Indexing & Abstracting
Full Text
ProcedureDOI Number : 10.36811/ojrmi.2021.110013Article Views : 156Article Downloads : 98
Biopince Full Core Biopsy Device for Percutaneous Lung Biopsy: A Retrospective Analysis of 184 Procedures Analyzing Complication Rates and Procedure Success
Robert Bernstein, MD, PhD* and Ryan Garrow, PA-C
University Diagnostic Medical Imaging, Bronx, NY (Bernstein, Garrow), USA
*Corresponding Author: Robert Bernstein, MD, PhD, University Diagnostic Medical Imaging, 1200 Waters Place Suite M108, Bronx, NY 10461; Email: Drdrbob1969@gmail.com
Article Information
Aritcle Type: Procedure
Citation: Robert Bernstein, Ryan Garrow. 2021. Biopince Full Core Biopsy Device for Percutaneous Lung Biopsy: A Retrospective Analysis of 184 Procedures Analyzing Complication Rates and Procedure Success. O J Radio Med Img. 4: 17-25.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2021; Robert Bernstein
Publication history:
Received date: 03 February, 2021Accepted date: 12 February, 2021
Published date: 15 February, 2021
Abstract
Background: Unlike fine needle aspiration, core needle biopsies allow the collection of intact tissue for pathological and molecular evaluation. In outpatient clinical practice, full core needle lung biopsy may be underused because of concerns that it might be too dangerous. We describe our experience using a full core device for percutaneous lung biopsy in a large cohort of patients. Research Question: Is percutaneous full core needle lung biopsy effective and safe in the outpatient setting?
Study Design and Methods: The analyzed population comprised patients with lung masses >1.1 cm who underwent percutaneous lung biopsy with a full core device. Analyzed data included core mass dimensions, distance from pleural edge to mass, lobe location, type, outcomes, and complications. Biopsy success was defined as adequate tissue acquisition for pathological evaluation that yielded a diagnosis. Biopsy procedures with incomplete data were excluded from this analysis.
Results: We analyzed data from 184 lung biopsies performed on 182 patients (mean age, 70±11.7 years). Most biopsies were parenchymal (54.9%). The overall diagnostic success rate was 98.4%. No complications were reported for 77.2% of biopsies. Minor complications occurred during 39 biopsies (21.2%) and were primarily pneumothorax (16.8%). Major complications occurred during 4 biopsies (2.1%): 3 patients with pneumothorax required emergency department (ED) management and 1 patient went to the ED for severe pain. All complications resolved within 24 hours without hospitalization or transfusion. Crosstabulation analyses showed no significant differences between the lung lobe locations in terms of rates of disposition and complications, and between the lesion types in terms of rates of disposition and complications.
Interpretation: Percutaneous lung biopsy performed using a full core biopsy device demonstrated a high rate of diagnostic success and a low risk of clinically significant procedural complications in an outpatient setting.
Background
Image-guided percutaneous needle biopsy is primarily performed using core (also called, cutting) needle or fine-needle aspiration (FNA) technologies and is an essential tool for the assessment of patients with pulmonary abnormalities either not assessable by bronchoscopy or in patients who cannot tolerate bronchoscopy [1]. The choice between these core or fine-needle technologies is dependent upon factors such as size and location of target lesion, purpose for the sample sought, and operator training and preference [2]. While core needle biopsy has been associated with a higher rate of complications than FNA [3], core needle biopsies may be better than FNA at providing diagnostic specimens for non-malignant lesions and for molecular analyses [4]. Unlike FNA, which provides samples suitable for cytological analyses, Full core needle biopsies allow the collection of intact tissue for full pathological evaluation including surface receptor and molecular analysis. In clinical practice, full core needle biopsy may be underused in the diagnosis of patients with lung lesions because of concerns that it might be too aggressive or dangerous in an outpatient setting.
Other authors have reported positive results using a tri-axial, end-cut system to perform full core image guided percutaneous biopsies in liver, lymph nodes, thyroid, lung, and other organs [5]. In the present retrospective study, we describe our experience using the same full core device for percutaneous lung biopsy in a large cohort of patients referred to our institution.
Methods
Patients
We retrospectively analyzed data from patients who underwent percutaneous lung biopsy with the Bio Pince full core, 18-gauge system (Argon Medical Device, Athens, TX) from February 5, 2009 to November 11, 2019, at our out-patient imaging facility (University Diagnostic Medical Imaging, Bronx, NY). Patients were considered for the procedure with lung masses greater than 1.1 cm and felt to be accessible by the radiologist. Each patient provided informed consent prior to the procedure. All data were de-identified prior to analysis.
