Indexing & Abstracting
Full Text
Review ArticleDOI Number : 10.36811/ojrmi.2019.110002Article Views : 3480Article Downloads : 65
Foreign Bodies in the Female Reproductive Tract
Suzanne M Czerniak*, and Jason Hao and Gary Israel
Department of Radiology and Biomedical Imaging, Yale School of Medicine, Yale New Haven Hospital, USA
*Corresponding author: Suzanne Czerniak, MD, PhD, Department of Radiology and Biomedical Imaging, Yale School of Medicine, Yale New Haven Hospital, Park Street PS340, 20 York Street, New Haven, CT 06510, USA, Tel: 508-769-8583; Email: suzanne.czerniak@yale.edu
Article Information
Aritcle Type: Review Article
Citation: Suzanne M Czerniak, Jason Hao, and Gary Israel. 2019. Foreign Bodies in the Female Reproductive Tract. O J Radio Med Img. 2: 12-22.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Suzanne M Czerniak
Publication history:
Received date: 31 January, 2019Accepted date: 15 February, 2019
Published date: 18 February, 2019
Abstract
Purpose:Most imaged foreign bodies of the female reproductive tract are intentionally placed, either by a medical professional or the patient herself. In many cases, these are reported as incidental findings, but occasionally studies are ordered specifically to locate wayward devices or assess for complications related to them. This requires radiologists to be able to correctly identify a wide range of foreign bodies, recognize their expected location, and assess for any associated complications. The purpose of this article is to familiarize the reader with a variety of foreign bodies and their usual positions in the female reproductive tract as well as their associated complications, if any.
Methods: A search was performed of our institutional database, Montage, to find examples of frequently encountered foreign bodies of the external genitalia, vagina, cervix, uterus, and fallopian tubes in their expected positions. Further searches were made to illustrate common complications related to each.
Results: Imaging of foreign bodies including external genital piercings, tampons, menstrual cups, pessaries, contraceptive rings, brachytherapy applicators, intra uterine contraceptive devices, and internal and external tubal closure devices were compiled across multiple modalities including x-ray, CT, MRI, and ultrasound. Complications including migration, perforation, and infection were reviewed.
Conclusion: Foreign bodies of the female reproductive tract are ubiquitous and should be readily recognized by radiologists. Comprehensive evaluation includes assessment for correct location and device-related complications.
Introduction
Most imaged foreign bodies of the female reproductive tract are intentionally placed, either by a medical professional or the patient herself. In many cases, these are reported as incidental findings, but occasionally studies are ordered specifically to locate wayward devices or assess for complications related to them. This requires radiologists to be able to correctly identify a wide range of foreign bodies, recognize their expected location, and assess for any associated complications. The purpose of this article is to familiarize the reader with a variety of foreign bodies and their usual positions in the female reproductive tract as well as their associated complications, if any. The appearance of a range of foreign bodies, including external genital piercings, tampons, menstrual cups, pessaries, contraceptive rings, brachytherapy applicators, intrauterine contraceptive devices, and internal and external tubal closure devices, across multiple modalities including x-ray, CT, MRI, and ultrasound are described below.
External Genitalia
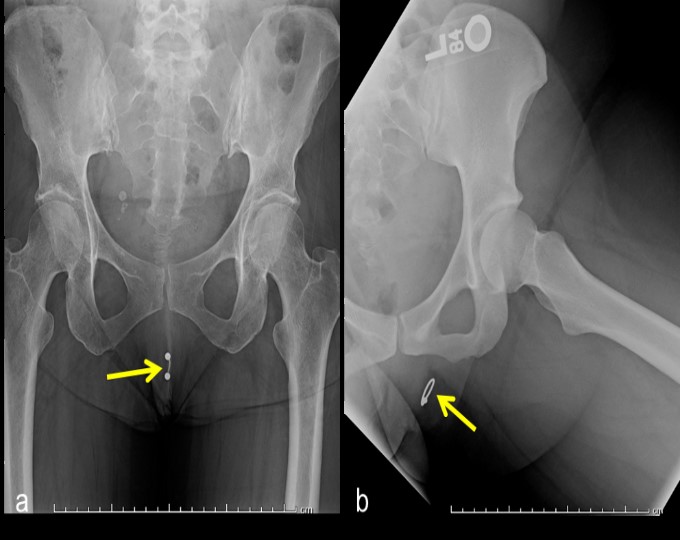
Genital piercing is undertaken for a variety of reasons: aesthetic, ethnic expression, and in some cases, as form of self-mutilation or body modification [1]. Genital piercing in women usually occurs in the soft tissues surrounding the vagina such as the clitoral hood, inner labia, and outer labia [2]. Piercings of the clitoris itself or perineal area are less common [1]. The jewelry used in such piercings is usually composed of a radiopaque material such as stainless steel or gold and may appear circular (ring, hoop) or linear (stud, barbell) in shape [2]. Genital piercings can here be seen as incidental findings on hip radiographs (Figure 1a,b). Complications arising from genital piercings include infection, keloid formation, and interference with barrier contraceptive use [1,2].
Figure 1: Linear, barbell shaped (a) and ring shaped (b) genital piercings.
