Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ojgor.2020.110014Article Views : 9Article Downloads : 17
Influence of Ulipristal Acetate in 3D-PW-Doppler parameters of patients with uterine myomas: a prospective observational pilot study
Patricia Diaz Ortega* and García-Manero Manuel
and García-Manero Manuel
Department of Obstetrics and Gynecology Hospital García Orcoyen, Spain
*Corresponding Author: Dr Patricia Díaz Ortega, Departamento de Obstetricia y Ginecología Hospital García Orcoyen, Calle Santa Soria, 22 CP 31200, Estella (Navarra), Spain, Email: patry.do80@gmail.com
Article Information
Aritcle Type: Research Article
Citation: Patricia Diaz Ortega, García-Manero Manuel. 2020. Influence of Ulipristal Acetate in 3D-PW-Doppler parameters of patients with uterine myomas: a prospective observational pilot study. O J Gyencol Obset Res. 2: 11-18.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2020; Patricia Diaz Ortega
Publication history:
Received date: 23 January, 2020Accepted date: 17 February, 2020
Published date: 19 February, 2020
Abstract
Introduction: uterine fibroids are the most common benign tumors of the female genital tract. They associate a varied symptomatology, from the absence of symptoms to more disabling bleeding or pain [1,2]. There are multiple treatments directed against different targets such as ulipristal acetate which has proven effective in reducing the size of the fibroids and their symptoms [7]. Our preliminary study seeks to find the relationship between ulipristal acetate and angiogenesis of uterine fibroids, by measuring ultrasound vascularization of the fibroid throughout the treatment.
Material and Methods: A prospective observational study has been designed, in which 24 patients with symptomatic uterine fibroids have been included and given 4 cycles of Ulipristal Acetate. The size and vascularization of the fibroids were measured at the beginning and end of treatment; as well as on several occasions throughout the follow-up. Myoma vascularization was measured by power doppler 3D ultrasound through different parameters that define the vascularization in a more objective way: Vascularization Index (VI), Flow Rate (FR), Flow Vascularization Index (FVI).
Result: A significant reduction in the size of the fibroids has been observed, as well as their vascularization in terms of vascularization indices measured by 3D PW ultrasound. These changes were evident at the end of treatment and were maintained over time.
Conclusion: There is a correlation between myoma vascularization and treatment with Ulipristal Acetate. SPRMs may provide effective treatment for women with symptomatic fibroids by two ways: supports selective progesterone receptor modulators and reduced angiogenesis. In addition, the use of vascularization markers of 3DPW ultrasound and the colour map allow us to monitor the response to medical treatment of myomas in a non-invasive and easily reproducible way.
Keywords: Ulipristal Acetate; Esmya®; Uterine fibroids; Angiogenesis; 3D Power Doppler Ultrasound
Introduction
Uterine fibroids are the most common type of benign tumor in women of reproductive age and originate in the muscle cells of the uterine wall [1]. Although it is possible to have a single fibroid, the most frequent scenario is that they are multiple and of different sizes that can vary from millimeters to several centimeters, even occupying the entire uterus and reaching a significant weight. The cause of myomas is unknown, however, it is known that their growth is influenced by female sex hormones (estrogens and gestagens) since their size normally grows during pregnancy and in the reproductive stage and decreases during menopause [5,9]. Uterine fibroids show a clinically significant prevalence of 20% with 40% peaks in women between 35 and 55 years of age. The majority are asymptomatic and do not require any type of treatment, but in those women in which symptoms develop, they have a significant negative impact on their physical and emotional well-being. Normally, 62% of women who have symptoms have more than one. The symptoms that appear are the following [2]:
- Changes in bleeding: irregular or prolonged bleeding, bleeding between periods or excessive bleeding resulting in anemia.
- Pelvic discomfort due to the compression of some adjacent organs (bladder, intestine, ureters).
- Pelvic pain caused by anatomical distortions of the uterus.
- Difficulties in conception or infertility.
- Difficulties in embryo implantation. During pregnancy, the presence of myomas can increase the risk of spontaneous abortion, premature births, as well as making labor more difficult.
