Indexing & Abstracting
Full Text
Case ReportDOI Number : 10.36811/ojor.2020.110009Article Views : 4Article Downloads : 5
Multiple Spinal Metastasis of a Thyroid Carcinoma Mimicking Tuberculous Spondylodiscitis: A case report and Literature Review
Lahjaouj M, Ahmed Brahim A*, Merzouki B, Oukessou YR, Abada, Rouadi S, Roubal M and Mahtar M
Department of ENT, “20 August” Hospital, Ibn Rochd University Hospital, Casablanca, Faculty of Medicine and Pharmacy, Casablanca, Morocco
*Corresponding Author: Ahmed Brahim Ahmedou, Department of ENT, “20 August” Hospital, Ibn Rochd University Hospital, Casablanca, Faculty of Medicine and Pharmacy, Casablanca, Morocco, Email: a.ahmedbrahim@gmail.com
Article Information
Aritcle Type: Case Report
Citation: Lahjaouj M, Ahmedbrahim A, Merzouki B, et al. 2020. Multiple Spinal Metastasis of a Thyroid Carcinoma Mimicking Tuberculous Spondylodiscitis: A case report and Literature Review. Open J Otolaryngol Rhinol. 2: 15-.22
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2020; Lahjaouj M
Publication history:
Received date: 24 November, 2020Accepted date: 08 December, 2020
Published date: 10 December, 2020
Abstract
Tuberculosis is a common disease in developing countries. It constitutes a major health problem. Several forms are described: Pleuro-pulmonary and extra-pulmonary forms. Osteoarticular tuberculosis represents 3 to 5% of all localizations. In case of no clinical improvement and worsening of symptoms, other diagnosis must be evoked, especially, tumoral causes. We describe the case of a woman who was treated for mediastinal lymph node tuberculosis with suspicion of tuberculosis spondylodiscitis, in whom preclinical investigations revealed a spinal metastasis of a papillary thyroid carcinoma.
Keywords: Tuberculous Spondylodiscitis; Papillary Thyroid Carcinoma; Spinal Metastases; Surgery; Iodineradiation therapy; Radiotherapy
Introduction
Tuberculosis is a slowly evolving bacterial infection whose prevalence continues to increase in developing countries, due to the overcrowding and the lower socio-economic level of the population. The most common form is the pleuropulmonary tuberculosis while the osteoarticular localization accounts only for 3 to 5% of all forms of tuberculosis [1]. The osteoarticular tuberculosis is localized at the thoracic and lumbar levels in 95% of cases, while the cervical spine is only affected in 5% of cases [2]. Although tuberculosis spondylitis has become the most frequent manifestation of osteoarticular tuberculosis, in the presence of multiple vertebral lesions, several causes are possible such as tumor lesions especially bone metastases present in 60% of cases. Bone metastasis are usually painful, it may cause spinal cord compression with neurologic signssometimes. Bone metastasis occursthrough hematogenous route of certain osteophilic tumors: thyroid, lungs, breast, prostate and kidneys. Therefore, it may reveal the disease or complicate its course since its discovery worsens the prognosis [3]. The incidence of bone metastasis in differentiated thyroid carcinomas represents only2 to 13% of cases. Bone metastasis in follicular carcinoma is three times more frequent (7 to 28%) compared to papillary carcinoma (1 to 7%) [4]. In our study, we report the case of a patient in whom a suspicion of tuberculosis spondylodiscitis was highly likely, but the clinical course has led the diagnosis to multiple bone metastases of papillary thyroid carcinoma.
Case Report
A 60-year-old female, with a medical history of cardiac pathology under treatment and a right thyroid lobectomy in 2012 (benign histopathological origin), presented for 8 months a painful backache without sphincter disorders in a context of night fever and a remarkable weight loss. MRI of the spine showed hypointensevertebral lesions -T1 and T2-at the cervical, thoracic and lumbar level as well as on the right iliac bone, enhanced after Gadolinium injection (Figure 1). Multiple cervical (lateral to trachea) lymph nodes and in Barrett’s lodgewere also noticed. Thoracoscopy with lymph node biopsy was performed. The histopathological examination was consistent with a tuberculosis lymphadenitis (epithelioid histolytic granuloma with central caseous necrosis). All vertebral lesions were thought to be secondary to tuberculosis adenitis and were considered to be due to tuberculousspon dylodiscitis for which the patient was put under anti-bacillary drugs for 9 months. Given the lack of improvement in general condition and neurological symptoms worsening, the diagnosis of tuberculosis has been questioned. A biopsy of the spinouts process of the second lumbar vertebrae (L2) was carried out.