Vagina/Cervix
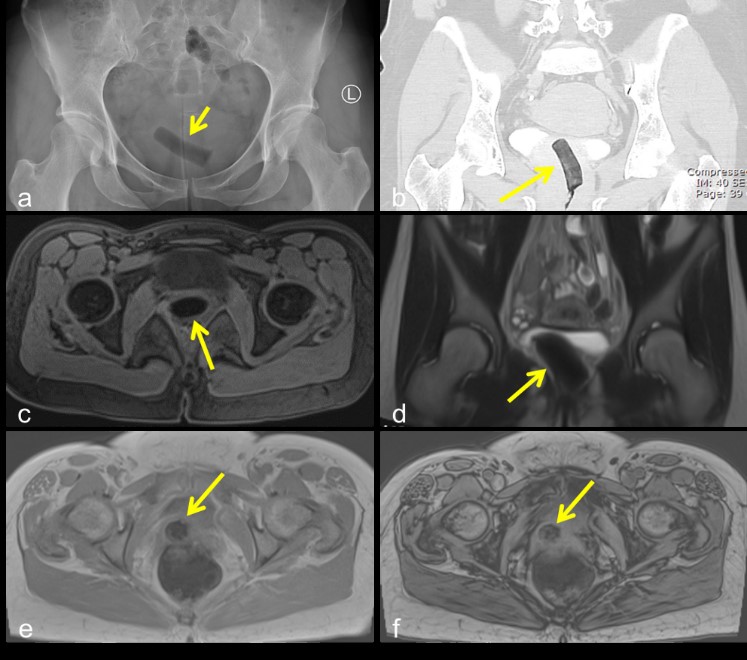
The first commercially marketed tampons became available in the 1930s, and were based on a Colorado physician’s design of surgical cotton with an accompanying cardboard applicator [3]. The purpose of a tampon is to absorb blood during menstruation, and so after placement a tampon will conform to the shape of the vaginal canal. They appear radiolucent on x-ray and CT, due to air trapped within the fibers of the tampon (Figure 2a,b). On MRI, tampons appear hypointense on both T1 and T2 weighted MRI images, again due to trapped air (Figure 2c,d). Blooming artifact (increase in hypointensity) can be seen on in-phase compared to out-of-phase images (when in-phase images are acquired with a longer echo time), as susceptibility artifact from the trapped air is more apparent with the longer echo times (Figure 2e,f). The most feared complication of tampon use is toxic shock syndrome [4].
Figure 2: Air trapped within tampon fibers causes a radiolucent appearance on x-ray (a) and CT (b). Tampons appear hypointense on T1 (c) and T2 (d) weighted MRI, again due to trapped air. Blooming (increase in hypointensity) can be seen on the in-phase (e) compared to out-of-phase images (f).
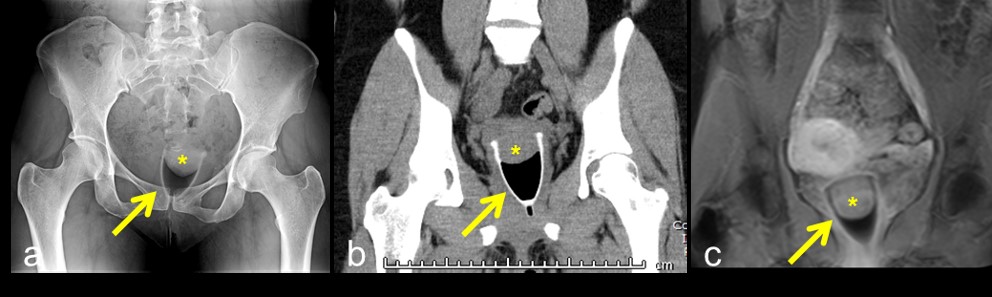
Menstrual cups have recently gained popularity as alternative to tampon use, despite becoming commercially available around the same time as tampons [5]. Modern menstrual cups tend to be made of a pliable plastic, and are inserted in a similar fashion to tampons. They function by catching menses in a small reservoir. The cup can then be removed, emptied, cleaned, and replaced. The expected location of a menstrual cup is within the vagina, with the wide end surrounding the cervix and the handle near the introitus. On x-ray and CT, it appears as a high-attenuation, cup-shaped structure (Figure 3a,b). The area between the cup and the cervix may appear lucent due to trapped air, or may contain an air-fluid level if menses are present. On T1-weighted MRI sequences, it appears as a hypointense, cup-shaped structure (Figure 3c). Complications of menstrual cup placement are radiographically occult, and include cramping and leakage [5].
Figure 3: A menstrual cup appears as a high-attenuation, cup-shaped structure (arrow) with gas between the cup and the cervix (asterix) on x-ray (a) and CT (b). It appears hypointense on T1-weighted MRI (c).
