Indexing & Abstracting
Full Text
Case ReportDOI Number : 10.36811/ojprm.2019.110003Article Views : 27Article Downloads : 23
Case report of an unexpected reaction associated with Lumacaftor/ivacaftor therapy for cystic fibrosis
Farrell C*, Coleman S and Casserly B
Respiratory Department, University Hospital Limerick, Limerick, Ireland
*Corresponding author: Dr Ciaran Farrell, Respiratory Department, University Hospital Limerick, Limerick, Ireland, Email: ciarandfarrell@gmail.com
Article Information
Aritcle Type: Case Report
Citation: Farrell C, Coleman S, Casserly B. 2019. Case report of an unexpected reaction associated with Lumacaftor/ivacaftor therapy for cystic fibrosis. Open J Pulm Respir Med. 1: 07-11.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Farrell C
Publication history:
Received date: 06 June, 2019Accepted date: 15 June, 2019
Published date: 16 June, 2019
Abstract
Lumacaftor/ivacaftor increases cystic fibrosis transmembrane conductance regulator (CFTR) activity and is an effective treatment for cystic fibrosis (CF) patients that are homozygous for the F508del mutation. We describe the case of an 18-year-old Irish man with F508del homozygous CF admitted to our centre with a rash affecting his arms and upper trunk. This occurred one day post completing a 14-day course of intravenous (IV) Piperacillin/Tazobactam and Tobramycin for an infective exacerbation of cystic fibrosis and day 9 post treatment with lumacaftor/ivacaftor. Skin punch biopsies were performed, and the features were consistent with erythema multiforme. Our patient received another dose of lumacaftor/ivacaftor 20 days after receiving the first dose. Ninety minutes after the receiving the dose, he developed a diffuse erythematous rash, similar to the initial presentation. Lumacaftor/ivacaftor was re-trailed once more 10 months later. Three hours after commencing treatment our patient developed a diffuse erythematous rash affecting his face, and upper torso along with conjunctival injection. This reaction was diagnosed as a cytokine release type reaction. There are no reported cases in the literature of a cytokine release type reaction to lumacaftor/ivacaftor resulting in its discontinuation. When assessing patients on lumacaftor/ivacaftor that have developed a rash, this should always be included in the differential as the underlying cause given its high incidence. Tezacaftor/ivacaftor is an alternative treatment for patients that have developed a severe reaction to lumacaftor/ivacaftor.
Keywords: Lumacaftor/ivacaftor, Cystic fibrosis, Adverse event, Rash, Cytokine release type reaction
Introduction
Lumacaftor/ivacaftor increases cystic fibrosis transmembrane conductance regulator (CFTR) activity and is an effective treatment for cystic fibrosis (CF) patients that are homozygous for the F508del mutation. This treatment has been shown to improve clinical outcomes (reduced rate of pulmonary exacerbations, increased forced expiratory volume in 1 second (FEV1) and improved nutritional status) [1]. Lumacaftor is a CFTR corrector that has been shown to correct CFTR misprocessing and increase the level of protein localized at the cell surface [2]. Ivacaftor elevates the likelihood of CFTR channels being open by increasing the amount of the time these channels remain open [3]. The combination of lumacaftor with ivacaftor is associated with a greater increase in chloride transport compared to either agent alone [1]. The most common adverse events associated with lumacaftor/ivacaftor are chest discomfort, dyspnea, hypertension and liver function deterioration in patients with advanced liver disease. A mild/moderate rash is a common adverse event affecting more than 1 in 50 patients. A severe rash or rash resulting in treatment discontinuation is not common, occurring in approximately 1 in 500 patients.
Case presentation
We describe the case of an 18-year-old Irish man with F508del homozygous CF admitted to our centre with a rash affecting his arms and upper trunk. This man’s CF had been complicated by CF-related diabetes, prior Burkholderia contaminans colonization (now eradicated) and persistently positive sputum cultures for Pseudomonas aeruginosa [4]. Regular medications include Creon 25,000 capsule four times daily, ursofalk 250 milligrams twice daily, pulmozyme 2.5 milligrams nebuliser once daily, and becotide inhaler 50 micrograms twice daily. His prior adverse drug reactions included severe back pain secondary to meropenem.