This study used preexisting information and did not involve collection and analysis of personal health information. An Institutional Review Board (Sterling IRB, Atlanta, GA) reviewed the study and determined that it was exempt from IRB review pursuant to the terms of the US Department of Health and Human Service’s Policy for Protection of Human Research Subjects at 45 CFR §46.104(d).
Biopsy
Upon referral to our institution for the biopsy procedure, we reviewed the patient clinical history and pre-procedure imaging. Pre-procedure planning was performed to evaluate the safety of the procedure and to determine the best route for percutaneous access to the lung mass. All the procedures were performed by one radiologist with over 10 years of experience in minimally invasive, image-guided interventional radiology.
During this outpatient procedure, patients were placed in the optimum position to provide the safest path for advancing the biopsy needle into their lesion. Using local anesthesia (1% or 2% lidocaine) and aseptic technique, the biopsy needle was advanced into the lung mass using computed tomography (CT) imaging guidance. Three to 4 specimens were obtained and immediately placed in formalin. The specimens were sent to off-site pathology laboratories. After removal of the biopsy needle, patients were observed for 1hour post procedure for possible complications before discharge.
Data Collection and Analysis
Collected de-identified data included date of procedure, patient age, biopsy parameters, biopsy outcomes, complications, patient disposition, and pathology findings. Biopsy-specific parameters collected included mass dimensions (x-axis, y-axis, z-axis; in mm), distance from pleural edge to mass (mm), lobe location (right or left, lower or upper), type (pleural, parenchymal, hilar), and outcomes (ie, sufficient or insufficient/non-evaluable specimen for diagnosis). Biopsy success was defined as obtaining adequate tissue acquisition for pathological evaluation that yielded a diagnosis. Biopsy procedures with incomplete data were excluded from this analysis (a corrupted file in the picture archiving and communication system limited third axis measurement).
We used descriptive and frequency statistics to summarize sample characteristics. The prevalence of complications was calculated as a crude rate using frequency statistics. Major complications were defined as requiring hospitalization, emergency room evaluation, or additional interventional procedure (chest tube or manual aspiration) and minor complications were defined as pneumo- or hemothoraces that did not require intervention (less than 10% and/or clinically insignificant). The statistical assumptions of normality and homogeneity of variance was assessed before using between-subjects analyses to answer the research questions regarding biopsy sample acquisition, location, and complications, if any. When the assumptions were met, we employed parametric unpaired t-tests to compare independent groups on continuous outcomes. Mean and standard deviation (SD) were reported and interpreted for the t-test analyses. When statistical assumptions were violated, then nonparametric Mann-Whitney U tests were used to test for significant difference between groups. Medians and interquartile ranges were analyzed for the non-parametric analyses. Chi-square analysis was performed when independent groups were compared on categorical outcomes. Statistical significance was assumed at an alpha value of 0.05 and all analyses were performed using SPSS Version 26 (IBM Corporation, Armonk, NY).
Results
Between February 5, 2009 and November 11, 2019, we performed 196 percutaneous full core needle biopsies on 193 patients; 3 patients underwent 2 biopsies each. Twelve procedures were excluded because of missing data (images not retrievable, not all dimensions described). Thus, we analyzed data from 184 biopsies performed on 182 patients who had a mean age (SD) of 70 (11.7) years. Table 1 summarizes the biopsy parameters and outcomes. The most common biopsy lobe sites were left upper (29.9%), right upper (28.8%), and right lower (19.6%). Most biopsies (54.9%) were parenchymal.
