Indexing & Abstracting
Full Text
Case ReportDOI Number : 10.36811/ojprm.2020.110011Article Views : 2Article Downloads : 1
Impinging problem of COVID 19 associated to obstructive sleep apnea and relevance of ventilatory strategies: A case report
M Louardi, H Ezzouine*, M Simou, A Khamboubi, I Mokhtari, A Raja, I Elkhaouri, A Mansour, Y Et-tahir, M Tabat, K Fahmaoui and B Charra
Medical Intensive Care Unit, Ibn Rushd University Hospital of Casablanca, Faculty of Medicine and Pharmacy-Hassan II University of Casablanca, Morocco
*Corresponding Author: Hanane Ezzouine, Medical Intensive Care Unit at Ibn Rushd University Hospital of Casablanca, Faculty of Medicine and Pharmacy-Hassan II University of Casablanca, Morocco, Email: mounir.louardi.11@gmail.com
Article Information
Aritcle Type: Case Report
Citation: M Louardi, H Ezzouine, M Simou, et al. 2020. Impinging problem of COVID 19 associated to obstructive sleep apnea and relevance of ventilatory strategies: A case report. Open J Pulm Respir Med. 2: 38-43.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2020; M Louardi
Publication history:
Received date: 02 October, 2020Accepted date: 13 October, 2020
Published date: 15 October, 2020
Abstract
Pneumonia due to SARS-CoV-2 has caused considerable morbidity and mortalityworldwide particularly amongst those with comorbidities. The most frequent comorbidities are hypertension, diabetes and cardiovascular disease. until now, few associations between obstructive sleep apnea syndrome (OSAS) and COVID-19 have been reported. So, through the column of this article, Given the limited number of clinical cases reported about obstructive sleep apnea and COVID-19, we would like to report a case and share some experiences.
Keywords: Obstructive Sleep Apnea; Obesity; COVID-19; Intensive care unit
Introduction
As the severe acute respiratory syndrome-coronavirus disease 2019 (SARS-COVID-19) pandemic is unfolding around the world, reports are being published identifying risk factors for severe and critical disease [1-3] are increasingly concerned about the high incidence of critical COVID-19 in overweight patients. Obstructive sleep apnea (OSA) is strongly associated with major comorbidities associated withsevere COVID disease namely hypertension, diabetes, cardiovascular diseaseand obesity [1] we suspect OSA (particularly with concurrent obesity) could potentially contribute to worsening hypoxemia and the cytokine storm that occurs in COVID patients given that both OSA and obesitymay be pro-inflammatory conditions [2-4].
Case Repor
We report the case of 45 years old man, with a history of obstructive sleep apnea and hypertension the history of the patient goes back to 11/07/20 by the appearance of a flu-like syndrome made of unencrypted fever and chills followed by the appearance of a dry cough and dyspnea of progressive aggravation, which motivated a consultation, the thoracic CT showed an aspect suggestive of viral infection, supplemented by a positive COVID PCR. patient was referred to a COVID-19 ICU. Examination shows a conscious patient with a GCS of 15, his blood pressure was at 120/80 mmHg and his heart rate was at HR 78 pulses per minute. His respiratory rate at 30c /min and 75% of arterial oxygen saturation at room air. However, we noticed bilateral humming groans on pleuropulmonary auscultation, without any other particular cardio-vascular signs. The chest-CT revealed air bronchogram associated with ranges of diffuse ground glass opacities at the level of the two pulmonary fields (figure 1), the viral origin of which is very likely with severe parenchyma involvement (50%).