According to the new FIGO classification, up to 9 types of fibroids are distinguished depending on their location (Figure 1).
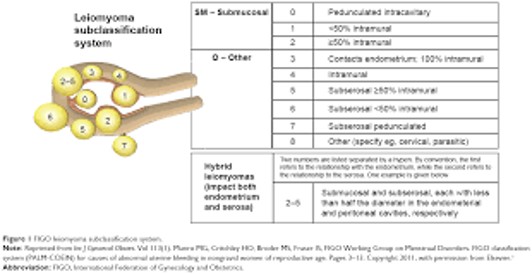
Figure 1: FIGO leiomioma sibclassification system.
Until recently, the Gold Standard of treatment to suppress the symptoms of myomas was the surgical removal of the myoma (myomectomy) or the uterus (hysterectomy). However, this type of treatment can be aggressive and pose compications for some patients. That is why today we opt for more conservative treatments, minimizing, and even avoiding, the risks and complications inherent to surgical intervention [3,4]. Ulipristal Acetate has shown good tolerability in patients and the ability to rapidly decrease bleeding, to reduce the volume of myomas, to adverse the effects of limited entity, and improves the level of pain and the patient´s quality of life. It is a therapeutic alternative for women with uterine fibroids that offers a long-term treatment option that can prevent surgery.Several studies have confirmed the efficacy and safety of intermittent and repeated long-term treatment of using Ulipristal Acetate (5 milligrams), in patients with symptomatic uterine fibroids. The effectiveness was demonstrated by reducing heavy bleeding, the size of myomas and pain, thus improving the quality of life of patients with a good safety profile [7].
Material and Methods
A prospective, observational study has been designed, using the medication under the conditions of normal use, in accordance with the indication established in its approved technical sheet. The assignment of a patient to a specific therapeutic strategy was not decided in advance by this protocol, but was determined by the usual practice of medicine, and the decision to prescribe Ulipristal Acetate was clearly dissociated from the decision to include the patient in the study. No intervention was applied to patients, either diagnostic or follow-up, which is not the usual clinical practice. Epidemiological methods were used to analyze the collected data. Patients had to meet all the inclusion criteria and not meet any of the exclusion criteria. The researcher obtained the informed consent of the patients prior to their inclusion in the study.
Inclusion criteria
o Women 18-50 years (both included).
o Women with symptomatic uterine fibroids (hypermenorrhea, pain and pressure).
o Candidates for treatment with Ulipristal Acetate.
o Patients who agree to participate in the study and have signed the Informed Consent.
Exclusion criteria
o Women <18 years.
o Women> 50 years.
o Patients eligible for surgery treatment.
o Patients for whom treatment with Ulipristal Acetate is contraindicated.
o Patients who cannot meet the study visits.
Criteria for discontinuation or premature withdrawal
Patients were considered in the study once the informed consent was signed. Discontinuation occurs when a patient included in the study, for any reason, leaves the study at any time, before the conditions set forth in the protocol are met, without having to offer any explanation or justification. Patients under study may also discontinue at any time at the discretion of the investigator or the promoter for safety or other reasons. Whenever the reasons for the discontinuation of a patient were known, they were recorded in the eCRD.
Equipment
The ultrasound study of fibroids was carried out with two types of Voluson ultrasound scanners from General Electrics. One of them is portable, however both of them use the same softwarwe, that allows a thorough study of both morphology, size, volume and the study of angiogenesis with the 2D and 3D Doppler.
The technical characteristics of the Doppler study are the following
3DPW combination of Power Doppler and 3D representation allowing an evaluation of the vascularization of myomas in its three dimensions. The VOCAL mode allows the calculation of myomas volumes. The concomitant use of the Power Doppler and the histogram function enables the reconstruction of the vascular network in a three-dimensional way. Also, it calculates the parameters that define the vascularization in a more objective way:
1) VI: Vascularization index. It studies the number of blood vessels in the study region.
2) FR: Flow rate. It evaluates the intensity of blood flow at the time of measurement.
3) FVI: Flow vascularization index. The density of vascularization determines the intensity of flow.