Figure 1: Coronal sections- T1 and T2-weighted MRI show multiple hypo intense lesions at cervical, thoracic and lumbar vertebrae (arrows).
Immunohistochemical study revealed a follicular variant of papillary thyroid carcinoma, expressing TTF1 and Thymoglobulin. Re-lecture of slides (histological examination) of the right thyroid lobectomyrevealed an encapsulated follicular variant of papillary thyroid carcinoma, with multiple foci of capsular rupture and vascular emboli (Figure 2, 3). The patient underwent completion thyroidectomy for the other half of the thyroid gland. Histopathological study showed a thyroid dystrophy. Amultidisciplinarydecision (ENT and Oncologists) was to complete the extension workup with a cervical-thoracic and abdominal CT scan which showed diffuse osteolytic and osteoblastic bone lesions, affecting the entire spine and both iliac bones, with an osteolyticlesion of the right pedicle of T7 and intramural invasion (Figure 4). Thymoglobulin rate was at 14,235 ng / ml. Given the inaccessibility of these diffuse bone lesions by neurosurgery, a third-level palliative analgesic treatment was considered with Iodine radiation therapy and radiotherapy. The follow-up was done at 2-4-6 months and then one year later. The monitoring was based mainly on thymoglobulin and TSH tests, a cervical ultrasound was considered in case of doubt of local recurrence. At 1-year Follow-up, we did not reveal any local recurrence on cervical imaging and a thymoglobulin rate was undetectable.
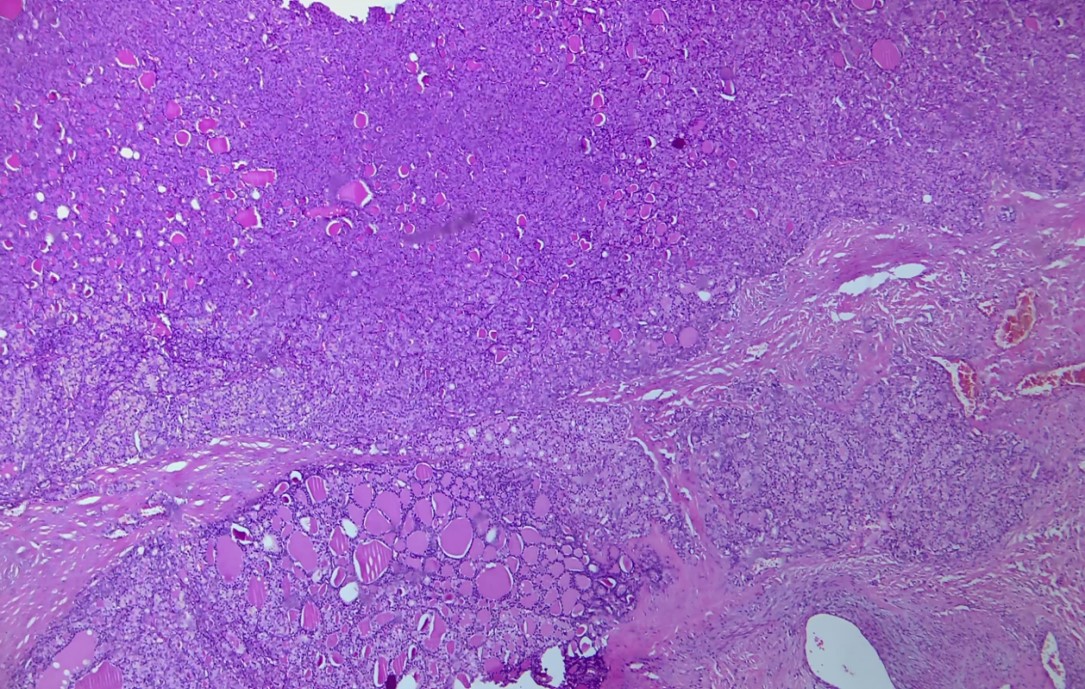
Figure 2: Follicular variant of thyroid neoplasm showing capsular invasion (HE* 40).
