaud’s phenomenon is caused by vasospasms and is not associated with vascular damage (1). Therefore, it can be hypothesized that NC in these patients should show a normal vascular morphology. However, there are several studies reporting capillaroscopic abnormalities in patients with PRP. In some cases, this can be explained by the persistently present vasospasm, in which the abnormalities are categorized according to the ‘borderline pattern’ (18). However, abnormalities can also be associated with vascular damage caused by aging, diabetes mellitus and cardiovascular disease [3,5,6]. These findings are supported by our results where an abnormal NC was found in 48% of the patients with PRP group (n=10/21). In addition, cardiovascular disease was present in 90% of the PRP patients with altered capillary morphology (n=9/10). This percentage was significantly lower in the group with normal NC (36%). These findings indicate that cardiovascular disease could contribute towards morphological changes of capillaries in absence of underlying systemic disease. Therefore, it appears that NC alone is inaccurate for characterizing the differences between PRP and SRP.
Effects of temperature changes
The inflammatory process in systemic diseases cause irreversible damage to vascular walls as a result of fibrosis formation. The affected vessels are often unable to appropriately constrict or dilate in reaction to stimuli such as cold [8]. Therefore, as TG provides functional information, it could be a valuable addition in the diagnostic process of SRP. Most studies have found skin temperature at baseline and the rewarming curve to be the most reliable parameters for differentiating between PRP and SRP [11,12,22]. Our findings support this by demonstrating that the rewarming curve in the SRP group had a lower slope than the curve in the PRP group. In addition, discordant to previous reports, we found that hand temperature in the SRP group did not decrease after the cold challenge. The absence is even more striking as our cold challenge test was performed with a more extreme temperature and the hands were exposed to this temperature longer than in most other cold challenge tests. The lack of response in hand temperature could be explained by the abnormal vascular reactivity, as the capillaries might not be able to constrict sufficiently. For all patients the mean temperature was significantly higher 10 minutes after cold induction than before cold induction. This finding supports the effectiveness of conditioning therapy by exposing the hands to extreme temperatures, both warm and cold, which has been implemented for over 2.000 years and has been described by Jobe et al, [23]. The efficacy could be explained by the induction of vasodilatation and therefore increased circulation. Furthermore, it has been describing that salicylates can have a dilating effect on vessels which can amplify the vasodilatory effect of the rewarming phase [24]. There was a faster speed of rewarming of the fifth digit in comparison to the other fingers in both groups. As there is no known difference in vascularization between the fingers, a potential explanation for this finding might be the smaller skin surface.
Limitations
A limitation of this study is the relatively small sample size. Although the 30 patients included in this study can be considered as a lower limit for statistical evaluation, the results indicate the clinical applicability and feasibility of the proposed workflow. In addition, the study population is predominantly female (90%). However, this is a similar distribution as the occurrence of RP in the general population and therefore we consider our population to be a representative sample. Furthermore, diagnosis was known or could be interpreted due to clinical symptoms, such as sclerodactyly and cotton’s papules by the rheumatologist performing the NC. Therefore, this could lead to a selection bias. However, this was not the case for the TG, as the images were taken and analyzed by two non-clinical researchers. Another limitation is the effect of incalculable internal and external stimuli on skin temperature, as this could affect the TG results. Finally, a higher frequency of temperature measurements could be helpful in estimating a more accurate evolution of temperature.
Future perspectives
Recent developments in TG equipment, such as low-cost TG cameras and applications for smartphones have increased the availability of this method, offering the prospect to implement this for the daily clinical routine [25,26]. In future, prospective studies should be conducted to investigate the change in vascular function over time to explore the possibility of predicting diagnosis (primary or secondary RP) and evaluating therapeutic efficacy such as intravenous proteinoids, endothelin receptor antagonist and sympathectomy. Furthermore, studies to investigate the cost-efficiency and clinical efficacy of this addition in clinical practice should be performed. Although the cold challenge test is the most commonly used, this could induce a Raynaud attack. A recent study has described performing TG using a heat challenge test. As this test is more patient friendly and has comparable accuracy in differentiating between PRP and SRP, future studies should aim to confirm these findings in a larger patient population [8].
Conclusion
Nailfold capillaroscopy and thermography can reliably be used to measure microvascular damage and dysfunction. Additional thermography is better suitable to differentiate between older patients with PRP and SRP. Furthermore, in presence of cardiovascular disease, thermography appears to be a reliable technique to complement nailfold capillaroscopy in differentiating between patients with PRP and SRP.
