Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ojrmi.2019.110001Article Views : 2719Article Downloads : 47
MRI validation of Post-Prostatectomy Radiotherapy Contouring
Mo Manji1, Juanita Crook2*, Leigh Bartha2 and Rasika Rajapakshe3
1Department of Radiation Oncology, British Columbia Cancer Agency, Centre for the Southern Interior, University of British Columbia, Kelowna BC, Canada
2Radiation Therapy, British Columbia Cancer Agency, Centre for the Southern Interior, University of British Columbia, Kelowna BC, Canada
3Medical Physics, British Columbia Cancer Agency, Centre for the Southern Interior, University of British Columbia, Kelowna BC, Canada
*Corresponding author: Juanita Crook MD FRCPC, University of British Columbia, BCCA Center for the Southern Interior, 399 Royal Avenue, Kelowna BC, Canada V1Y 5L3, Tel: 250 712 3958; Fax: 250 712 3911; Email: jcrook@bccancer.bc.ca
Article Information
Aritcle Type: Research Article
Citation: Mo Manji, Crook J, Bartha L, et al. 2018. MRI validation of Post-Prostatectomy Radiotherapy Contouring. O J Radio Med Img. 1: 01-09.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2018; Mo Manji
Publication history:
Received date: 11 December, 2018Accepted date: 17 December, 2018
Published date: 27 December, 2018
Abstract
Introduction: Level One Evidence has established the indications for, and importance of, adjuvant radiotherapy following radical prostatectomy. Several guidelines have addressed delineation of the prostate bed but with variable specification of the inferior border relative to the penile bulb or to the first CT slice distal to visible urine in the bladder neck. This work determines the correlation between the caudal aspect of the anastomosis shown by the tip of the urethrogram cone and MRI anatomy.
Materials and Methods :
Sixteen patients receiving adjuvant radiotherapy following prostatectomy underwent diagnostic MRI in addition to planning CT with Urethrogram. The CT Reference Slice, tip of urethrogram cone and superior aspect of penile bulb were delineated.
Results:
MRI clearly demonstrates the penile bulb but not the anastomosis. In these 16 patients, the tip of the urethrogram cone was a median 3.9 mm cranial to the penile bulb (range 0-10.3 mm).
Conclusion: We show marked variability in the distance between penile bulb and the tip of the urethrogram cone. In all sixteen patients, placing the inferior border of the CTV 15mm cranial to the penile bulb would have failed to treat the caudal aspect of the anastomosis, a frequent site of local relapse, that cannot be reliably landmarked by any other anatomic structure. Individualizing the treatment volume to patient anatomy is the only way to ensure consistent coverage without treating a larger than necessary volume in many patients. We recommend the use of planning urethrogram to minimize the potential for geographic miss.
Keywords: Prostate cancer; Radiotherapy; Adjuvant radiotherapy; Salvage radiotherapy; Radiotherapy planning
Introduction
Prostate cancer is the most frequently diagnosed non-cutaneous malignancy in males in Canada and the United States [1,2] and the second leading cause of cancer-related death in North American men. Although radical prostatectomy (RP) is one of the main curative options for localized prostate cancer, radiotherapy plays an important role in treatment. Technological advances in imaging and computer software have improved the planning and delivery of radiotherapy. External beam radiation is often prescribed after surgery, as either adjuvant or salvage, to improve outcome in patients with positive margins and/or pT3 disease. From 2010 to 2014, the proportion of men with Gleason 9-10 disease undergoing RP has increased by 56% and the incidence of positive margins has increased by 19% [3], thus increasing the need for effective adjuvant/salvage radiotherapy.
Level One Evidence has established the indications for, and importance of, adjuvant radiotherapy following radical prostatectomy. Several guidelines have addressed delineation of the prostate bed but with variable specification of the inferior border. The EORTC definition specifies 15 mm cranial to the penile bulb, while the RTOG and trans-Tasman groups locate the inferior border 5-12 mm caudal to the first CT slice distal to visible urine in the bladder neck.