|
Characteristics |
Data |
|
Target lesion dimensions, mma |
|
|
X-axis |
24.00 (19.00) |
|
Y-axis |
24.00 (21.00) |
|
Z-axis |
26.50 (20.00) |
|
Depth (distance from pleural edge to mass), mma |
1.00 (15.00) |
|
Lobe/location, n (%) |
|
|
Left/lower |
29 (15.8) |
|
Left/ upper |
55 (29.9) |
|
Right/lower |
36 (19.6) |
|
Right/middle |
11 (6.0) |
|
Right/upper |
53 (28.8) |
|
Type, n (%) |
|
|
Parenchymal |
101 (54.9) |
|
Pleural |
5 (2.7) |
|
Pleural/Parenchymal |
78 (42.4) |
|
a Median (interquartile range). |
|
Table 2 summarizes biopsy diagnostic outcomes and complications. The overall diagnostic success rate was 98.4% and only 3 biopsies (1.6%) provided non-diagnostic samples. No complications were reported for 78.5% of biopsies. Minor complications occurred during 39 biopsies (21.2%) and included 31 (16.8%) cases of pneumothorax, 6 (3.3%) cases of hemothorax, and 1 (0.5%) case each of hemoptysis and severe pain. Major complications occurred during 4 biopsies (2.1%) and included 3 patients with pneumothorax that required emergency department (ED) management and 1 patient who went to the ED for severe pain. This last patient had a 2% pneumothorax (classified as a minor complication) that did not require intervention. Among the 3 patients who went to the ED for pneumothorax, 2 patients had chest tube decompression, 1 had chest tube re-expansion, and 1 was managed clinically. None of the 4 patients with major complications required greater than 24 hours of observation. None of the patients experienced bleeding issues requiring hospitalization or transfusion.
Table 2: Biopsy Outcomes (N=184). |
|
|
Outcome per biopsy, n (%) |
Data |
|
Diagnostic outcome of biopsy |
|
|
Diagnostic |
181 (98.4) |
|
Non-diagnostic |
3 (1.6) |
|
Complications, n (%) |
|
|
No complication |
142 (77.2) |
|
Minor complication |
39 (21.2) |
|
Pneumothorax |
31 (16.8) |
|
Hemothorax |
6 (3.3) |
|
Hemoptysis |
1 (0.5) |
|
Severe pain |
1 (0.5) |
|
Major complications |
4 (2.1%) |
|
ED visit to manage pneumothorax |
3 (1.6%) |
|
ED visit to manage pain |
1 (0.5%) |
|
ED = emergency department. |
|
As shown in Table 3, between biopsy comparisons found no statistically significant differences between the complication groups (no complication versus complication) for age (t(179) = 0.51; P = 0.61), x-axis (U = 2693.5; P = 0.79), y-axis (U = 2705.0; P = 0.83), and Z-axis (U = 2716.0; P = 0.86). There was a statistically significant difference between the complication groups for the distance from pleural edge to mass (U = 1831.0; P = 0.001). As shown in Table 4, Chi-square cross-tabulation analysis found no significant differences between the different lobes for disposition (P = 0.87) and complications (P = 0.57). Additional cross-tabulation analyses using Mann-Whitney U tests revealed no differences between the types for disposition (P = 0.79) and complications (P = 0.13) (Table 5).
Table 3: Comparison of Biopsies with and without Complications. |
|||
|
Variable |
With Complication (n = 39) |
Without Complication (n = 142) |
P Value |
|
Patient age, ya |
70.31 (11.86) |
69.23 (11.16) |
0.61 |
|
Biopsy core dimensions, mmb |
|||
|
X-axis |
23.0 (19.0) |
25.0 (23.0) |
0.79 |
|
Y-axis |
24.0 (19.0) |
23.0 (25.0) |
0.83 |
|
Z-axis |
26.5 (18.0) |
24.0 (22.0) |
0.86 |
|
Distance from pleural edge to mass, mmb |
0.0 (9.0) |
15.0 (27.0) |
0.001 |
|
a Mean (SD). b Median (interquartile range). |
|||
Table 4: Lobe Cross-Tabulation. |
|||||
|
Lobe Level |
Disposition, n (%) |
With Complication, n (%) |
|||
|
|
Good |
Hemothorax |
Pneumothorax |
Non-diagnostic |
|
|
Left Lower |
21 (72.4) |
1 (3.4) |
7 (24.1) |
0 (0.0 ) |
8 (27.6) |
|
Left Upper |
43 (78.2%) |
0 (0.0%) |
12 (21.8%) |
0 (0.0%) |
12 (21.8%) |
|
Right Lower |
29 (80.6%) |
2 (5.6%) |
4 (11.1%) |
1 (2.8%) |
6 (17.1%) |
|
Right Middle |
7 (63.6%) |
1 (9.1%) |
3 (27.3%) |
0 (0.0%) |
4 (36.4%) |
|
Right Upper |
42 (79.2%) |
1 (1.9%) |
8 (15.1%) |
2 (3.8%) |
9 (17.6%) |
|
P Value 0.87 0.57 |
|||||
Table 5: Type Cross-Tabulation. |
|||||
|
Type Level |
Disposition |
With Complication, n (%) |
|||
|
|
Good |
Hemothorax |
Pneumothorax |
Non-diagnostic |
|
|
Parenchymal |
73 (72.3%) |
4 (4.0%) |
23 (22.8%) |
1 (1.0%) |
27 (27.0%) |
|
Pleural |
4 (80.0%) |
0 (0.0%) |
1 (20.0%) |
0 (0.0%) |
1 (20.0%) |
|
Pleural/Parenchyma |
65 (83.3%) |
1 (1.3%) |
10 (12.8%) |
2 (2.6%) |
11 (14.5%) |
|
P value 0.79 0.13 |
|||||
An analysis of parenchymal versus pleural/parenchymal types in relation to rates of complications reveled a statistically significant difference (P = 0.045), with a much higher rate (n = 27; 27.0%) in the arenchymal group compared with the pleural/parenchymal group (n = 11; 14.5%).