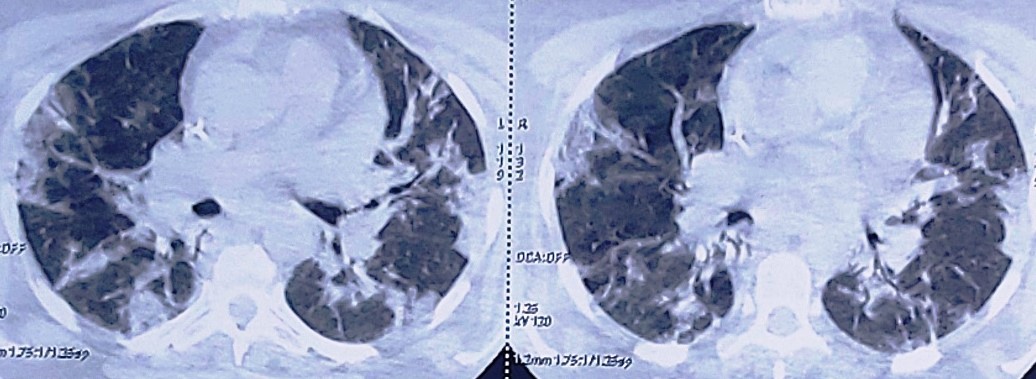
Figure 1: Chest-CT scan revealing air bronchogram associated with ranges of diffuse ground glass opacities.
The ECG showed sinus rhythm at 78bpm, PR fixed at 0.18, fine QRS without repolarization disorders. corrected QT was at 400 msec.
The echocardiography showed for the left ventricle that is normal in size with normal wall thickness and systolic function, an ejection fraction at 60%, without neither dyskinesia nor thrombosis. For the right ventricle,there were no valvular leakage or stenosis, a right ventricle systolic function associated with a concentric left ventricular hypertrophy. Both aorta and the inferior vena cava are normal. The patient's biological assessment revealed a white blood cell number of 27,860 cells/mm3 (Neutrophils 25970/mm3, lymphocytes 700/mm3), hemoglobin of 13.7g/dl, thrombocytes at 384,000 cells/mm3. Prothrombin time and partial thromboplastin time were normal (TP at 61% and TCA at 37,5s for a witness of 30s) Natremia:141mmol/l, kalemia: 4.5mmol/l, 31 g/l of albumin, correct liver and renal function (urea: 9.16 mmol/l and creatinine: 86 mmol/l, ASAT : 30 IU/l and ALT: 36 IU/l), fasting blood sugar at 1.76 g/l, C-reactive protein at 184 mg/l, Troponin at 2.5ug/l, ferritin at 2000 ng\ml Therapeutic management included oxygen therapy, we maintained his initial home parameters of Continuous positive airway pressure (CPAP) for his obstructive sleep apnea syndrome by night .However it became insufficient for his oxygen and ventilation needs. On the 6th day, his ventilatory state worsened as had more difficulties for breathing spontaneously therefore he was intubated and ventilated. Medical treatment associated Hydroxychloroquine 200 mg 3 times a day, Azythromycin 500mg the first day then 250mg per day, methylprednisone at 80mg a day for 7 days and curative anticoagulation treatment including enoxaparin 100 UI\kg (1mg\kg) twice a day.
Discussion
Obstructive sleep apnea (OSA) syndrome is a common disorder due to pharyngeal collapse during sleep resulting in frequent awakenings and therefore disrupted sleep and excessive day time sleepiness. Continuous positive airway pressure (CPAP) represents the main treatment. for OSA, CPAP has proved its efficiency in reducing symptoms, cardiovascular morbidity and mortality. However, the challenge for practitioners further more for intensivists, studies showed one quarter of patients OSA had severe COVID pneumonia [5-6]. Therefore, OSA particularly with concurrent obesity could potentially contributeto worsening hypoxemia and the cytokine storm that occurs in COVID patients. also, obesity may contribute to hypoxemia by reducing end-expiratory lung volume OHS often coexists with OSAHS. OHS, as a special manifestation of pathological obesity, is characterized by extremely excessive obesity, daytime sleepiness, hypoxia and hypercapnia, dyspnea, secondary erythrocytosis, systemic and pulmonary hypertension, etc. [3]. OHS is sometimes misdiagnosed as chronic obstructive pulmonary diseases or cardiac diseases. However, OHS patients usually do not have chronic respiratory diseases or pulmonary emphysema detectable by a chest X-ray. The pathogenesis of OHS consists mainly of (a) excessive obesity causing such a huge accumulation of adipose tissue in thoracic and abdominal cavities that the ventilation is limited by elevated diaphragm [8,9] or (b) a reduction in the sensitivity of the respiratory center to PaCO2 change [10].