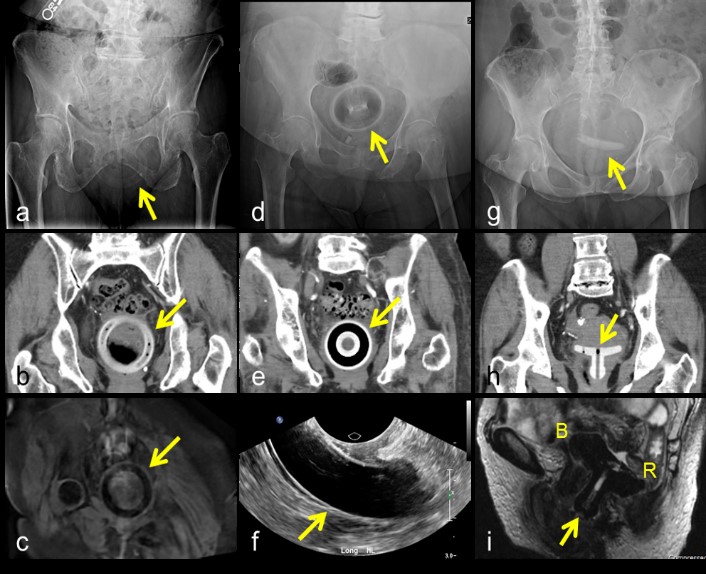
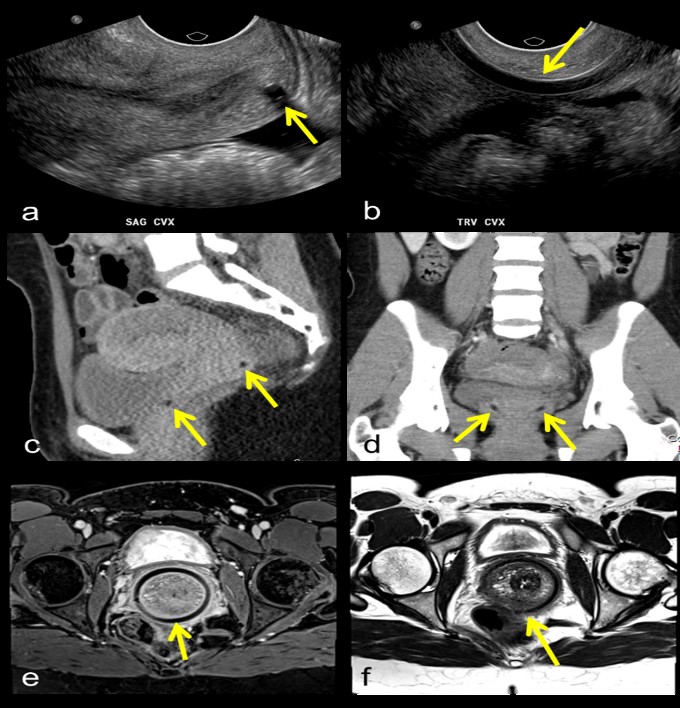
Pessaries are devices used to alleviate pelvic organ prolapse by mechanical means, and have been in use since the time of Hippocrates [6]. They remain a popular option for patients who are either unwilling or unable to undergo surgery [7]. Pessaries come in a variety of shapes and sizes, based on whether they are used to treat uterine prolapse, stress urinary incontinence, cystocele, or some combination [8]. The most commonly prescribed of these are the ring, donut, and Gelhorn pessaries, which are used to treat mild to moderate uterine prolapse [7,8]. Once placed, they are located within the vagina, adjacent to the cervix. Most pessary variants encircle the cervix, resting in the vaginal fornixes. Pessaries are usually composed of silicone and appear as high-attenuation structures on x-ray and CT (Figure 4a,b,d,e,g,h). In the case of ring- and Gelhorn-type pessaries, perforations in the body of the pessary may contain air and appear lucent. In the case of the donut-type pessary, the core may appear lucent. On ultrasound, ring- and donut-type pessaries appear as anechoic structures surrounded by an echogenic rim directly underneath a transvaginal probe (Figure 4f). Pessaries appear hypointense on both T1 and T2 weighted images (Figure 4c). Common complications of pessary use include discomfort, vaginal discharge, bleeding, and constipation [9]. Rarely, more severe complications can arise. There have been multiple case reports of neglected pessaries eroding through the vaginal wall and causing vesicovaginal or rectovaginal fistulas, requiring surgical removal (Figure 4i) [10,11].
Figure 4: Ring pessary on x-ray (a), CT (b), and T1-weighted MRI (c); Donut pessary on x-ray (d), CT (e), and transvaginal ultrasound (f); Gelhorn pessary on x-ray (g) and CT (h); Sagittal T2-weighted MR image of Gelhorn pessary (arrow) eroding into bladder (B) and rectum (R) (i).
Contraceptive vaginal rings are thin, flexible devices that, after insertion into the vagina, release ethinyl estradiol and etonogestrel in a controlled manner. Their efficacy and tolerability are similar to that of combined oral contraceptives (COCs) [12]. Unlike COCs, however, they are exchanged monthly by the patient at home. Their expected location is therefore anywhere within the vagina, depending on user placement. On ultrasound and CT, contraceptive rings appear as thin, anechoic or hypodense structures, respectively (Figure 5a-d). Contraceptive rings also appear hypointense on both T1- and T2- weighted MRI (Figure 5e,f). The major complication of contraceptive ring placement is expulsion; however, there have been several case reports of inadvertent placement into the bladder [13-14].
Figure 5: Contraceptive ring on ultrasound (a,b), CT (c,d) and T1- (e) and T2- (f) weighted images.