Statistical analysis
Expected statistical analyzes and study populations
The analyzes were performed based on the available data, without using ‘missing’ value substitution techniques and describing the number of ‘missing’ in each analysis. Descriptive analyzes of all variables were performed separately and depending on the character of the variable, the following will be represented: o in continuous variables: mean, standard deviation, P25, median, P75, maximum and minimum and missing. o in categorical variables: % with respect to total of each category.
Scheduling visits
The planning of visits in this study consisted of a first visit in which the patient's data were recorded, the methodology of the study was explained and informed consent was given; four more visits were made, coinciding with the successive cycles of treatment with ulipristal acetate. The research protocol was carried out in the Gynecological Unit of the Mendebaldea Health Center.
Method
Each of the 24 patients included in the study underwent an exhaustive anamnesis and a liver profile analysis before starting treatment with ulipristal acetate. Successive liver profile control analyses were performed throughout the treatment according to the recommendations of the European Committee for Risk Assessment in Pharmacovigilance (PRAC) [1,2]. One fibroid per patient was studied, in this case, the one with the highest vascularization at the beginning of the study, measured by 2D and 3D doppler ultrasound. All fibroids were intramural-submucosal. At the first visit of the study, the ultrasound determination of the vascularization of myomas was carried out by means of the color map study with 2D Doppler and 3D Doppler parameter analysis. In all cases an exploration with identical frequency and "pass filter" was performed; as well as a multiplanar and surface representation of the myoma in 3D. In the subsequent visits the color map was studied determining the following categories: null, sparse, moderate and abundant vascularization. Both at the beginning and at the end of the study, vascularization rates were added with 3D power doppler ultrasound.
Ethical approval
The researcher carried out the study in accordance with the basic ethical principles contained in the Helsinki Declaration of the World Medical Association on ethical principles for medical research in humans, and in its subsequent revisions. Copies of the latest version, as well as any updates that may be made during the course of the study, can be freely found on the World Medical Association website: http://www.wma.net/en/ 30publications /10policies/b3/index.html. The study was carried out in accordance with the protocol and with standardized work procedures (PNTs) that ensure compliance with the Standards of Good Clinical Practice (BPC). In compliance with Order SAS / 3470/2009, of December 16, this study will be submitted for consideration by an accredited Research Ethics Committee. Being an observational post-authorization study, it is exempt from the mandatory subscription of insurance.
Research Ethics Committee
The protocol, the patient information sheet, the proposed informed consent form and any other information for the patients was reviewed and approved by the Clinical Research Ethics Committee of Navarra, in accordance with Order SAN/3470/2009 of 16th December, which publishes the guidelines for post-authorization studies of an observational nature for medicines for human use, and with the other applicable regulations . Any modification of the protocol, other than administrative changes, will need an amendment to the protocol that must be approved by this Committee.
Result
24 patients between the ages of 33 and 52, with an average age of 45, who met the inclusion criteria were enrolled in a prospective observational study. All the patients were diagnosed with uterine fibroids and presented subsidiary treatment symptoms. The most common symptom was hypermenorrhea, in 90% of patients, followed by pelvic pain in 7% and asthenia secondary to chronic anemia in 3%. Treatment with ulipristal acetate was administered at the dose of 5mg per day for 3 months, repeated for 4 cycles, in accordance with the recommendations in the data sheet. In each of the patients, the myoma with intramural-submucosal characteristics with greater vascularization was studied by means of a 2D/3D ultrasound scan at the beginning of the study. The average size of fibroids at the beginning of the treatment was 44,87 mm (range of 10 to 100mm) (Table 1).