Our recent publication reported considerable variation in the location and length of the anastomosis and in our experience, the caudal aspect of vesico-urethral junction can only be clearly delineated with retrograde urethrogram. [4]. This report explores the correlation between the caudal aspect of the anastomosis shown by the tip of the urethrogram cone and the MRI anatomy.
Body
Materials and Methods
Sixteen patients (median age 63: range 56-71) scheduled to receive post prostatectomy adjuvant radiotherapy (6600 cGy in 33 fractions) underwent diagnostic magnetic resonance imaging (MRI 1.5 T, Signa, GE Healthcare, Chicago Illinois, 16 Channel Cardiac Full, T1:TR 680/TE 8. Echo Train Length 4, Flip Angle 90, Slice thickness/spacing 3.0/3.0, Band width 120, matrix 256/224. T2: TR 4600/TE 120, Echo Train length 26, Flip angle 8, Slice thickness/spacing 3.0/3.0, Band width 195, matrix 320x320), using an FrFSE T2 sequence with images obtained in axial, sagittal and coronal planes; all with 5 mm slice thickness and 1 mm gap. Subsequently, planning helical computed tomography with urethrogram was performed with 2.5 mm slice thickness for the purpose of radiotherapy treatment planning. The patient was instructed to have a comfortably full bladder and an empty rectum. Urethrogram was performed by retrograde injection of 5-10 cc of Hypaque sterile X-ray contrast and applying a Cunningham clamp to the penile shaft to retain the contrast during the subsequent scan. Contouring and planning were based on MRI-CT fusion.
All image sets were anonymized and the authors (MM and JC) independently contoured the most caudal CT slice showing urine in the bladder neck to define the CT reference slice, located one slice caudally, as well as identifying the tip of the urethrogram cone and the cranial aspect of the penile bulb as seen on MRI.
Results
Although 1.5 T MRI clearly demonstrates the penile bulb, it was not helpful in visualization of the anastomosis. Sixteen sets of readings were obtained. In all sixteen of these patients, the tip of the urethrogram cone was located cranial to the superior aspect of the penile bulb but there was marked variability in the distance between the penile bulb and urethrogram tip (0-10.3 mm, median 3.9 mm) (Figure 1,2).
Figure 1: 2 cases with CT-MRI fusion with urethrogram showing variation in the distance between penile bulb and urethrogram tip. Figure 1a, the penile bulb is 7 mm caudal to the tip of the urethrogram cone and in Figure 1b the penile bulb is at the same level as the urethrogram cone (0 mm).
Figure: 1a
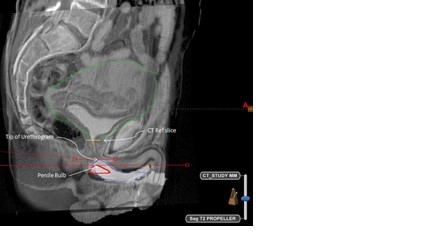
Figure: 1b

Figure 2: Waterfall plot showing the distribution of distance mm between the superior aspect of the penile bulb and the tip of the urethrogram cone for 16 patients.
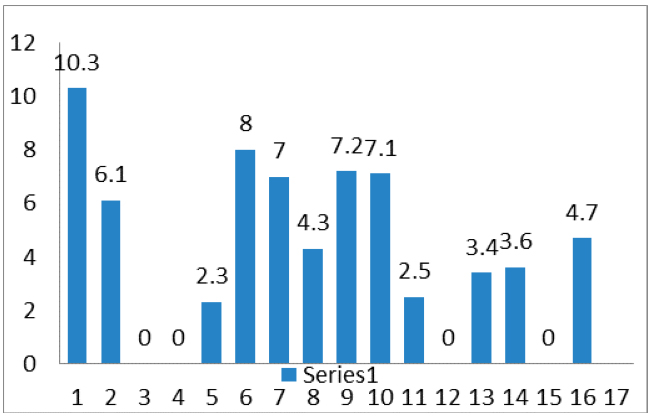
Figure 3: Waterfall plot of 16 patients showing the distribution of distance (mm) between the CT- reference slice and tip of the urethrogram cone.