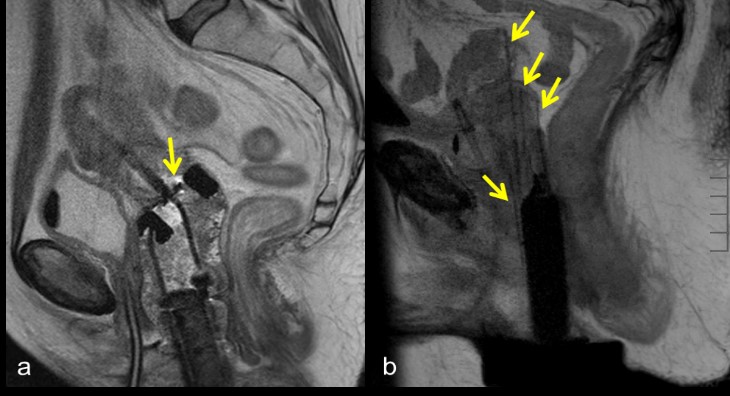
Brachytherapy applicators are used in combination with external beam radiation therapy and/or chemotherapy to deliver high doses of radiation to cervical cancer tumors [15]. Applicators may be placed using an intracavitary or interstitial approach, or a combination of the two. Intracavitary brachytherapy is most commonly used [15]. In this setup, an intrauterine tube, called a tandem, is passed through the vagina and cervix into the uterus to the level of the fundus. Two colopostats (ovoids) are then placed in the lateral vaginal fornices, on either side of the cervix; together, this is referred to as a tandem and ovoid (T & O) configuration. Alternatively, a ring may be used to encircle the cervix; in this case the configuration is called tandem and ring (T & R) (Figure 6a). Intracavitary brachytherapy is usually employed when tumor location and size permits the appropriate placement of a T & O or T & R configuration, such that the area reached by radiation will entirely encompass the tumor [15]. If the tumor is large or intracavitary applicators cannot be appropriately positioned, interstitial brachytherapy may be considered [15]. In interstitial brachytherapy, multiple applicators are passed directly into affected tissues using a transperineal template. This allows for more flexible delivery of radiation to encompass a larger area of disease (Figure 6b). The most severe complication of a misplaced brachytherapy applicator is unintentional radiation delivery to surrounding tissues, including the rectum and bladder. Improper positioning my also result in an insufficient radiation dose to targeted tissues [15]. To this end, an MRI or CT scan is usually performed after applicator insertion for treatment planning [16].
Uterus
Intrauterine devices (IUDs) are reversible, highly effective forms of contraception used by approximately 14% of women worldwide [17]. IUDs can be broadly classified into 2 types: copper, non-hormonal devices, and hormonal devices that release a low dose of levonorgestrel [18]. There are a variety of IUD shapes, including T-framed, U-framed, frameless, and ring-shaped. The most frequently used type of IUD varies from country to country: in the US, T-framed devices are most common (Figure 7a-f), while in China, stainless steel rings are most common (Figure 7g-i) [17]. Devices such as the Dalkon Shield and Lippe’s Loop are no longer actively in use, but may still occasionally be seen in an older patient (Figure 7j,k) [19]. IUDs generally appear as high-attenuation structures on x-ray and CT, hyperechoic on ultrasound imaging, and hypointense on T1- and T2-weighted MRI.
The correct location for an IUD is entirely within the endometrial cavity [18]. In the case of T-framed IUDs, the arms of the “T” should be at the fundus, with the stem ending above the internal cervical os [18]. Strings, used for IUD retrieval, exit the external cervical os and terminate in the vagina [18]. The most common complication of IUDs is displacement, where the position of the IUD shifts within the endometrial cavity [20]. This affects up to 25% of women using IUDs, and is associated with decreased contraceptive efficacy and an increased likelihood of expulsion. Uterine expulsion, where the IUD moves through the cervix into the vagina, occurs in approximately 3-10% of IUD users [20,21]. Partial expulsion may also occur, and is more common younger women (ages 13-19) [22]. As contraceptive efficacy of the IUD depends on correct placement within the endometrial cavity, users with an expelled or partially expelled IUD are at risk of pregnancy. However, even with a correctly-positioned IUD in place, pregnancy can occur in up to 1% of users [22]. Another complication of IUD use is embedment, where the device erodes into the uterine endometrium and myometrium, but does not perforate the serosa [20]. This occurs in up to 18% of users [20,23]. Treatment includes antibiotics and removal of the device, by surgical hysteroscopy if necessary [24]. The most severe complication of IUD use, complete perforation, is also the rarest, occurring in approximately 0.1% of users [20,25]. In this case, the device penetrates the endometrium, myometrium, and serosa, and may escape the uterus to float freely within the peritoneal cavity (Figure 7f). Management includes antibiotics and surgical removal [20,24].
Figure 7: Appropriately positioned T-framed IUD with stem seen completely within the endometrial cavity on sagittal ultrasound (a) and with arms at the fundus on transverse ultrasound (b); position is confirmed with 3D rendering (c). T-framed IUD within the pelvis on x-ray (d) and within the uterus on CT (e). Complete perforation with intraperitoneal T-framed IUD adjacent to the right side of the uterus (asterix) on hysterosalpingogram (f). Ring-shaped IUD within the pelvis on x-ray (g) and appropriately positioned completely within the endometrial cavity on transverse ultrasound (h) and 3D rendering (i). Lippe’s Loop IUD within the pelvis on x-ray (j) and within the uterus on CT (k).