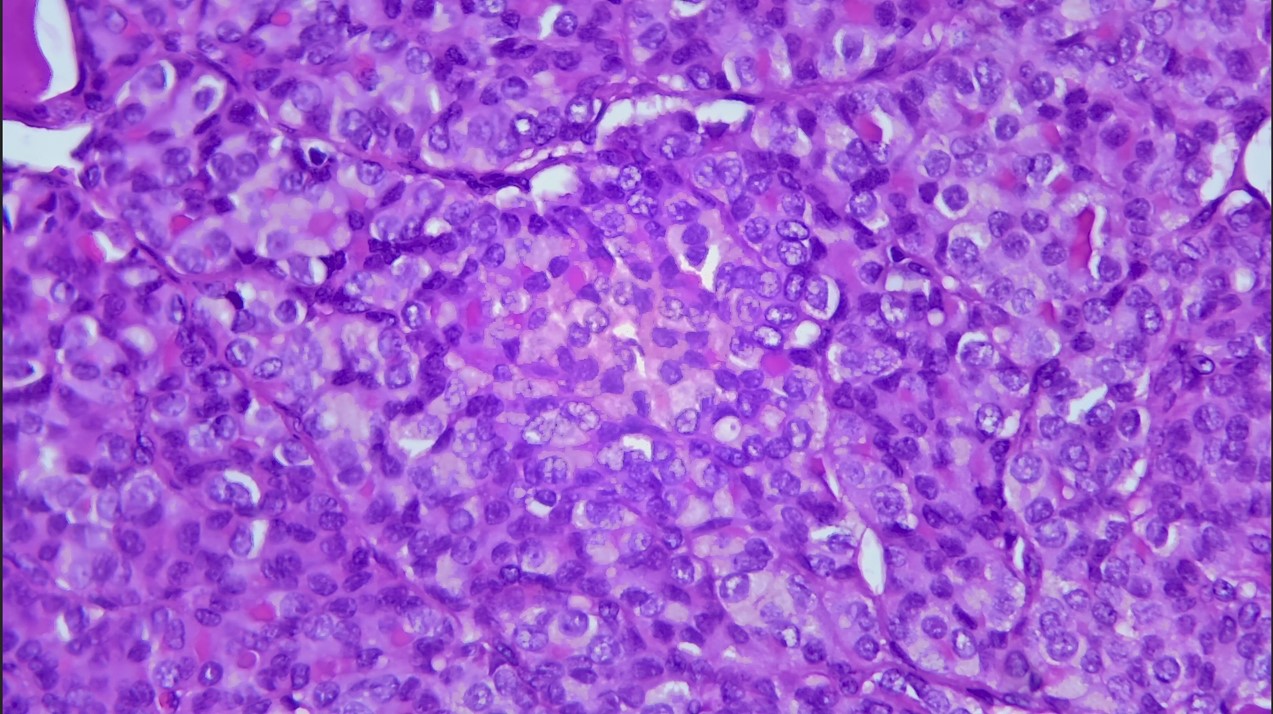
Figure 3: Nuclear atypia of papillary thyroid carcinoma: increased nuclear cytoplasmic ratio, nuclear crowding overlap, nuclear grooves and nuclear chromatin clearing (HE * 400).