During his admission the rash worsened affecting his upper limbs, lower limbs and trunk (Figures 1a-c). He was one day post completing a 14-day course of intravenous Piperacillin/Tazobactam and Tobramycin for an infective exacerbation of cystic fibrosis and day 9 post treatment with lumacaftor/ivacaftor. He was tachycardic and pyrexical (39.2 0C). On auscultation of lungs there were crepitations in the right base. He later developed lip swelling and a sore throat. White cell count (WCC) was 6.9 * 109/litre on admission but increased to 15.9 * 109/litre after one day. C-reactive protein (CRP) increased from 65 milligram/litre to 297 milligram/litre after one day. Chest x ray (CXR) was reported as ring shadowing and parenchymal opacification particularly in the upper zones in keeping with known cystic fibrosis. He was commenced on intravenous hydrocortisone, oseltamivir and ciprofloxacin. He continued to deteriorate, developing cheilitis, facial swelling, odynophagia, breathing difficulty, conjunctival injection and headache. He was transferred to the high-dependency unit for a central line and closer monitoring. Blood and urine cultures were negative. Serology showed previous immunity to Epstein Barr Virus and varicella zoster. Nasopharyngeal swabs were negative for influenza, parainfluenza, adenovirus, human metapneumovirus and respiratory syncytial virus. The rash progressed with the formation of flaccid bullae in the right upper arm and antecubital fossa. This was treated with 3 hourly applications of paraffin gel. Skin punch biopsies of the left lower abdomen and left thigh showed marked dermal oedema, and the overlying epidermis showed some vascular changes with necrotic keratinocytes. The patient showed a normal stratum corneum (Figures 2a and b). These features are consistent with erythema multiforme. The patient remained in the high dependency unit for a total of 5 days and was subsequently transferred back to the cystic fibrosis unit. On discharge from the high dependency unit, the patient had extensive skin desquamation and a severely oedematous scrotum.
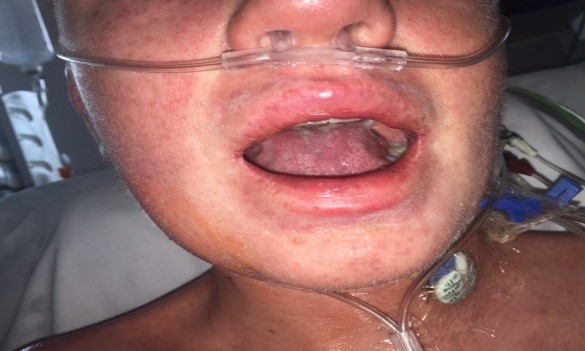
Figure 1a: Images of face.
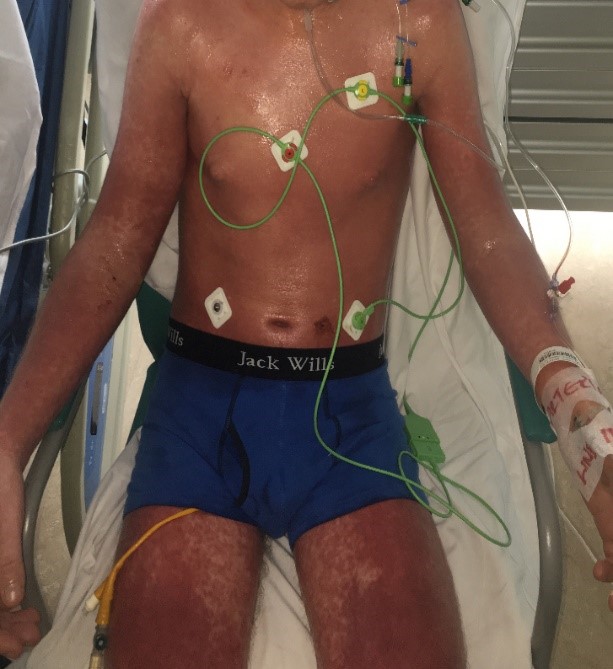
Figure 1b: Images of trunk, upper and lower limbs.
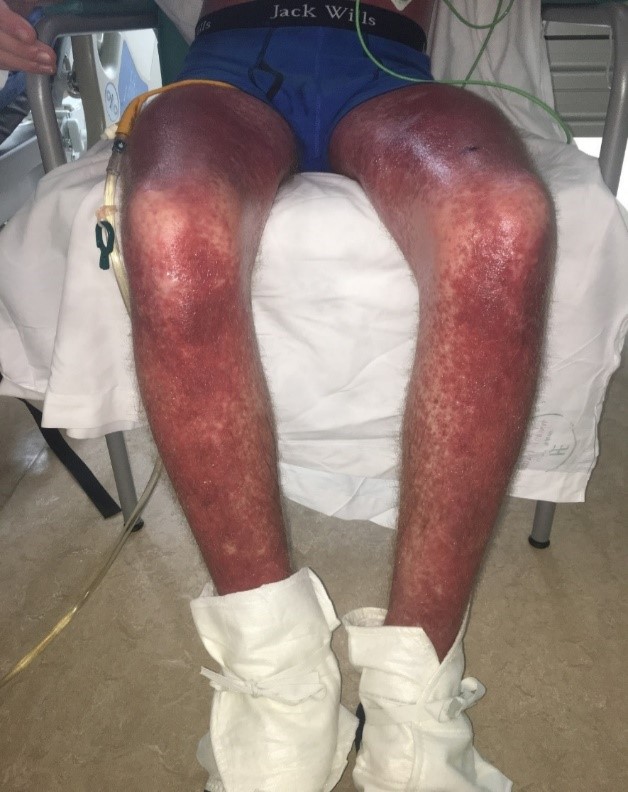
Figure 1c: Images of lower limbs.