Hypoventilation is caused by upper airways narrowness or obstruction in OSAS but by pulmonary ventilation limitation in OHS. Therefore, ventilation for OHS with coexisting OSAHS should ideally keep the UA patent to prevent UA obstruction or narrowness and enlarge tidal volume to prevent CO2 retention.
BiPAP ventilation has been served as an effective treatment for OHS patients with OSAHS. However, for such patients, their need for EPAP levels is varied from awake to sleep since in patients with OSAHS their OSA and hypopnea events selectively occur only when they are a sleep. Although a relatively higher EPAP is needed during sleep, CO2 retention is also easier to occur if IPAP keeps unchanged because of reduced P difference. However, if IPAP level is set too high, patients can feel discomfort followed by a poorer compliance with NIV treatment. Auto-trilevel PAP promotes CO2 discharge by a lower EPAP at the early expiratory phase and eliminates residual OSA events with an adjustable higher EEPAP towards the end expiratory phase under the precondition that IPAP was kept unchanged during inspiration. In the current study, two BiPAP modes were applied in order to compare with auto-trilevel NIV mode. In mode 1, with a high enough P difference, CO2 retention previously existed was effectively eliminated. However, this resulted in a relatively higher occurrence of residual OSA events. Although in mode 2, the residual OSA events were noticeably removed with a higher EPAP level, a higher PaCO2 was resulted in. Auto-trilevel PAP looks like a product of combination of BliPAP and auto-EPAP models. The three PAP levels delivered by the auto-trilevel PAP ventilator, (IPAP, EPAP, and EEPAP) have unique functions. A relatively high IPAP with a lower EPAP corrects CO2 retention and a relatively elevated EEPAP reduces residual OSA because UA is easier to collapse at the end stage of expiration when intrinsic airway pressure tends to be quite low. By auto-regulating EPAP based on patients’ real-time ventilation volume, auto-trilevel PAP ventilation is available for synchronized removal of both CO2 retention and residual OSA events [7]. Both OSA and obesity hypoventilation can cause important hypoxemia, which could worsen hypoxemia and the cytokine storm that can occur in COVID pneumonia which can cause ARDS and multiorgan failure, given that both OSA and obesity may be pro-inflammatory conditions [2-3] Angiotensin converting enzyme 2 (ACE2) is the entry receptor of SARS-CoV-2 [11]. The expression of ACE and dysregulation of renin angiotensin system in OSAS patient has been proven [12], as severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) enters human cells by binding to the angiotensin converting enzyme 2 (ACE-2) receptor [13]. This receptor is expressed in heart, lungs, kidneys, and intestines, thereby providing a multimodal entry point for the virus to infiltrate the body. Also, ACE-2 receptor concentrations are higher in adipose tissue that might be vulnerable to SARS-CoV-2 This presents a risk for adverse outcomes for obese patientswith more adipose tissue and a greater number of ACE-2 receptorsin comparison with their non-obese counterparts. Alterations of adipose tissue distribution and function linked to obesity have been shown to promote production of pro-inflammatory cytokines and induce chronic systemic inflammation [14]. The release of cytokines exacerbates activation of kinase receptors, triggering a positive feedback of inflammation and metabolic dysfunction which puts obese patients with COVID-19 at greater risk for a cytokine storm and therefore organ failure. Although obesity-specific clinical data are lacking, general findingsprovide evidence supporting the cytokine storm concept