In this sixteen patient cohort, the mean distance between Bladder CT Reference
Slice and the tip of the urethrogram cone was 17.8mm (range: 8.5 mm to 34.1 mm) (Figure 3). If the caudal limit of CTV is defined 8 mm caudal to the CT Reference Slice, in no patient would the CTV volume extend far enough caudally to cover the tip of the urethrogram cone. With the CTV caudal limit defined at 12mm below the CT Reference Slice, 31% (5/16 patients) had adequate coverage and this increased to 44% (7/16 patients) when CTV inferior limit was defined at 15 mm below the CT Reference Slice (Table 1).
| Patient # | CT Ref Slice to tip of Urethrogram cone (mm) | CT Ref Slice +8 mm | CT Ref Slice +12 mm | CT Ref Slice +15 mm | |
|---|---|---|---|---|---|
| 1 | 34.1 | 26.1 | 22.1 | 19.1 | |
| 2 | 22.2 | 14.2 | 10.2 | 7.2 | |
| 3 | 15.9 | 7.9 | 3.9 | 0.9 | |
| 4 | 12.0 | 4.0 | 0 | -0.3 | |
| 5 | 18.7 | 10.7 | 6.7 | 3.5 | |
| 6 | 16.0 | 8.0 | 4.0 | 1.0 | |
| 7 | 9.8 | 1.8 | -2.2 | -5.2 | |
| 8 | 20.1 | 12.1 | 8.1 | 5.1 | |
| 9 | 14.0 | 6.0 | 2.0 | -1.0 | |
| 10 | 10.8 | 2.8 | -1.2 | -4.2 | |
| 11 | 28.9 | 20.9 | 16.9 | 13.9 | |
| 12 | 31.4 | 23.4 | 19.4 | 16.4 | |
| 13 | 11.1 | 3.1 | -0.9 | -3.9 | |
| 14 | 8.5 | 0.5 | -3.5 | -6.5 | |
| 15 | 14.2 | 6.2 | 2.2 | -0.8 | |
| 16 | 17.2 | 9.5 | 5.5 | 2.5 | |
| tip within CTV | 0/16 | 5/16 | 7/16 | ||
| Table 1: Measurements for 16 patients in the study. Zero and negative numbers indicate that the inferior limit of the CTV falls at or caudal to the tip of the cone. With the lower limit of the CTV defined 8mm below the CT Reference Slice, no patient (0%) has the CTV volume extend as far as the tip of the urethrogram cone. With the CTV inferior limit defined at 12mm below the CT Reference Slice, this increases to 31% (5/16 patients) and at 15mm to 44% (7/16 patients) We recommend that the caudal limit of the CTV be 5mm caudal to the tip of the urethrogram. | |||||
Discussion
Optimal treatment planning of adjuvant or salvage post radical prostatectomy radiotherapy is essential to avoid geographic miss of the intended target. Increasing conformality and use of Intensity Modulated Radiation therapy (IMRT) or Volumetric Modulated Arc therapy (VMAT) to spare adjacent organs-at-risk may improve tolerance of treatment but could paradoxically decrease efficacy if the target volume is not correctly defined. Evaluation of post prostatectomy pathological specimens has revealed tumor to be present distally in 75% of specimens and the apical surgical margin is that most commonly involved [5,6]. Modern imaging with 18F -Fluorocholine PET/CT is used increasingly for assessment of local recurrence following prostatectomy. Wang et al found two thirds of the relapses occur in the vicinity of the vesico-urethral anastomosis [7]. El Kabbaj et al used 18F -Fluorocholine PET/CT imaging to show that more than half of suspected relapses occurred at the anastomotic site [8].