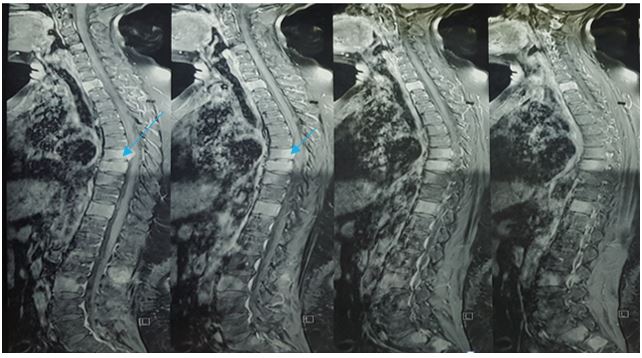
Figure 4: Coronal T1-weighted MRI shows multiple vertebral lesions with intradural invasion and the onset of spinal cord compression at T7 level.
Discussion
Tuberculosis (TB) is a major public health problem in the international community, the World Health Organization (WHO) estimated at 10.4 million cases in 2016 [5]. Extra pulmonary tuberculosis (EPTB) does not exceed 15% of all TB patients reported for many years [5,6]. However, there are some differences between WHO regions. In Morocco, WHO estimates for the year 2016 that approximately 36,000 people have been affected by tuberculosis, with an estimated incidence of 103 cases per 1,00,000 persons [1]. Between 1980 and 2016, the proportion of reported EPTB cases increased from 23% to 46% [7]. Spondylodiscitis (SD) or vertebral osteomyelitis (VOM) usually involves the inflammation or infection of the intervertebral disc and the adjacent vertebrae. The aetiologic agents of VOM may be bacteria, fungi or parasites. Its incidence varies according to different geographical areas and underlying diseases [8]. When the infecting agent is Mycobacterium tuberculosis complex, it is called Tuberculous Spondylodiscitis [9]. The disease is popularly known as Pott’s spine. The name traces back its origin from the description of tuberculosis infection of the spine by Sir Percival Pot in his monograph in 1779 [10]. Diagnosis of spinal tuberculosis depends on presence of clinical characteristics and neuroimaging findings. The accurate diagnosis requires the demonstration of acid-fast bacilli under microscope or culture of the biopsied tissue. Polymerase chain reaction is also an effective method for bacteriological diagnosis of tuberculosis [11]. The absence of improvement or even worsening of clinical signs under anti-bacillary treatment is an essential parameter to question the diagnosis of tuberculosis and considering other causes such as tumors, mainly osteophilicones (kidney, breast, prostate thyroid and lung), therefore, start an additional investigation according to the patient's history. In our case, the patient had a history of a right lobectomy which should not be neglected. Fortuitous discovery of a differentiated thyroid cancer (DTC) following isolated bone metastases constitutes 24% of cases, [12]. DTC accounts for the vast majority (85-98%) of thyroid malignancies and bone metastasis incidence in its case is low 2-13% [13]. Papillary Thyroid Carcinoma (PTC) derives from thyroid follicular epithelial cells. It’s the most common malignant thyroid neoplasm [14]. It accounts for 77% of DTC and
has a low incidence of spinal metastasis(SM) 1-7% of cases, while follicular thyroid cancer (FTC) which accounts for 15% of all DTC, has an incidence of bone metastasis of 7-20% of cases secondary to the hematogenous diffusion [15]. Conversely, a large series made by Tickooand al. showed that the majority of bone metastases are related to papillary carcinoma. [16]. Bone metastases most often occur in old age people: the average age is 65 years [17], predominantly in females [16]. Bone metastasis may be revealed by) multiple symptoms such as pain, swelling and pathologic fracture. Distant metastases can involve all segments of the skeleton, electively localized at the level of the axial skeleton, pelvis, shoulder blades and ribs, sternum, femur, and skull base [18]. Bone metastasis related to Papillary Thyroid Carcinoma remains latent and does not or grow very slowly; it is associated with a low mortality rate with a relative survival at 5 years that varied from 93% to 100% in stages I, II and III [19]. While lymph node metastases are often present at the time of diagnosis (average of 50%, may reach 85-90% in some series), hematogenous spread is rather a rare and late event [20]. Metastatic thyroid tumors cause considerable morbidity [21]. They indicate an advanced stage of disease and are usually associated with a poor prognosis and reduced response to treatment [22]. Classic clinical symptoms appear with the progression of metastatic disease of the spine and are the consequence of infiltration and / or compression of par vertebral, bone and neural tissues [23]. Magnetic resonance