References
1. Herrick A, Muir L. 2014. Raynaud’s phenomenon (secondary). BMJ Clin. Evid. Ref.: https://pubmed.ncbi.nlm.nih.gov/25322727/
2. Belch J, Carlizza A, Carpentier PH, et al. 2017. ESVM guidelines – the diagnosis and management of Raynaud’s phenomenon. Vasa. 46: 413-423. Ref.: https://pubmed.ncbi.nlm.nih.gov/28895508/ https://doi.org/10.1024/0301-1526/a000661
3. Ocampo-Garza SS, Villarreal-Alarcón MA, Villarreal-Treviño AV, et al. 2019. Capilaroscopia: una herramienta diagnóstica valiosa. Actas Dermosifiliogr. Ref.: https://pubmed.ncbi.nlm.nih.gov/30851874/ https://doi.org/10.1016/j.ad.2018.10.018
4. Pavlov-Dolijanovic S, Damjanov NS, Stojanovic RM, et al. 2012. Scleroderma pattern of nailfold capillary changes as predictive value for the development of a connective tissue disease: a follow-up study of 3,029 patients with primary Raynaud’s phenomenon. Rheumatol. Int. 32: 3039-3045. Ref.: https://pubmed.ncbi.nlm.nih.gov/21901350/ https://doi.org/10.1007/s00296-011-2109-2
5. Sanchez-Garcia ME, Ramirez-Lara I, Gomez-Delgado F, et al. 2018. Quantitative evaluation of capillaroscopic microvascular changes in patients with established coronary heart disease. Med. Clin. (Barc). 150: 131-137. Ref.: https://pubmed.ncbi.nlm.nih.gov/28870422/ https://doi.org/10.1016/j.medcli.2017.06.068
6. Maldonado G, Guerrero R, Paredes C, et al. 2017. Nailfold capillaroscopy in diabetes mellitus. Microvasc. Res. 112: 41-46. Ref.: https://pubmed.ncbi.nlm.nih.gov/28274735/ https://doi.org/10.1016/j.mvr.2017.03.001
7. Jones BF. 1998. A reappraisal of the use of infrared thermal image analysis in medicine. IEEE Trans. Med. Imaging. 17: 1019-1027. Ref.: https://pubmed.ncbi.nlm.nih.gov/10048859/ https://doi.org/10.1109/42.746635
8. Chojnowski M. 2017. Infrared thermal imaging in connective tissue diseases. Reumatologia/Rheumatology. 1: 46-51. Ref.: https://pubmed.ncbi.nlm.nih.gov/28386141/ https://doi.org/10.5114/reum.2017.66686
9. Ring EFJ, Ammer K. 2012. Infrared thermal imaging in medicine. Physiol. Meas. 33: 33-46. Ref.: https://pubmed.ncbi.nlm.nih.gov/22370242/ https://doi.org/10.1088/0967-3334/33/3/r33
10. van der Weijden MAC, van Vugt LM, Valk D, et al. 2017. Exploring thermography: a promising tool in differentiation between infection and ischemia of the acra in systemic sclerosis. Int. J. Rheum. Dis. 20: 2190-2193. Ref.: https://pubmed.ncbi.nlm.nih.gov/27038005/ https://doi.org/10.1111/1756-185x.12859
11. Horikoshi M, Inokuma S, Kijima Y, et al. 2016. Thermal Disparity between Fingers after Cold-water Immersion of Hands: A Useful Indicator of Disturbed Peripheral Circulation in Raynaud Phenomenon Patients. Intern. Med. 55: 461-466. Ref.: https://pubmed.ncbi.nlm.nih.gov/26935364/ https://doi.org/10.2169/internalmedicine.55.5218
12. Cherkas LF, Carter L, Spector TD, et al. 2003. Use of thermographic criteria to identify Raynaud’s phenomenon in a population setting. J. Rheumatol. 30: 720-722. Ref.: https://pubmed.ncbi.nlm.nih.gov/12672189/
13. Murray AK, Moore TL, Manning JB, et al. 2009. Noninvasive imaging techniques in the assessment of scleroderma spectrum disorders. Arthritis Rheum. 61: 1103-1111. Ref.: https://pubmed.ncbi.nlm.nih.gov/19644893/ https://doi.org/10.1002/art.24645