External beam radiation therapy (EBRT) is often prescribed after surgery to improve outcome in patients with high risk pathology. Three mature phase 3 randomized trials of adjuvant EBRT versus observation have reported a reduction in biochemical failure, local recurrence and clinical progression [9-11]. The RTOG, Canadian and Australian/New Zealand Groups have addressed the delineation of the prostate bed target volume based on CT imaging. The vesico-urethral anastomosis is identified as the first CT slice caudal to visible urine in the bladder neck (CT Reference Slice) [12-14]. The Radiotherapy Oncology Group (RTOG) recommends the CTV limit be placed 8-12 mm caudal to this while the Genito-urinary Radiation Oncologists of Canada (GUROC) recommend the inferior limit to be 8 mm caudal to the CT reference slice. They also explored using a fiducial seed placed by a radiologist under trans-rectal ultrasound guidance but, based on data from 12 patients showing good agreement between the fiducial and the 8mm limit, fiducial placement was deemed unnecessarily invasive. The Australian/New Zealand group recommend a 5 mm margin and the EORTC suggest placing the caudal limit of the CTV 15 mm cranial to the penile bulb [15].
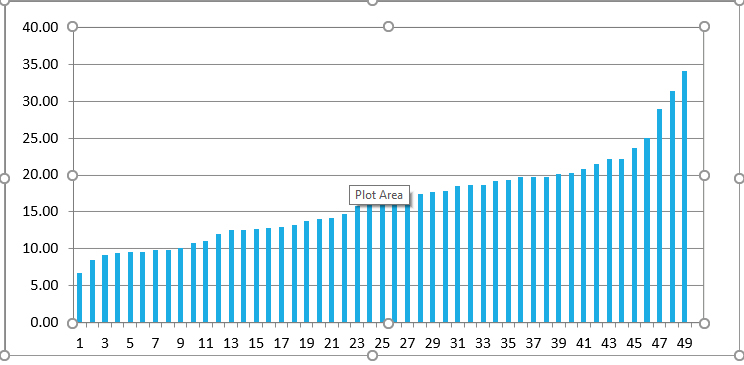
Our previous work looked at the target volume definition with respect to the above guidelines and their relationship to the previous standard practice of localizing the anastomosis using retrograde urethrogram. Our contouring and planning practice is based on MRI-CT fusion. Although prostate mobility can be an issue when treating the intact prostate, post prostatectomy the anastomosis is relatively stable due to post- operative scaring and fibrosis. When a urethrogram is incorporated into CT- planning, there is considerable variation seen in the distance between the tip of the urethrogram cone and the recommended CT guidelines. It appears that only urethrogram clearly and unambiguously delineates the caudal aspect of the vesico-urethral anastomosis (VUA). Our previously published data from 33 post prostatectomy patients, comparing VUA identification by urethrogram to the current guidelines, showed that for 79% the tip of the urethrogram cone is >12 mm caudal to CT Reference Slice. (4) The data from our current study of 16 patients validates these findings. Adding these 2 cohorts to produce a total of 49 post- prostatectomy patients, showed that for 78% of the patients, the tip of the urethrogram is > 12mm caudal to CT Reference Slice (Figure 4).
Figure 4: Waterfall plot of 49 patients showing the distribution of distance between the CT-reference slice and tip of the urethrogram cone.
The logistics of incorporating urethrogram into CT simulation for post RP radiotherapy clearly poses some challenges for many departments. It was hoped that MR simulation might obviate the need for a urethrogram by adequately demonstrating the anastomosis. We found that the penile bulb was very well delineated by 1.5T MRI but unfortunately the anastomosis was not clearly visualized. Furthermore, there was marked variability in the distance between the penile bulb (anatomical landmark used by EORTC guidelines) and the tip of the urethrogram cone, with a range of 0 to 10.3 mm cranial to the penile bulb. Thus, if the caudal border of the CTV is placed 15 mm cranial to the penile bulb as the EORTC guideline recommends, none of the patients would have had adequate coverage of the anastomosis (Figure 5). A radiotherapy technologist can easily be taught and certified in the method of retrograde urethrography that we employ, or conversely, a urology catheter technician could be assigned the task to relieve the Radiation Oncologist from the necessity of attending the CT-simulation.