imaging (MRI) is the gold-standard imaging modality in spinal metastasis diagnosis. It is an extremely detailed multilane imaging, allowing the visualization of metastatic infiltration and/or compression of par vertebral, bone and neural tissues [24-25]. Computed tomography (CT) imaging is an excellent way to assess the spine and all bone structures. With high degree of accuracy (90% sensitivity, 100% specificity), it can identify metastatic lesions, and vertebral destruction [26]. Bone scintigraphy is used to screen bone metastasis. Despite its high sensitivity (62-89%), it should be noted that bone scintigraphy measures abnormalities in bone metabolism, therefore, does not have a high specificity in identifying spinal metastases [27]. Therapeutic interventions should first and foremost target the integrity of the spine to avoid neurological complications. By doing so, they can ensure safe control of symptoms, such as pain, paralysis and their impact on daily activities[28]. Treatment modalities may be palliative or curative, using Iodine ablation therapy, selective remobilization therapy (SET), bisphosphonates, surgery as well as small molecular therapy is being discussed [29]. Iodine radiation therapy is the mainstay of thyroid cancer management Curiously, Van Tolland al. demonstrated that radioiodine ablation reduces pain on analogue scale for pain [30]. The absorption of radioactive iodine is a prognostic factor for metastatic disease. Tumors which do not function represent an entity further down the path of malignant transformation and are resistant to the dose of radiation delivered by radioactive iodine sequestration [31]. Selective remobilization therapy (SET) represents an attractive therapy making a rapid improvement even if transient of symptoms. Eustatia Rotten and al. [32], suggested that symptomatic control and progression arrest is achievable in 59% of embolizations.In reality, however, this does not equate to 59% of patients receiving neurological and pain benefit [33]. SET alone induces tissue hypoxia and is a tumor growth stimulus. The combination of SET with radiation therapy may confer synergistic benefit [34,35]. The role of surgery in bone metastasis of DTC is questionable. Quan and al. suggest that surgery is indicated for patients with intractable pain, cord compression, neurological deficit or cervical instability [34]. The prognosis of patients with distant thyroid cancer metastases is generally poor, with an average 4-year survival rate after diagnosis of metastasis of 40% and an overall 10-year survival rate of 27% for bone metastases of DTC [35]. Despite the low survival rate, DTC with bone metastases compared to other malignant tumors with bone metastases, still maintains a better prognosis and therefore justifies aggressive treatment [36,37].
Conclusion
Osteolytic vertebral lesions in a context of a confirmed tuberculosis diagnosis, which contrast with poor progress under adequate treatment, should not rule out other diagnoses, especially, tumoral causes as the associations possible. DTCs are among some head and neck cancers with an excellent prognosis with 10-year survival rates of up to 95%. Survival rate drops by half if metastases occur. Multimodal treatment including surgery, iodine radiation therapy, and sometimes radiotherapy provides good disease control and improved survival rate.
References
1. BEZZA A, NIAMANE R, BENBOUAZZA K, et al. 1998. Latuberculosesterno-claviculaire. A propos de deux cas. Rev. Rhum. 65.
2. LARGET-PIET B, MARTIGNYS J. 1995. p o n dylodiscitebactérienne : Etiologi e, diagnostic, évolution, pro n o s t i c, traitement. Revue du praticien. Paris. 45.
3. Professeur Jean-Philippe Vuillez. Tumeursosseusessecondaires (154e)/Octobre 2003http://www-sante.ujf-grenoble.fr/SANTE/)
4. Muresan MM, Oliver P, Leclere J, et al. 2008. Bone metastases from differentiated thyroid carcinoma. EndocrRelat Cancer. 15: 37-49. Ref.: https://pubmed.ncbi.nlm.nih.gov/18310274/
5. Julia Lienard, Fredric Carlsson. 2017. Murine Mycobacterium marinum Infection asr a Model for Tuberculosis. World Health Organization. WHO; Geneva, Switzerland: 2017. Global Tuberculosis Report-2017. Ref.: https://pubmed.ncbi.nlm.nih.gov/27914088/