14. Cutolo M, Pizzorni C, Secchi ME, et al. 2008. Capillaroscopy. Best Pract. Res. Clin. Rheumatol. 22: 1093-1108. Ref.: https://pubmed.ncbi.nlm.nih.gov/19041079/ https://doi.org/10.1016/j.berh.2008.09.001
15. Cutolo M, Melsens K, Herrick AL, et al. 2018. Reliability of simple capillaroscopic definitions in describing capillary morphology in rheumatic diseases. Rheumatol. (United Kingdom). 57: 757-759. Ref.: https://pubmed.ncbi.nlm.nih.gov/29361155/ https://doi.org/10.1093/rheumatology/kex460
16. Cutolo M, Sulli A, Smith V. 2013. How to perform and interpret capillaroscopy. Best Pract. Res. Clin. Rheumatol. 27: 237-248. Ref.: https://pubmed.ncbi.nlm.nih.gov/23731933/ https://doi.org/10.1016/j.berh.2013.03.001
17. Maricq HR, LeRoy EC. Patterns of finger capillary abnormalities in connective tissue disease by "wide-field" microscopy. Arthritis Rheum. 16: 619-628. Ref.: https://pubmed.ncbi.nlm.nih.gov/4742842/ https://doi.org/10.1002/art.1780160506
18. Cutolo M, Sulli A, Secchi ME, et al. 2007. The contribution of capillaroscopy to the differential diagnosis of connective autoimmune diseases. Best Pract. Res. Clin. Rheumatol. 21: 1093-1108. Ref.: https://pubmed.ncbi.nlm.nih.gov/18068864/ https://doi.org/10.1016/j.berh.2007.10.001
19. Smith V, Vanhaecke A, Herrick AL, et al. 2019. Fast track algorithm: How to differentiate a “scleroderma pattern” from a “non-scleroderma pattern”. Autoimmun. Rev. 18: 102394. Ref.: https://pubmed.ncbi.nlm.nih.gov/31520797/ https://doi.org/10.1016/j.autrev.2019.102394
20. Boulon C, Devos S, Mangin M, et al. 2017. Reproducibility of capillaroscopic classifications of systemic sclerosis: results from the SCLEROCAP study. Rheumatology. 56: 1713-1720. Ref.: https://pubmed.ncbi.nlm.nih.gov/28957554/ https://doi.org/10.1093/rheumatology/kex246
21. van Roon AM, van Roon AM, Stel AJ, et al. 2020. Assessing recovery after cold challenge and thumb involvement can help to rule out systemic sclerosis in patients presenting with Raynaud’s phenomenon. Scand. J. Rheumatol. 49: 137-140. Ref.: https://pubmed.ncbi.nlm.nih.gov/31637927/ https://doi.org/10.1080/03009742.2019.1643911
22. Anderson ME, Moore TL, Lunt M, et al. 2007. The ‘distal-dorsal difference’: a thermographic parameter by which to differentiate between primary and secondary Raynaud’s phenomenon. Rheumatology. 46: 533-538. Ref.: https://pubmed.ncbi.nlm.nih.gov/17018538/ https://doi.org/10.1093/rheumatology/kel330
23. Jobe JB, Beetham WP, Roberts DE, et al. 1985. Induced vasodilation as a home treatment for Raynaud’s disease. J. Rheumatol. 12: 953-956. Ref.: https://pubmed.ncbi.nlm.nih.gov/4087271/
24. Ying Z, Giachini FRC, Tostes RC, et al. Salicylates dilate blood vessels through inhibiting PYK2-mediated RhoA/Rho-kinase activation. Ref.: https://pubmed.ncbi.nlm.nih.gov/19276129/ https://doi.org/10.1093/cvr/cvp084
25. Kanazawa T, Nakagami G, Goto T, et al. 2016. Use of smartphone attached mobile thermography assessing subclinical inflammation: a pilot study. J. Wound Care. 25: 177-182. Ref.: https://pubmed.ncbi.nlm.nih.gov/27064366/ https://doi.org/10.12968/jowc.2016.25.4.177
26. Xue EY, Chandler LK, Viviano SL, et al. 2018. Use of FLIR ONE Smartphone Thermography in Burn Wound Assessment. Ann. Plast. Surg. 80: 1. Ref.: https://pubmed.ncbi.nlm.nih.gov/29489530/ https://doi.org/10.1097/sap.0000000000001363




