Figure 5: Using the EORTC recommendation to place the caudal border of CTV 15 mm cranial to the penile bulb misses the tip of the urethrogram cone by variable amounts, 8 mm in Figure 5a and 15 mm in Figure 5b.
Figure: 5a

Figure: 5b

In patients who develop biochemical relapse after radical prostatectomy, early salvage radiotherapy performed at PSA relapse [16,17] is an appropriate curative salvage option. Advanced imaging with 68Ga-PSMA (prostate specific membrane antigen) -PET imaging, as reported by Habl et al, can influence staging and radiotherapy management in these patients [18]. 68Ga PSMA-PET scan can be a guide for radiation treatment in post prostatectomy recurrence. Habl et al used MRI imaging for radiotherapy volume definition, and found that the location of relapse in the prostate bed after radical prostatectomy is frequently at the level of the anastomosis between the bladder neck and the urethra [19-22]. This confirms the importance of adequately treating the VUA and thus objective definition of VUA is critical. As we have shown marked variation of the location of the caudal aspect of VUA, and since MRI is not helpful in visualizing the anastomosis, we recommend adding urethrogram to ensure adequate coverage inferiorly of the prostate bed for individualized treatment delivery. Both our studies support the use of planning urethrogram to delineate the caudal aspect of VUA and to minimise the potential for geographic miss.
The three randomised trials of adjuvant Radiation Therapy (RT) vs. observation established the caudal limit of VUA using urethrogram (9-11) while the current guidelines use surrogate anatomic references that don’t relate consistently to the location of the anastomosis and don’t take into account anatomic variability. Over three quarters of patients, in our previously published report, had greater than 12 mm between the tips of the urethrogram cone and recommended CT landmark for VUA and over 50% had a distance greater than 15 mm (4). The recommended caudal limit is 5-12 mm below the CT landmark. One of the issues may be that the anatomical length of the anastomosis is quite variable due to post- surgical scarring, surgical technique and/or pelvic floor tone. Without the benefit of the urethrogram, the prostate bed coverage can be compromised. Guidelines should be validated against the previously used standard which established the value of post prostatectomy radiotherapy.
Conclusion
Careful target definition is critical for optimal delivery of post radical prostatectomy radiotherapy. Several guidelines have been developed to address the delineation of prostate bed target volume with variable definition of the caudal border but none of these have been validated against the previously accepted standard, retrograde urethrogram. The location of local relapse in the prostate bed after radical prostatectomy is frequently at the anastomosis between the bladder neck and the urethra. Urethrogram delineates clearly the caudal aspect of vesico-urethral anastomosis for the definition of the inferior border of the CTV. Data from our previous publication showed marked variability in the distance between the CT Reference Slice and tip of the urethrogram cone and data from the current study utilizing MRI shows marked variability in the distance between the penile bulb and the tip of the urethrogram cone. In all sixteen patients, placing the inferior border of the CTV 15mm cranial to the penile bulb would have failed to treat the caudal aspect of the anastomosis. In our experience, neither the penile bulb nor the CT reference slice reliably landmark the site of the vesico-urethral anastomosis in the individual patient. We recommend the use of a planning urethrogram for treatment planning of post prostatectomy radiotherapy.
References
- Canadian Cancer Society.Cancer.ca/Canadian Cancer Statistics 2017 http://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%
20cancer%20statistics/Canadian-Cancer-Statistics-2017-EN.pdf?la=en (accessed 10 April 2018) [Ref.] - Jemal A, Siegel R, Ward E, et al. 2008. Cancer statistics, 2008. CA Cancer J Clin. 58: 71-96. [Ref.]
- Lester Coll NH, Bledsoe TJ, Johnson S, et al. 2017. Increasing rate of positive margins in men with high-risk prostate cancer undergoing radical prostatectomy. Int J Radiat Oncol Biol Phys. 96: 208. [Ref.]