In general, the imaging approach to a missing IUD begins with ultrasound evaluation to assess intrauterine placement [20,24]. If embedment is noted, the IUD should be removed. If perforation is detected, with a portion of the IUD outside the uterus, cross-sectional imaging such as CT or MRI of the abdomen and pelvis may be considered for further localization [20,24]. Similarly, if the device is not seen within the uterus on ultrasound, but appears to be extra-uterine on plain radiographs, cross-sectional imaging may be helpful [20,24]. If the device cannot be identified on ultrasound or plain radiographs, complete expulsion may be assumed [20,24].
Fallopian Tubes
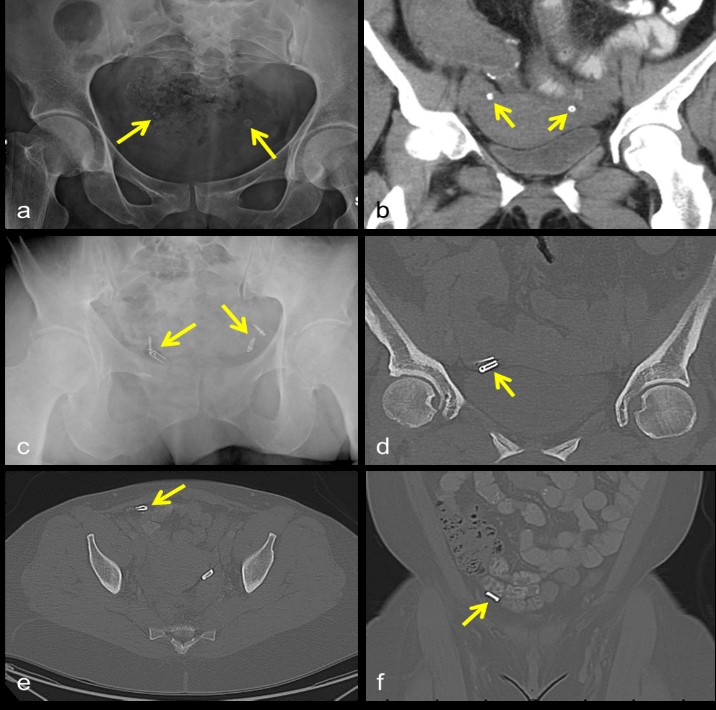
Tubal sterilization is a permanent form of birth control that can be achieved in multiple ways. Laparoscopic electrocoagulation, using either a unipolar or bipolar approach, results in the removal of several centimeters of the fallopian tube at the level of the isthmus [26]. The Pomeroy method of ligation involves tying a suture around a loop of fallopian tube, then severing the encircled segment. Salpingectomy or partial salpingectomy may also be performed. These methods of sterilization are largely radiographically occult. By contrast, laparoscopic placement of silicone rings impregnated with barium sulfate (tubal ring) or tubal ligation clips, either silicone-lined titanium clips (Filshie Clip) or toothed clips locked with a stainless steel spring (variously referred to as spring, Hulka, or Wolf clips) across the isthmic portions of the fallopian tubes may be seen radiographically (Figure 8a-d) [26-28]. On MRI, tubal ligation clips cause susceptibility artifact and are usually not well seen.
The most severe complications of laparoscopic tubal ligation tend to occur during or immediately after the procedure, and are related to general anesthesia or the laparoscopic approach [29]. Post-procedurally, infections of the pelvic organs or the wound itself have been reported but appear to be uncommon, occurring in less than 1% of women [29-30]. Complications occurring more frequently after Filshie clip or tubal ring placement include migration and mesosalpingeal injuries, respectively, and are also rare [27,31]. Filshie clip migration (Figure 8e,f) has an incidence of 0.1-0.6% [27,31], and in several cases expulsion through the rectum, urethra, or vagina has been reported [27]. Mesosalpingeal and tubal injuries more commonly occur with tubal ring placement, a finding that has been attributed to the method of ring placement: a loop approximately 2 cm of fallopian tube is drawn through the ring, which can result in tubal transection or mesosalpingeal hematoma [27]. Unplanned pregnancy occurs equally, albeit rarely, after tubal ring or clip placement (1.7 per 1000 women) [27]. Ectopic pregnancy can also occur after all methods of tubal sterilization, at a rate of 2.4-2.9/1000 procedures [32].
Figure 8: Tubal rings in expected location within the pelvis on x-ray (a) and CT (b). Appropriately positioned toothed clips locked with a stainless steel spring, variously referred to as spring, Hulka, or Wolf clips, on x-ray (c) and CT (d). Filshie clip migration into the peritoneal cavity on axial (e) and coronal (f) CT images.