with COVID-19 non-survivors having significantly higher concentrations of interleukin-6, a pro-inflammatory cytokine that regulates homeostasis and inflammation, compared with survivors [15]. There is a debate on the effectiveness of various interventions to improve NIV in patients. However, it is plausible that interventions used to improve CPAP adherence in OSA [16] may also be effective to improve adherence to NIV in patients with OHS. Therefore, educational interventions (ie, verbal or audiovisual information), enhanced support by regular meetings, telephone follow-up, or interactive applications for encouraging continued use of NIV or behavioral interventions designed to promote adherence in patients with OHS with long-term NIV therapy. the presence of OSA is associated with decreased lung function, decreased lung-transfer factor for carbon monoxide, and, importantly, increased lung inflammation [17]. These conditions may explain the high risk of pneumonia in patients with OSA [18]. Further supporting our hypothesis that OSA is an additional risk for the development of severe disease in patients with COVID-19 is the observation that patients with OSA associated with cardiac surgery are at risk of developing adult respiratory distress syndrome [19]. Furthermore, comorbidities as hypertension, heart failure, coronary artery disease, cerebrovascular diseases, diabetes mellitus, and obesity-those are also risk factors for mortality in COVID-19-are commonly seen in OSAS patients [20-21]. Fibrotic changes is present after COVID-19 [22] and fibrosis is shown to be a risk factor for OSAS. Currently, there is no direct evidence to support OSA as an independent risk factor for severe COVID-19 infection, but some inferences can be made from data on ARDS. Guidelines advocate for early endotracheal intubation in patients with severe hypoxemia in favor of NIV [23-24]. NIV can delay recognition of decompensation and put the patient under unnecessary risk. Currently, the World Health Organization (WHO) recommends NIV of no longer than 1 hour to avoid delaying intubation [25].
Conclusion
COVID19 seems to lead to a more morbid course amongst the elderly, especially patients various comorbidities as hypertension, diabetes, and cardiovascular disease; to that list, we should now add obesity and by extension obstructive sleep apnea. Additional research into these potential mechanisms of increased morbidity in OSA patients with COVID-19 seems prudent, given the basic patient characteristic observations.
Authors’ contribution
All the authors contributed equally in drafting of the manuscript. All the authors read and agreed to the final manuscript.
References
1. Jordan AS, McSharry DG, Malhotra A, et al. 2014. Adult obstructive sleep apnoea. 383: 736-747. Ref.: https://pubmed.ncbi.nlm.nih.gov/23910433/
2. Lévy P, Kohler M, McNicholas WT, et al. 2015. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers. 1: 15015. Ref. : https://pubmed.ncbi.nlm.nih.gov/27188535/
3. Pierce AM, Brown LK. 2015. Obesity hypoventilation syndrome: currenttheories of pathogenesis. CurrOpinPulm Med CurrOpinPulm Med. 21: 557-622. Ref.: https://pubmed.ncbi.nlm.nih.gov/26390338/
4. Heymsfield SB, Wadden TA. 2017 Jan.Mechanisms, Pathophysiology, and Management of Obesity. 376: 254-266. Ref.: https://pubmed.ncbi.nlm.nih.gov/28099824/
5. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. 2020. Covid-19 in Critically Ill Patients in the Seattle Region - Case Series. Ref.: https://pubmed.ncbi.nlm.nih.gov/32227758/
6. Arentz M, Yim E, Klaff L, et al. 2020. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 inWashington State. Ref.: https://pubmed.ncbi.nlm.nih.gov/32191259/