- Manji M, Crook J, Schmid M, et al. 2016. Target volume definition for post prostatectomy radiotherapy: Do the consensus guidelines correctly define the inferior border of the CTV? Rep Pract Oncol Radiother. 21: 525-531. [Ref.]
- Byar DP, Mostofi FK. 1972. Carcinoma of the prostate: prognostic evaluation of certain pathologic features in 208 radical prostatectomies. Examined by the step-section technique. Cancer. 30: 5-13. [Ref.]
- Stamey TA, Villers AA, McNeal JE, et al. 1990. Positive surgical margins at radical prostatectomy: importance of the apical dissection. J Urol. 143: 1166-1173. [Ref.]
- Wang J, Kudchadker R, Choi S, et al. 2014. Local recurrence map to guide target volume delineation after radical prostatectomy. Pract Radiat Oncol. 4: 239-246. [Ref.]
- El Kabbaj O, Robin P, Bourhis D, et al. 2018. Target definition in salvage postoperative radiotherapy for prostate cancer: 18F-fluorocholine PET/CT assessment of local recurrence. Acta Oncol. 57: 375-381. [Ref.]
- Wiegel T, Bottke D, Steiner U, et al. 2009. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 27: 2924-2930. [Ref.]
- Bolla M, van Poppel H, Collette L, et al. 2005. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911). Lancet. 366: 572-578. [Ref.]
- Thompson IM, Tangen CM, Paradelo J, et al. 2006. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 296: 2329-2335. [Ref.]
- Michalski JM, Lawton C, El Naqa I, et al. 2010. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 76: 361-368. [Ref.]
- Wiltshire KL, Brock KK, Haider MA, et al. 2007. Anatomic boundaries of the clinical target volume (prostate bed) after radical prostatectomy. Int J Radiat Oncol Biol Phys. 69:1090-1099. [Ref.]
- Sidhom MA, Kneebone AB, Lehman M, et al. 2008. Post-prostatectomy radiation therapy: consensus guidelines of the Australian and New Zealand Radiation Oncology Genito-Urinary Group. Radiother Oncol. 88: 10-19. [Ref.]
- Poortmans P, Bossi A, Vandeputte K, et al. 2007. Guidelines for target volume definition in post-operative radiotherapy for prostate cancer, on behalf of the EORTC Radiation Oncology Group. Radiother Oncol. 84: 121-127. [Ref.]
- Pfister D, Bolla M, Briganti A, et al. 2014. Early salvage radiotherapy following radical prostatectomy. Eur Urol. 65: 1034-1043. [Ref.]
- Stephenson AJ, Scardino PT, Kattan MW, et al. 2007. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 25: 2035-2041. [Ref.]
- Habl G, Sauter K, Schiller K, et al. 2017. (68) Ga-PSMA-PET for radiation treatment planning in prostate cancer recurrences after surgery: Individualized medicine or new standard in salvage treatment. Prostate. 77: 920-927. [Ref.]
- Silverman JM, Krebs TL. 1997. MR imaging evaluation with a transrectal surface coil of local recurrence of prostatic cancer in men who have undergone radical prostatectomy. AJR Am J Roentgenol. 168: 379-385. [Ref.]
- Connolly JA, Shinohara K, Presti JC Jr, et al. 1996. Local recurrence after radical prostatectomy: characteristics in size, location, and relationship to prostate-specific antigen and surgical margins. Urology. 47: 225-231. [Ref.]
- Leventis AK, Shariat SF, Slawin KM. 2001. Local recurrence after radical prostatectomy: correlation of US features with prostatic fossa biopsy findings. Radiology. 219: 432-439. [Ref.]
- Sella T, Schwartz LH, Swindle PW, et al. 2004. Suspected local recurrence after radical prostatectomy: endorectal coil MR imaging. Radiology. 231: 379-385. [Ref.]




