6. World Health Organization. WHO; Geneva, Switzerland: 2016. Global Tuberculosis Report-2016.
7. Bennani K, KhattabiA, Akrim M. 2019. Evaluation of the yield of histopathology in the diagnosis of lymph node tuberculosis in Morocco, 2017: cross-sectional study. JMIR Publ. Health Surveill. 9: 5. Ref.: https://pubmed.ncbi.nlm.nih.gov/31599732/
8. Mete B, Kurt C, Yilmaz MH, et al. 2012. Vertebral osteomyelitis: eight years’ experience of 100 cases. RheumatolInt. 32: 3591-3597. Ref.: https://pubmed.ncbi.nlm.nih.gov/22095392/
9. Fitzgerald DW, Sterling TR, Haas DW. 2015. Mycobacterium tuberculosis.In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 8th ed. Philadelphia: Churchill Livingstone. 2787-2818.
10. Dobson J, Percivall Pott. 1979. Ann R Coll Surg Eng. 50: 54-65. Ref.: https://pubmed.ncbi.nlm.nih.gov/4550865/
11. Ravindra K, Dilip Singh S, Chhatrapati S. 2011. Spinal tuberculosis: A review Medical University, Lucknow, Uttar Pradesh, India. The Academy for Spinal Cord Injury Professionals.
12. Haq M, Harmer C. 2005. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome. Clinical Endocrinology. 63: 87-89. Ref.: https://pubmed.ncbi.nlm.nih.gov/15963067/
13. Wexler JA. 2011. Approach to the Thyroid Cancer Patient with Bone Metastases.J ClinEndocrinolMetab. 96: 2296-2307. Ref.: https://pubmed.ncbi.nlm.nih.gov/21816796/
14. Kum RO, Aygenç E, Somuk BT, et al. 2015. A unique case of intranasal metastasis from occult poorly differentiated thyroid carcinoma.Balkan Med J. 32: 316-319. Ref.: https://pubmed.ncbi.nlm.nih.gov/26185723/
15. Wexler JA. 2011. Approach to the Thyroid Cancer Patient with Bone Metastases. JClinEndocrinol Metab. 96: 2296-2307. Ref.: https://pubmed.ncbi.nlm.nih.gov/21816796/
16. Ali Ender Ofluo?lu, YakupSancarBar??. 2013. ErhanEmelMultiple Skull and Intracranial Metastasis of Follicular Thyroid Carcinoma: Case Report Causa Pedia. 2: 357.
17. Mahtar M, Kadiri M, Destouli M, et al. 2002. Métastase mandibulaire révélatrice d’un cancer de la thyroïde. A propos de deux cas.Rev. Stomatol. Chir. Maxillofac. 103: 120-123. Ref.: https://pubmed.ncbi.nlm.nih.gov/11997740/
18. Satish K. Tickoo, Anastassios G, et al. 2000. Bone Metastasis from Thyroid Carcinoma. A Histopathologic Study with clinical Correlates. Arch Pathol. 124. Ref.: https://pubmed.ncbi.nlm.nih.gov/11035572/
19. Statistiques de survie pour le cancer de la glande thyroïde
20. Rosahl SK, Erpenbeck V, Vorkapic P, et al. 2000. Solitary follicular thyroidcarcinoma of the skull base and its differentiation from ectopic adenoma–review, use of galectin-3 and report of a new case. Clin NeuroNeurosurg. 102: 149-155. Ref. : https://pubmed.ncbi.nlm.nih.gov/10996713/
21. Tokuhashi Y, Ajiro Y, Oshima M. 2009. Algorithms and Planning in MetastaticSpine Tumors.OrthopClin N Am. 40: 37. Ref.: https://pubmed.ncbi.nlm.nih.gov/19064054/
22. Pittas AG, Adler M, Fazzari M, et al. 2000. Bone metastases from thyroid carcinoma: clinicalcharacteristics and prognostic variables in one hundred forty-sixpatients. Thyroid. 10: 261-268. Ref.: https://pubmed.ncbi.nlm.nih.gov/10779141/