|
|
N |
Lower limit |
Upper limit |
Average |
Standard deviation |
|
Age (years) |
24 |
33,00 |
52,00 |
45,4583 |
4,22188 |
|
Size 1 (mm) |
24 |
10,00 |
100,00 |
44,8750 |
23,52300 |
|
N valid |
24 |
|
|
|
|
|
Table 1: Statistical descriptive data. Average age of patients enrolled. Average size of fibroids (Size 1: measurement made at the beginning of the study). |
|||||
|
|
Average |
N |
Standard deviation |
Standard error |
|
|
Pair 1 |
Size 1(mm) |
45,0870 |
24 |
24,02823 |
5,01023 |
|
Size 2(mm) |
29,0000 |
24 |
16,96788 |
3,53805 |
|
|
Table 2: Paired sample statistics. Size1: Size of fibroids at baseline. Size2: Size of fibroids after tratment with ulipristal acetate. |
|||||
|
|
Average |
Standard deviation |
Standard error |
Lower |
Upper |
t |
Bilateral signification |
|
Pair 1 VI1-VI2 |
1,59750 |
2,92863 |
,59780 |
,36085 |
2,83415 |
2,672 |
,014 |
|
Pair 2 FR1-FR2 |
8,07271 |
10,51189 |
2,14573 |
3,63393 |
12,51149 |
3,762 |
,001 |
|
Pair 3 FVI1-FVI2 |
1,43975 |
1,83332 |
,37422 |
,66561 |
2,21389 |
3,847 |
,001 |
|
Table 3: Paired sample test. VI: Vascularization index FR: Flow rate. FVI: Flow vascularization index. The suffix "1" refers to the measurement made at the beginning of the study, before starting the treatment; the suffix "2" refers to the measurement made after finishing the treatment with ulipristal acetate. The difference between values 1 and 2 is calculated for each patient; the average of the values is used for statistical analysis of the data. |
|||||||
VI, FR and FVI were measured before and after the treatment with Ulipristal treatment. In addition, several measurements of these parameters were made throughout the follow-up of these patients. A decrease in the size of fibroids was observed, from 45?24mm to 29?16mm on average after the treatment with Ulipristal Acetate (Table 2). as we have reflected in another parallel study. Likewise, a decrease in vascularization was observed, with a significant modification of all the parameters studied with 3DPW Ultrasound, Vascularization index (VI), Flow rate (FR), Flow vascularization index (FVI) (Table 3), and therefore a correlation was found between treatment with ulipristal acetate and a decrease in vascularization rates, VI (p=0,014); FR (p=0,001); FVI (p=0,001). In addition, this decrease observed in these parameters was not modified after the suspension of the treatment, findings we discussed later.
Discussion
The analysis of the data shown leads us to conclude that ulipristal acetate modifies the vascularization map of myomas reducing it significantly. This statement is corroborated by the analysis of the color map and confirmed by the measurement of myomas vascularization indices.
Being a prospective study, we can affirm that the effect of ulipristal acetate remains over time, not only in regard to the volume of the myoma but also in the vascularization presented by them. In summary we can affirm that the UPA causes a decrease in vascularization directly, which gives it a new route of action in the treatment of fibroids. The data of our study coincides with those of Czuczwar P [11] which already observed a decrease in vascularization parameters after three months of treatment. In our study, we can affirm that the decrease in vascularization not only occurs at the beginning of treatment but also occurs throughout the chronic intermittent cycle and that vascularization is not influenced by time without treatment. It should be taken in to account that due to a health alert, some patients were on a rest period for more than the two months indicated in the data sheet. This setback did not cause increases in ultrasound vascularization in any of the 24 cases studied. To our knowledge, this is the first study that analyzes the vascularization of myomas by 2D and 3D Doppler study after treatment with ulipristal acetate in chronic intermittent regime. Most of the literature papers analyze the effect of UPA [4,5,8] on vascularization after three months of use. However, we have been affected by a health alert which was resolved in a favourable manner. It has affected the follow-up of the patients and has caused the two-month pause periods to be delayed to 5 months before starting another cycle. During this period of pause, patients with ultrasound were monitored and no changes in vascularization were observed with respect to that of the previous cycle. This leads us to reaffirm that the effect of UPA on the vascularization of myomas measured over time does not regenerate rapidly. In the literature reviewed there is the possibility of biases when performing ultrasound investigations by different researchers [6,9,10]. In our case, this possibility was avoided since all measurements were made by the same MGM researcher with more than 15 years of experience. In the reviewed papers, the high VI and VFI rates correlate with high cellular activity while the lower levels correlate with ischemic necrosis (Minsart AF) [9]. The cases studied in this research have high levels of VI and VFI according to the literature reviewed that decrease significantly after the administration of UPA in an intermittent chronic manner. With the results obtained, we believe that the effect of UPA on myomas is double by blocking progesterone receptors and by decreasing vascularization by decreasing angiogenesis. In addition, the use of vascularization markers of 3DPW ultrasound and the color map allow us to monitor the response to medical treatment of myomas in a non-invasive and easily reproducible way. The determination of vascularization rates prior to medical treatment can determine the degree of response to it, which in the future will optimize the indications of medical treatment [12,13].