Discussion
In this retrospective analysis of 184 full core percutaneous needle lung biopsies, we report a 98.4% diagnostic success rate and a good safety profile, with a low incidence of clinically significant complications. Most (78.5%) of the biopsies we performed did not cause any complications. Minor complications occurred in 21.2% of biopsies and were primarily pneumothorax (16.8%) and hemothorax (3.3%) which resolved spontaneously. Only 4 biopsies (2.1%) caused major complications, classified as such because ED visits were required to manage pneumothorax in 3 patients or severe pain in 1 patient. All major and minor complications resolved within 24 hours of observation. There were no pneumothoraxes or bleeding issues necessitating hospitalization or transfusion. Cross-tabulation analyses showed no significant differences between the different lobe locations in terms of rates of disposition and complications, and between the lesion types in terms of rates of disposition and complications.
The 98.4% diagnostic success rate we report here compares well with similar analyses reported in the literature. A large study by Hee et al. [6], on the diagnostic accuracy of transthoracic needle lung biopsies reported a lower rate of non-evaluable results with core needle biopsy compared with FNA (1.3% versus 8.9%, respectively. Mills et al. [7], reported an 87.2% technical success rate in a single-institution retrospective study of consecutive percutaneous CT-guided core needle biopsies of lung lesions. In a retrospective study of CT-guided percutaneous lung biopsies, Beslic et al. [8], reported rates of definitive diagnoses for 79.6% of patients who had FNA and 96.8% of patients who had core needle biopsy.
The 21.2% overall complication rate and 16.8% rate of pneumothorax seen in our study falls within the range reported in other studies of percutaneous lung biopsies. A review by Winokur et al. [2], noted that pneumothorax rates in CT-guided percutaneous lung biopsy studies ranged from 17% to 26.6 %. The Heerink et al. [3], 2017 meta-analysis of CT-guided transthoracic lung biopsy reported pooled complication rates of 38.8% for core biopsy and 24.0% for FNA. A retrospective analysis by Yeow and colleagues [9] reported that pneumothorax (23%), hemoptysis (4%), and chest tube insertion (1%) were the most common complications of CT-guided percutaneous coaxial needle lung biopsies.
Full core device differs from other core biopsy devices in that they remove cylindrical specimens of tissue unlike side notch-style devices which remove partial cylindrical specimens. Full core biopsy devices allow for larger quantity of tissue obtained per biopsy when compared to side- notch style devices of the same gauge.
The main limitations of the present study are its retrospective single-center design and small sub-group sample size for the cross-tabulation analyses. Because the same experienced radiologist performed all the procedure, our results many not be reproducible in other settings. Accuracy of the diagnosis is also dependent on the experience of the cytology/histopathology personnel.
In conclusion, percutaneous lung biopsy performed using a full core biopsy device demonstrated higher rates of diagnostic success with no greater risk of procedural complications when compared to the literature for procedural success and complications utilizing side-notch style biopsy devices (Figure 1-4).