23. Wexler JA. 2011. Approach to the Thyroid Cancer Patient with Bone Metastases.J ClinEndocrinolMetab. 96: 2296-2307. Ref.: https://pubmed.ncbi.nlm.nih.gov/21816796/
24. Harel R, Angelov L. 2010. Spine metastases: current treatments and futuredirections. Eur J Cancer. 46: 2696-2707. Ref.: https://pubmed.ncbi.nlm.nih.gov/20627705/
25. Molina CA, Gokaslan ZL, Sciubba DM. 2012. Diagnosis and managementof metastatic cervical spine tumors. OrthopClin Nam. 43: 75. Ref.: https://pubmed.ncbi.nlm.nih.gov/22082631/
26. Perrin RG, Laxton AW. 2004. Metastatic spine disease: epidemiology, pathophysiology, and evaluation of patients. NeurosurgClin Nam. 15: 365. Ref.: https://pubmed.ncbi.nlm.nih.gov/15450871/
27. Molina CA, Gokaslan ZL, Sciubba DM. 2012. Diagnosis and managementof metastatic cervical spine tumors. OrthopClin Nam. 43: 75. Ref.: https://pubmed.ncbi.nlm.nih.gov/22082631/
28. Tokuhashi Y, Ajiro Y, Oshima M. 2009. Algorithms and Planning in MetastaticSpine Tumors.OrthopClin N Am. 40: 37. Ref.: https://pubmed.ncbi.nlm.nih.gov/19064054/
29. Quan GM, Pointillart V, Palussière J, et al. 2012. Multidisciplinary treatmentand survival of patients with vertebral metastases from thyroidcarcinoma. Thyroid. 22:125-130. Ref.: https://pubmed.ncbi.nlm.nih.gov/22176498/
30. Van Tol KM, Hew JM, Jager PL, et al. 2000. Embolization in combination with radioiodine therapyfor bone metastases from differentiated thyroid carcinoma. ClinEndocrinol (Oxf ). 52: 653-659. Ref.: https://pubmed.ncbi.nlm.nih.gov/10792347/
31. Hindie E, Paolo Zanotti-Fregonara, Isabelle Keller, et al. 2007. Bone metastases of differentiated thyroid cancer: Impactof early I-131-based detection on outcome. EndocrRelat Cancer. 14: 799-807. Ref.: https://pubmed.ncbi.nlm.nih.gov/17914109/
32. Eustatia-Rutten CF, Romijn JA, Guijt MJ, et al. 2003. Outcome of palliative embolization of bonemetastases in differentiated thyroid carcinoma. J Clin Endocrinol Metab. 88: 3184-3189. Ref.: https://pubmed.ncbi.nlm.nih.gov/12843163/
33. De Vries MM, Adrienne C M Persoon, Pieter L Jager, et al. 2008. Embolization therapy of bone metastases fromépithelial thyroid carcinoma: effect on symptoms and serumthyroglobulin. Thyroid. 18: 1277-1284. Ref.: https://pubmed.ncbi.nlm.nih.gov/18991486/
34. Quan GM, Pointillart V, Palussière J, et al. 2012. Multidisciplinary treatmentand survival of patients with vertebral metastases from thyroidcarcinoma. Thyroid. 22: 125-130. Ref.: https://pubmed.ncbi.nlm.nih.gov/22176498/
35. Siti A, ArepenIrfan M. 2016. Massive bilateral mandibularmetastasis from papillary thyroid carcinoma. EJENTAS. 12: 004.
36. Schlumberger M, Tubiana JP, Fragu P, et al. 1986. Long-term results of treatment of 283patients with lung and bone metastases from differentiated thyroidcarcinoma. J ClinEndocrinolMetab. 63: 960-967. Ref.: https://pubmed.ncbi.nlm.nih.gov/3745409/
37. Ramadan S, Ugas MA, Berwick RJ. 2012. Spinal metastasis in thyroid cancer. Head Neck Oncol . Ref.: https://pubmed.ncbi.nlm.nih.gov/22730910/




