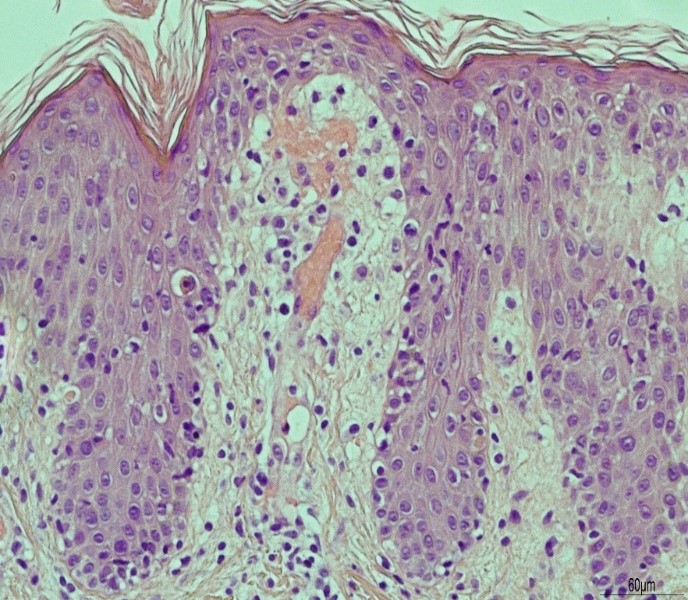
Figure 2a:
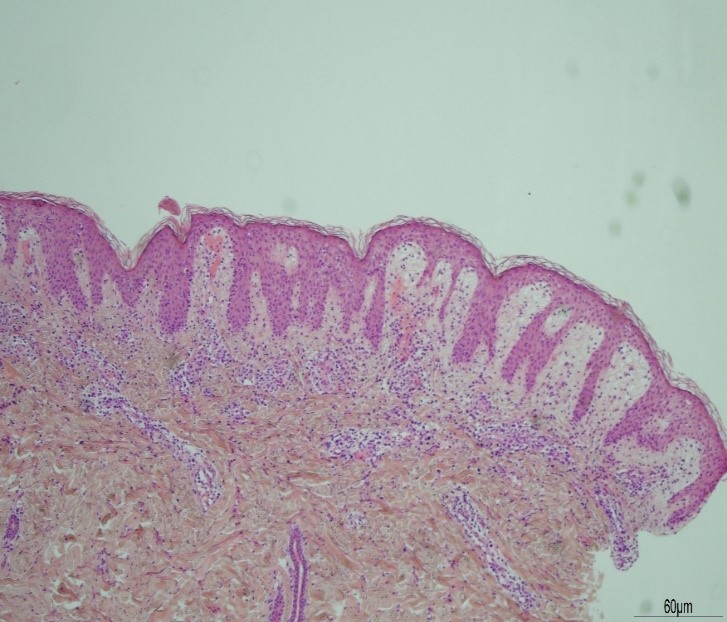
Figure 2b:
During this admission our patient received another dose of lumacaftor/ivacaftor 20 days after receiving the first dose. At this point, or patient was haemodynamically stable, WCC was normal at 8.0 * 109/litre and CRP 13 milligrams/litre. Ninety minutes after the receiving the dose, he developed a diffuse erythematous rash, similar to the initial presentation. Over the following 5 days there was extensive desquamation: superficially on both hands, the upper arms, and legs. Our patient was discharged home 3 weeks following admission. Lymphocyte transformation testing performed after the first reaction confirmed a drug reaction that was most likely secondary to Piperacillin/tazobactam. It was advised that all beta-lactam antibiotics be avoided. Our patient was referred to an immunologist for desensitization but was deemed unsuitable given the limitations of diagnostic testing to determine the offending drug as well as the risk of a more severe reaction.
At this point, it was unclear if lumacaftor/ivacaftor contributed to the reaction, and this treatment was re-initiated 10 months later. The patient was admitted to our centre and administered the drug. His vital signs on commencement were temperature 36.4 0C, heart rate 101 beats per minute, blood pressure 126/74 millimetres of mercury and respiratory rate 20 breaths per minute. Three hours later, our patient developed “a red face”. On examination, his face and upper torso showed a diffuse erythematous rash, along with conjunctival injection. He was febrile (39.2 0C), tachycardic (150 beats per minute) and hypoxic (SaO2 84% on room air). A diffuse wheeze and stridor were audible on auscultation. High-flow oxygen therapy was initiated, and he was given 200 milligrams hydrocortisone and 10 milligrams chlorphenamine intravenously. The emergency team was contacted, and they arranged for the patient’s transfer to the high dependency unit. The WCC prior to drug infusion was 12.3 * 109/litre which increased to 32.4 * 109/litre (predominantly a neutrophilia) the following day. CRP increased from 17-201 milligrams/litre two days later. CXR was reported as increasing peri bronchial thickening and infiltrates and upper zones of both lungs compared to prior examinations.