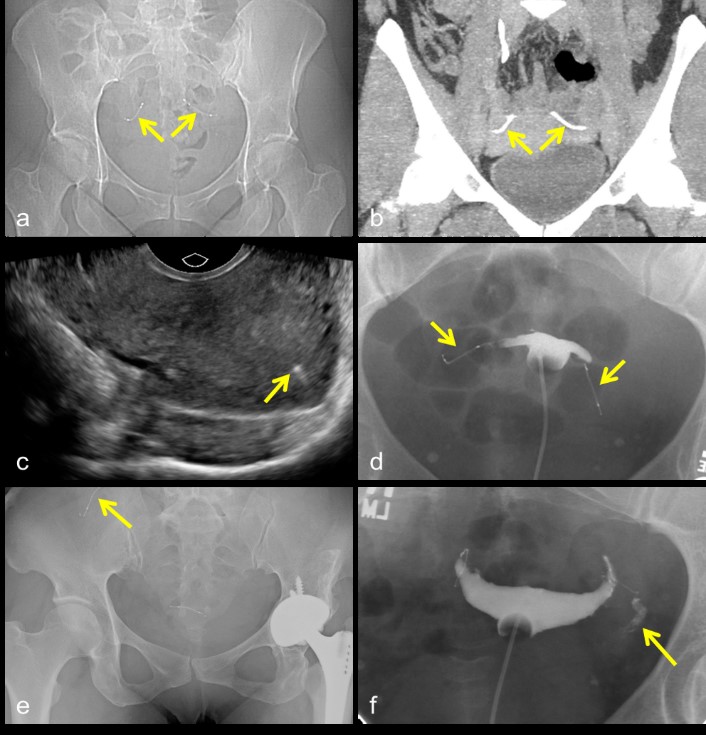
By contrast to the laparoscopic or minilaparotomy methods described above, transcervical placement of micro-inserts into the proximal portion of the fallopian tubes utilizes a hysteroscopic approach. Once placed, the micro-inserts generate local fibrosis within the fallopian tubes, resulting in occlusion and thus sterilization [33-34]. Composed of titanium, stainless steel, and nickel, micro-inserts are approximately 4 cm in length and resemble a coiled spring [34,35]. They are visible on plain radiographs and CT images, as well as pelvic ultrasound, as optimal placement of the device results in a small portion protruding from the tubal ostium into the uterus (Figure 9a-c) [33,35,36]. After micro-insert placement, a follow up hysterosalpingogram (HSG) should be obtained 90 days later to ensure complete tubal occlusion by fibrosis [37,38]. During the intervening time, women using the device are counseled to continue an alternate form of contraception [33]. If the 90 day HSG shows total occlusion, backup contraception can be stopped; if tubal patency is maintained, then the alternate form of contraception should be continued and the patient should return for another HSG in 6 months (Figure 9d,f) [33,37].
The complications following micro-insert placement are rare, but include chronic pain, infection, nickel allergy, malpositioning (including device migration and expulsion), and unintended pregnancy [39,41]. Device migration into the abdomen is particularly rare, occurring in only 0.04-0.1% of users (Figure 9e) [39,41,42]. In most cases, intra-abdominal migration is asymptomatic. Laparoscopic removal of the device(s) may be considered, however, as there have been several reports of serious complications, including bowel obstruction or perforation [33,39,42]. Unintended pregnancy after micro-insert placement is also uncommon, affecting approximately 0.25% of users after 5 years of follow up [43]. Findings from multiple studies suggest that most of these pregnancies are either already present at the time of device placement, due to lack of backup contraception during the 90 days following placement, or the result of missed or inaccurate follow up imaging [39,44].
Figure 9: Micro-inserts in expected location within the pelvis on x-ray (a) and CT (b). Sagittal ultrasound image shows a small portion of the micro-insert visible within the uterus (c). Hysterosalpingogram demonstrating complete occlusion of the Fallopian tubes, with no spillage of intra-uterine contrast into the peritoneal cavity (d). Micro-insert migration on x-ray (e). Failed occlusion of the left Fallopian tube with free spillage of contrast into the peritoneal cavity (f).
Other
Occasionally, foreign bodies are found in the female reproductive tract (usually the vagina) that do not serve a medical or personal care purpose (Figure 10). These objects vary widely, ranging from bottle caps to illicit drugs [45]. They are most frequently seen in children, but may also be present in adults who are mentally incompetent, seeking sexual pleasure, or who are simply curious [46]. The most common complication of a long-retained vaginal foreign body is excessive vaginal discharge, which may be foul-smelling or blood-tinged [45,47]. In a young girl who has not inserted the object herself, sexual abuse must be considered [47].
Figure 10:
Spring that was self-inserted into the vagina.

Conclusion
Foreign bodies of the female reproductive tract are ubiquitous and should be readily recognized by radiologists. Their appearance on x-ray, CT, MRI, and ultrasound images varies widely, based on each object’s composition. External genital piercings may be seen in the soft tissues surrounding the vagina such as the clitoral hood, inner labia, and outer labia, and appear radiopaque on x-ray and CT imaging. Tampons, menstrual cups, pessaries, contraceptive rings, and brachytherapy applicators may all be seen in the vagina, in some cases abutting or passing through the cervix. Trapped air within tampon fibers appears radiolucent on x-ray and CT, hypointense on both T1 and T2 weighted MRI, and demonstrates blooming on images obtained with longer echo times. Menstrual cups, composed of pliable plastic, appear as high-attenuation structures on x-ray and CT and hypointense structures on T1-weighted MRI sequences. Pessaries are usually composed of silicone and appear high-attenuation on x-ray and CT, as anechoic structures surrounded by an echogenic rim on ultrasound, and hypointense on both T1 and T2 weighted images. On ultrasound and CT, contraceptive rings appear as thin, anechoic or hypodense structures, respectively; on both T1- and T2- weighted MRI they appear hypointense. Brachytherapy applicators can be placed in several different configurations, and have a complex appearance on both CT and MRI. IUDs should be located entirely within the endometrial cavity, and appear high-attenuation on x-ray and CT, hyperechoic on ultrasound imaging, and hypointense on T1- and T2-weighted MRI. Tubal closure devices, including rings, clips, and micro-inserts, should be centered in the isthmic portions of the fallopian tubes bilaterally, and appear high-attenuation on x-ray and CT, as well as hyperechoic on ultrasound imaging.