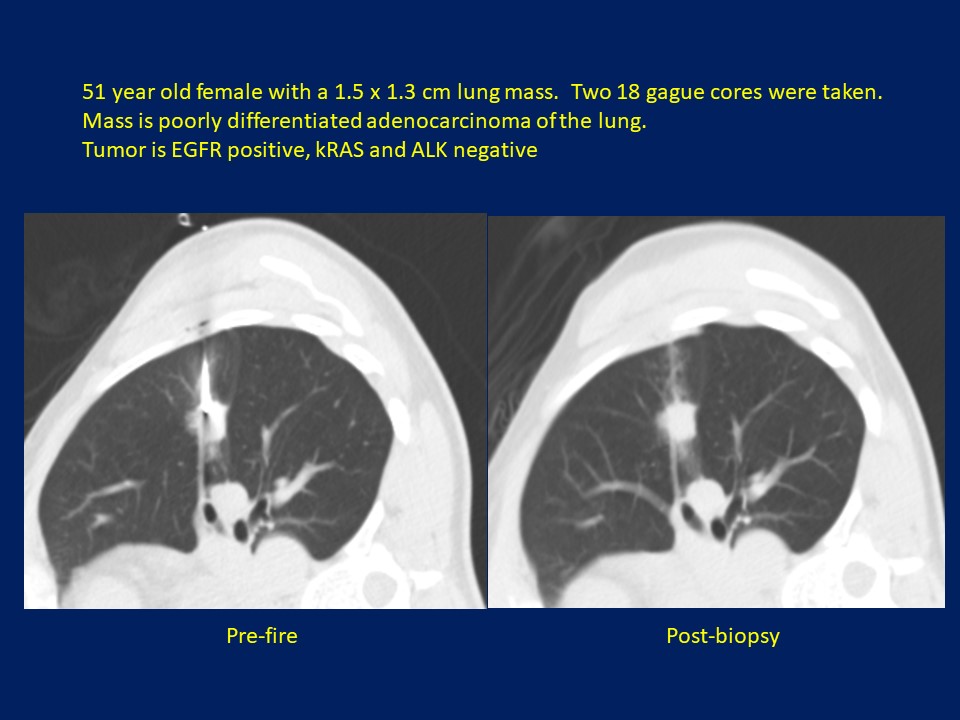
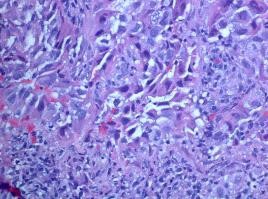
Figure 2: 51-year-old female with a 1.5 x 1.3 cm lung mass. Two 18 gague cores were taken. Mass is poorly differentiated adenocarcinoma of the lung. Tumor is EGFR positive, kRAS and ALK negative.
Pre-fire biopsy image Post-biopsy image
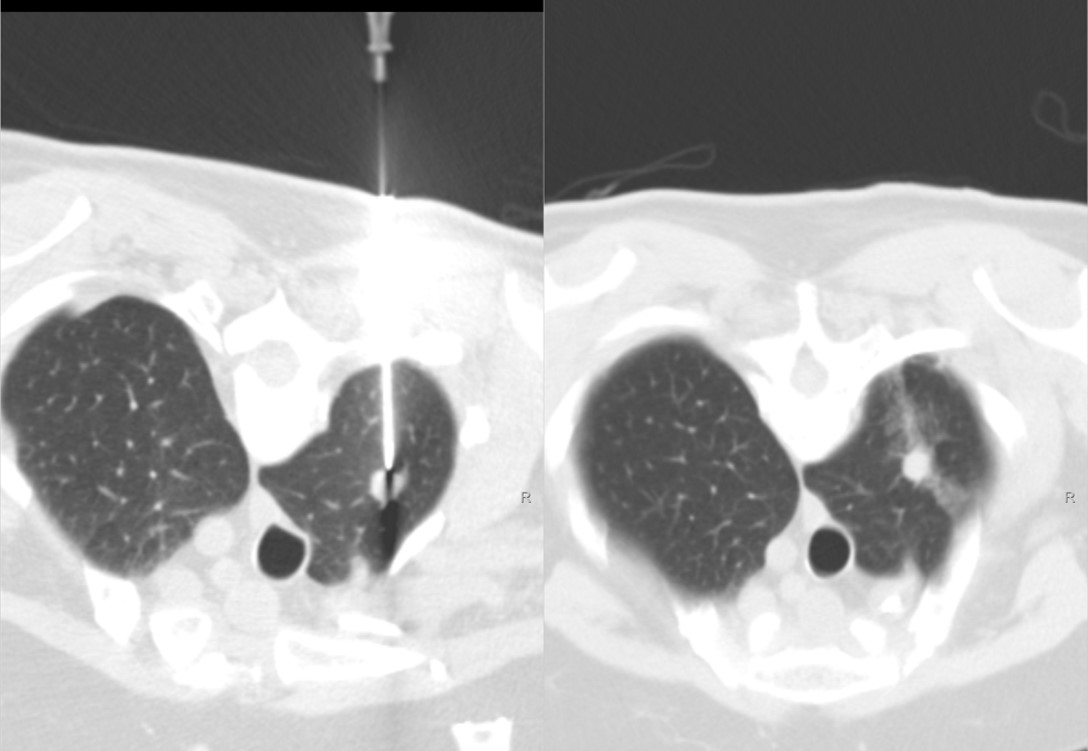
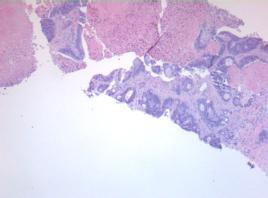
Figure 3: 79-years-old female with history of colon cancer and new 1.1 cm hypermetabolic lung apex mass. Mass is metastatic colonic adenocarcinoma.