At 10 hours post-admission to the high dependency unit, our patient became more distressed vomited once with a worsening rash. IV hydrocortisone was increased to 200 mg QDS, and a 10 mg stat dose of chlorphenamine was given. He was transferred to the Intensive care unit (ICU). At this point, the patient was erythematous throughout his whole body. Dermatology consultant reviewed, and their impression was of a cytokine release reaction secondary to lumacaftor/ivacaftor. They advised paraffin gel and lacrilube. Our patient was discharged from the ICU one day later to a general ward. He continued to be pyrexical and tachycardic over the next couple of days but slowly improved. He was discharged on day 6 of admission. Our patient is currently avoiding lumacaftor/ivacaftor and all beta lactam antibiotics including tazocin, ceftazidime, Augmentin, flucloxacillin, meropenem and cephalosporins. His pulmonary function tests have been stable (FEV1 29% predicted over the last 5 months), and few pulmonary exacerbations of his CF have occurred since these two reactions. However, appropriate antibiotic treatment options are limited, with suitable regimens including ciprofloxacin, vancomycin and aztreonam.
Discussion
There are no reported cases in the literature of a cytokine release type reaction to lumacaftor/ivacaftor resulting in its discontinuation. Consulting the TRAFFIC, TRANSPORT and PROGRESS trials, rash is a common adverse event of lumacaftor/ivacaftor. In the TRAFFIC and TRANSPORT trial, 17 out of 738 patients (2.3%) developed a rash that was likely secondary to lumacaftor/ivacaftor treatment. The rash was described as severe in one (0.13 %) of these patients, and treatment was discontinued in 2 (0.27%). In the PROGRESS trial, rash occurred in 4 out of 675 patients (0.59%). The rash was described as severe in 1 patient (0.15%) but none of the treatments were discontinued.In a safety and efficacy programme carried out in the United States 9.1% had an adverse event secondary to rash [5]. In contrast, another study with Ivacaftor monotherapy showed no adverse events due to rash [6].
One limitation of this study is the lack of biopsy confirmation or photographs for the second and third reactions. However, symptoms began 1.5 hours and 3 hours after commencing the infusion in the second and third reaction, respectively, indicating a strong likelihood that lumacaftor-Ivacaftor is the causative drug. An alternative to lumacaftor/ivacaftor is Tezacaftor/ivacaftor. This treatment also targets the underlying cause of the disease and has been shown to improve FEV1 and reduce the rate of pulmonary exacerbations [7], while the incidence of rash is much lower at 0.6% (Phase 3 clinical trials on Tezacaftor/ivacaftor). We are planning on trailing our patient on Tezacaftor/ivacaftor under observation in a hospital setting in the coming months. When assessing patients on lumacaftor/ivacaftor that have developed a rash, this should always be included in the differential as the underlying cause given its high incidence. Tezacaftor/ivacaftor is an alternative treatment for patients that have developed a severe reaction to lumacaftor/ivacaftor.
References
- Wainwright CE, Elborn JS, Ramsey BW, et al. 2015. Lumacaftor–Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N Engl J Med. 373: 220-231. [Ref.]
- Van Goor F, Hadida S, Grootenhuis PD, et al. 2011. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci USA. 108: 18843–18848. [Ref.]
- Yu H, Burton B, Huang CJ, et al. 2012. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J Cyst Fibros. 11: 237-245. [Ref.]
- Coutinho CP, Barreto C, Pereira L, et al. 2015. Incidence of Burkholderia contaminans at a cystic fibrosis centre with an unusually high representation of Burkholderia cepacia during 15 years of epidemiological surveillance. J Med Microbiology. [Ref.]
- Taylor-Cousar J, Niknian M, Gilmartin G, et al. 2016. Effect of ivacaftor in patients with advanced cystic fibrosis and a G551D-CFTR mutation: Safety and efficacy in an expanded access program in the United States, Journal of Cystic Fibrosis.[Ref.]
- Clancy JP, Rowe SM, Accurso FJ, et al. 2012. Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. 67: 12-18. [Ref.]
- Taylor-Cousar JL, Anne Munck, McKone EF, et al. 2017. Tezacaftor–Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. N Engl J Med. 377: 2024–2035. [Ref.]




