Comprehensive evaluation of foreign bodies includes assessment for correct location and device-related complications. Complications of genital piercings, tampons, and menstrual cups are largely radiographically occult. Pessaries, when left in place for long periods of time, can erode through the vaginal walls creating vesicovaginal or rectovaginal fistulas. Several cases of contraceptive rings accidentally placed into the urinary bladder have been reported. Suspected partial expulsion, embedment, or complete perforation of an IUD may be further worked up with a combination of ultrasound, x-ray, and/or cross-sectional imaging. Migration is a rare complication of both tubal ligation clips and micro-inserts, and can be seen on x-ray and CT images.
References
- Koenig LM, Carnes M. 1999. Body piercing: medical concerns with cutting?edge fashion. Journal of General Internal Medicine. 14: 379-385. [Ref.]
- Meltzer DI. 2005. Complications of body piercing. American Family Physician. 72. [Ref.]
- Lamb K, Berg N. 1985. Tampon use in women with endometriosis. Journal of community health. 10: 215-225. [Ref.]
- Davis JP, Chesney PJ, Wand PJ, et al. 1980. Toxic-shock syndrome: epidemiologic features, recurrence, risk factors, and prevention. New England Journal of Medicine. 303: 1429-1435. [Ref.]
- North BB, Oldham MJ. 2011. Preclinical, clinical, and over-the-counter postmarketing experience with a new vaginal cup: menstrual collection. Journal of Women's Health. 20: 303-311. [Ref.]
- Lamers BH, Broekman BM, Milani AL. 2011. Pessary treatment for pelvic organ prolapse and health-related quality of life: a review. International urogynecology journal. 22: 637-644. [Ref.]
- Cundiff GW, Weidner AC, Visco AG, et al. 2000. A survey of pessary use by members of the American Urogynecologic Society. Obstetrics & Gynecology. 95: 931-935. [Ref.]
- Viera AJ, Larkins-Pettigrew M. 2000. Practical use of the pessary. American family physician. 61: 2719-2716. [Ref.]
- Sarma S, Ying T, Moore KH. 2009. Long?term vaginal ring pessary use: discontinuation rates and adverse events. BJOG: An International Journal of Obstetrics & Gynaecology. 116: 1715-1721. [Ref.]
- Arias BE, Ridgeway B, Barber MD. 2008. Complications of neglected vaginal pessaries: case presentation and literature review. International Urogynecology Journal. 19: 1173-1178. [Ref.]
- Hanavadi S, Durham-Hall A, Oke T, et al. 2004. Forgotten vaginal pessary eroding into rectum. Annals of the Royal College of Surgeons of England. 86: 18. [Ref.]
- Oddsson K, Leifels-Fischer B, de Melo NR, et al. 2005. Efficacy and safety of a contraceptive vaginal ring (NuvaRing) compared with a combined oral contraceptive: a 1-year randomized trial. Contraception. 71: 176-182. [Ref.]
- Bhaduri M, Carr LK, Dunn S, et al. 2009. The vaginal ring: expelled or misplaced?. Journal of Ultrasound in Medicine. 28: 259-261. [Ref.]
- Gabarró ST, Vizcaíno MA, Toro OA. Et al. 2009. Accidental introduction of a contraceptive vaginal ring into the urinary bladder. International Urogynecology Journal. 20: 1511-1513. [Ref.]
- Banerjee R, Kamrava M. 2014. Brachytherapy in the treatment of cervical cancer: a review. International journal of women's health. 6: 555-564. [Ref.]
- Vargo JA, Beriwal S. 2014. Image-based brachytherapy for cervical cancer. World journal of clinical oncology. 5: 921-930. [Ref.]
- Buhling KJ, Zite NB, Lotke P, et al. 2014. Worldwide use of intrauterine contraception: a review. Contraception. 89: 162-173. [Ref.]
- Gabriel ID, Tudorache ?, Vl?d?reanu S, et al. Birth Control and Family Planning Using Intrauterine Devices (IUDs). [Ref.]
- Aniulien? R, Aniulis P. 2014. Lippes Loop intrauterine device left in the uterus for 50 years: case report. BMC women's health. 14: 97. [Ref.]
- Boortz HE, Margolis DJ, Ragavendra N, et al. 2012. Migration of intrauterine devices: radiologic findings and implications for patient care. Radiographics. 32: 335-352. [Ref.]
- Teal SB, Romer SE, Goldthwaite LM, et al. 2015. Insertion characteristics of intrauterine devices in adolescents and young women: success, ancillary measures, and complications. American journal of obstetrics and gynecology. 213: 515. [Ref.]