7. Su M, Huai D, Cao J, et al. 2018. Auto-trilevel versus bilevel positive airway pressure venti- lation for hypercapnicoverlap syndrome patients. SleepBreath. 22: 65-70. Ref.: https://pubmed.ncbi.nlm.nih.gov/28612267/
8. Zerah F, Harf A, Perlemuter L, et al. 1993. Effects of obesity on respiratoryresistance. Chest. 103: 1470-1476. Ref.: https://pubmed.ncbi.nlm.nih.gov/8486029/
9. Pankow W, Podszus T, Gutheil T, et al. 1998. Expiratory flow limitation and intrinsic positive end-expiratory pressure in obesity. J Appl Physiol. 85: 1236-1243. Ref.: https://pubmed.ncbi.nlm.nih.gov/9760311/
10. Steier J, Jolley CJ, Seymour J, et al. 2009. Neural respiratory drive in obesity. Thorax. 64: 719-725. Ref.: https://pubmed.ncbi.nlm.nih.gov/19386586/
11. South AM, Diz D, Chappell MC. 2020. COVID-19, ACE2 and thecardiovascular consequences. Am J Physiol Heart CircPhysiol. 318: 1084-1090. Ref.: https://pubmed.ncbi.nlm.nih.gov/32228252/
12. Barceló A, Elorza MA, Barbé F, et al. 2001. Angiotensin converting enzyme in patients with sleepapnoea syndrome: plasma activity and gene polymorphisms. Eur Respir J. 17: 728-732. Ref.: https://pubmed.ncbi.nlm.nih.gov/11401071/
13. Augoustides JG. 2020. The renin-angiotensin-aldosterone system in coronavirus infectiond current considerations during the pandemic. J Cardiothorac Vasc Anesth. 34: 1717. Ref.: https://pubmed.ncbi.nlm.nih.gov/32360010/
14. Rodr?´guez-Hern!andez H, Simental-Mend?´a LE, Rodr?´guez-Ram?´rez G, et al. 2013. Obesity and inflammation:epidemiology, risk factors, and markers of inflammation. Int J Endocrinol. 678159. Ref.: https://pubmed.ncbi.nlm.nih.gov/23690772/
15. Zhou F, Yu T, Du R, et al. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 395: 1054. Ref.: https://pubmed.ncbi.nlm.nih.gov/32171076/
16. Wozniak DR, Lasserson TJ, Smith I. 2014. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adultswith obstructive sleepapnoea. Cochrane DatabaseSystRev. 1: CD007736.
17. Rouatbi S, Ghannouchi I, Kammoun R, et al. 2020. The ventilatory and diffusion dysfunctions in obese patients with and without obstructive sleepapnea-hypopnea syndrome. 2020: 8075482.
18. Su VY, Liu CJ, Wang HK, et al. 2014. Sleepapnea and risk of pneumonia:anationwide population-basedstudy. CMAJ. 186: 415e2. Ref.: https://pubmed.ncbi.nlm.nih.gov/24591276/
19. Memtsoudis S, Liu SS, Ma Y, et al. 2011. Perioperativepulmo- naryoutcomes in patients with sleepapnea afternon cardiac surgery. AnesthAnalg. 112: 113e21. Ref.: https://pubmed.ncbi.nlm.nih.gov/21081775/
20. Zhou F, Yu T, Du R, et al. 2020. Clinical course and risk factors formortality of adult inpatients with COVID-19 in Wuhan, China: aretrospective cohort study. Lancet. 395: 1054-1062. Ref.: https://pubmed.ncbi.nlm.nih.gov/32171076/
21. Pinto JA, Ribeiro DK, Cavallini AF, et al. 2016. Comorbidities associated with obstructive sleep apnea: a retrospectivestudy. Int Arch Otorhinolaryngo. l20: 145-150. Ref.: https://pubmed.ncbi.nlm.nih.gov/27096019/
22. Ye Z, Zhang Y, Wang Y, et al. 2020. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. https://doi.org/10.1007/s00330-020-06801-0
23. Sorbello M, El-Boghdadly K, Di Giacinto I, et al. 2020. The Italian coronavirus disease 2019 outbreak:recommendations from clinical practice. Anaesthesia. Ref.: https://pubmed.ncbi.nlm.nih.gov/32221973/
24. Wax RS, Christian MD. 2020. Practicalrecommendations for critical care and anesthesiology teams caring for novel corona- virus (2019-nCoV) patients. Can J Anaesth. 67: 568-576. Ref.: https://pubmed.ncbi.nlm.nih.gov/32052373/
25. World Health Organization. Clinical care of severe acute respiratory infections - Tool kit. Availableat: https://www. who.int/publications-detail/clinical-care-of-severe-acute-respiratory-infections-tool-kit. Published 2020. Updated April 11, 2020.




