Pre-fire biopsy image Post-biopsy image
Figure 4: 79-years-old female with history of colon cancer and new 1.1 cm hypermetabolic lung apex mass. Mass is metastatic colonic adenocarcinoma.
Acknowledgements
Author Contributions: RB is the guarantor of the content of the article and takes full responsibility for the accuracy of its content, including the data analysis. All authors contributed to the study design, data collection, and manuscript preparation.
Other Contributions: The authors also thank Robert Eric Heidel, PhD (University of Tennessee Graduate School of Medicine) for statistical analysis; and Monica Nicosia, PhD (Nicosia Medical Writer LLC) for medical writing and editing services, funded by Argon Medical.
Abbreviations
CT = computed tomography
ED = emergency department
FNA = fine needle aspiration
IRB = institutional review board
SD = standard deviation
References
1. Sakai H, Takeda M. 2019. Percutaneous transthoracic needle biopsy of the lung in the era of precision medicine. Journal of thoracic disease. 11: S1213-S1215. Ref.: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6560553/ Doi: https://dx.doi.org/10.21037%2Fjtd.2019.03.20
2. Winokur RS, Pua BB, Sullivan BW, et al. 2013. Percutaneous lung biopsy: technique, efficacy, and complications. Seminars in interventional radiology. 30: 121-127. Ref.: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3709987/ Doi: https://dx.doi.org/10.1055%2Fs-0033-1342952
3. Heerink WJ, de Bock GH, de Jonge GJ, et al. 2017. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. European radiology. 27:138-148. Ref.: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5127875/ Doi: https://dx.doi.org/10.1007%2Fs00330-016-4357-8
4. de Margerie-Mellon C, de Bazelaire C, de Kerviler E. 2016. Image-guided biopsy in primary lung cancer: Why, when and how. Diagnostic and interventional imaging. 97: 965-972. Ref.: https://pubmed.ncbi.nlm.nih.gov/27481575/ Doi: https://doi.org/10.1016/j.diii.2016.06.016
5. Diederich S, Padge B, Vossas U, et al. 2006. Application of a single needle type for all imageguided biopsies: results of 100 consecutive core biopsies in various organs using a novel tri-axial, end-cut needle. Cancer imaging: the official publication of the International Cancer Imaging Society. 6: 43-50. Ref.: https://pubmed.ncbi.nlm.nih.gov/16766268/ Doi: https://doi.org/10.1102/1470-7330.2006.0008
6. Lee KH, Lim KY, Suh YJ, et al. 2019. Diagnostic accuracy of percutaneous transthoracic needle lung biopsies: a multicenter study. Korean journal of radiology. 20: 1300-1310. Ref.: https://pubmed.ncbi.nlm.nih.gov/31339018/ Doi: https://doi.org/10.3348/kjr.2019.0189
7. Mills M, Choi J, El-Haddad G, et al. 2017. Retrospective analysis of technical success rate and procedure-related complications of 867 percutaneous CT-guided needle biopsies of lung lesions. Clinical radiology. 72: 1038-1046. Ref.: https://www.clinicalradiologyonline.net/article/S0009-9260(17)30409-9/abstract#secsectitle0030 Doi: https://doi.org/10.1016/j.crad.2017.07.023
8. Beslic S, Zukic F, Milisic S. 2012. Percutaneous transthoracic CT guided biopsies of lung lesions; fine needle aspiration biopsy versus core biopsy. Radiology and oncology. 46: 19-22. Ref.: https://pubmed.ncbi.nlm.nih.gov/22933975/ Doi: https://doi.org/10.2478/v10019-012-0004-4
9. Yeow KM, Su IH, Pan KT, et al. 2004. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest. 126: 748-754. Ref.: https://pubmed.ncbi.nlm.nih.gov/15364752/ Doi: https://doi.org/10.1378/chest.126.3.748




