- Aoun J, Dines VA, Stovall DW, et al. 2014. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstetrics & Gynecology. 123: 585-592. [Ref.]
- Shipp TD, Bromley B, Benacerraf BR. 2010. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. Journal of Ultrasound in Medicine. 29: 1453-1456. [Ref.]
- Edwards S, Lackritz KD. 2018. Managing the Misplaced Intrauterine Device. Topics in Obstetrics & Gynecology. 38: 1-5. [Ref.]
- Heinemann K, Reed S, Moehner S, et al. 2015. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 91: 274-279. [Ref.]
- Ryder RM, Vaughan MC. 1999. Laparoscopic tubal sterilization: Methods, effectiveness, and sequelae. Obstetrics and gynecology clinics of North America. 26: 83-97. [Ref.]
- Sokal D, Gates D, Amatya R, et al. 2000. Two randomized controlled trials comparing the tubal ring and Filshie clip for tubal sterilization. Fertility and sterility. 74: 525-533. [Ref.]
- Dominik R, Gates D, Sokal D, et al. 2000. Two randomized controlled trials comparing the Hulka and Filshie Clips for tubal sterilization. Contraception. 62: 169-175. [Ref.]
- Ouzounelli M, Reaven NL. 2015. Essure hysteroscopic sterilization versus interval laparoscopic bilateral tubal ligation: a comparative effectiveness review. Journal of minimally invasive gynecology. 22: 342-352. [Ref.]
- Jamieson DJ, Hillis SD, Duerr A, et al. 2000. Complications of interval laparoscopic tubal sterilization: findings from the United States Collaborative Review of Sterilization. Obstetrics & Gynecology. 96: 997-1002. [Ref.]
- Mumme AM, Cham J. 2015. Filshie clip migration with multiple groin hernias: a case report. Journal of medical case reports. 9: 187. [Ref.]
- Malacova E, Kemp A, Hart R, et al. 2014. Long-term risk of ectopic pregnancy varies by method of tubal sterilization: a whole-population study. Fertility and sterility. 101: 728-734. [Ref.]
- Kerin JF, Cooper JM, Price T, et al. 2003. Hysteroscopic sterilization using a micro?insert device: results of a multicentre Phase II study. Human Reproduction. 18: 1223-1230. [Ref.]
- Valle RF, Carignan CS, Wright TC. 2001. Tissue response to the STOP microcoil transcervical permanent contraceptive device: results from a prehysterectomy study. Fertility and sterility. 76: 974-980. [Ref.]
- Hastings-Tolsma M, Nodine P, et al. 2006. Essure: hysteroscopic sterilization. Journal of midwifery & women's health. 51: 510-514. [Ref.]
- Hurskainen R, Hovi SL, Gissler M, et al. 2010. Hysteroscopic tubal sterilization: a systematic review of the Essure system. Fertility and sterility. 94: 16-19. [Ref.]
- Guiahi M, Goldman KN, McElhinney MM, et al. 2010. Improving hysterosalpingogram confirmatory test follow-up after Essure hysteroscopic sterilization. Contraception. 81: 520-524. [Ref.]
- Shah V, Panay N, Williamson R, et al. 2011. Hysterosalpingogram: an essential examination following Essure hysteroscopic sterilisation. The British journal of radiology. 84: 805-812. [Ref.]
- Adelman MR, Dassel MW, Sharp HT. 2014. Management of complications encountered with Essure hysteroscopic sterilization: a systematic review. Journal of minimally invasive gynecology. 21: 733-743. [Ref.]
- Mino M, Arjona JE, Cordon J, et al. 2007. Success rate and patient satisfaction with the Essure™ sterilisation in an outpatient setting: a prospective study of 857 women. BJOG: An International Journal of Obstetrics & Gynaecology. 114: 763-766. [Ref.]
- Povedano B, Arjona JE, Velasco E, et al. 2012. Complications of hysteroscopic Essure® sterilisation: report on 4306 procedures performed in a single centre. BJOG: An International Journal of Obstetrics & Gynaecology. 119: 795-799. [Ref.]
- Rezai S, LaBine M, Roberts G, et al. 2015. Essure microinsert abdominal migration after hysteroscopic tubal sterilization of an appropriately placed Essure device: dual case reports and review of the literature. Case reports in obstetrics and gynecology. [Ref.]
- Ríos-Castillo JE, Velasco E, Arjona-Berral JE, et al. 2013. Efficacy of Essure hysteroscopic sterilization–5 years follow up of 1200 women. Gynecological Endocrinology. 29: 580-582. [Ref.]
- Cleary TP, Tepper NK, Cwiak C, et al. 2013. Pregnancies after hysteroscopic sterilization: a systematic review. Contraception. 87: 539-348. [Ref.]
- Nwosu EC, Rao S, Igweike C, et al. 2005. Foreign objects of long duration in the adult vagina. Journal of obstetrics and gynaecology. 25: 737-739. [Ref.]
- Hunter TB, Taljanovic MS. 2003. Foreign bodies. Radiographics. 23: 731-757. [Ref.]
- Stricker T, Navratil F, Sennhauser FH. 2004. Vaginal foreign bodies. Journal of paediatrics and child health. 40: 205-207. [Ref.]




