Conclusion
In view of the results of this preliminary study, we can conclude that ulipristal acetate, whose efficacy in treating the symptoms of uterine fibroids is well known, acts at the level of myoma angiogenesis, reducing its vascularization. These changes are cumulative and are maintained over time, even after treatment is completed. The 3DPW ultrasound, through different parameters, proves to be a safe, non-invasive and easily reproducible measurement instrument; to assess these changes in the vascularization of fibroids, and would allow us to monitor the effectiveness of treatment, response to it and compliance with therapy. These findings, despite the small sample size of our preliminary study, open a door to further studies on the role of ulipristal acetate in angiogenesis; as well as the role of 3DPW ultrasound in the assessment and monitoring of angiogenesis.
Declarations of interest
All authors have seen and approved the final version of the manuscript being submitted. They warrant that the article is the authors' original work, hasn't received prior publication and isn't under consideration for publication elsewhere.
Key message
We demonstrate the effect of ulipristal acetate on the decrease of myoma vascularization, measuring it with 3D Power Doppler Ultrasound through different parameters: VI, FR and FVI; and we monitor those changes over time with the same procedure.
Abbreviation
3DPWUS: 3D Power Doppler Ultrasound
UPA: Ulipristal Acetate
VI: Vascularization Index
FR: Flow Rate
FVI: Flow Vascularization Index
References
1. Gupta S, et al. 2008. Clinical Presentation of fibroids. Best Pract Res Clin Obstet Gynaecol. 22: 615-626. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/18372219
2. Stovall DW, et al. 2001. Clinical symptomatology of uterine leiomyomas. Clin Obstet Gynecol. 44: 364-371. Ref.: https://bit.ly/2STEEWD
3. Reshef Tal, James H Segars. 2014. The role of angiogenic factors in fibroid pathogenesis: potential implications for future therapy. Human Reproduction Update. 20: 194-216. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/24077979
4. Olufowobi O, Sharif K, Papaionnou S, et al. 2004 Are the anticipated benefits of myomectomy achieved in women of reproductive age? A five-year review of the results at a tertiary hospital. J. Obstet Gynaecol. 24: 434-440. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/15203587
5. Reshef Tal, James H Segars. 2014. The role of angiogenic factors in fibroid pathogenesis: potential implications for future therapy. Human Reproduction Update. 20: 194-216. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/24077979
6. Borahay MA, Al-Hendy A, Kilic GS, et al. 2015. Signaling Pathways in Leiomyoma: Understanding Pathobiology and Implications for therapy. Mol Med. 21: 242-256. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/25879625
7. Nieuwenhuis LL, Keizer AL, Stoelinga B, et al. 2017. Fibroid vascularization assessed with three-dimensional Power Doppler ultrasound is a predictor for uterine fibroid growth: a prospective study. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/28211610
8. Esmya® 5mg data sheet. 2012.Date of first autorization: 23th february.
9. Minah Kim, Hyeung Ju Park, Jae Won Seol, et al. 2013. VEGF-A regulated by progesterone governs uterine angiogénesis and vascular remodelling during pregnancy. EMBO Mol Med. 5: 1415-1430. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/23853117
10. Minsart AF, Ntoutoume Sima F, Vandenhoute K, et al. 2012. Does three-dimensional power Doppler ultrasound predict histopathological findings of uterine fibroids? A preliminary study. Ultrasound Obstet Gynecol. 2012. 40: 714-720. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/22581621
11. Ferrara KW, Merritt CR, Burns PN, et al. 2000. Evaluation of tumor angiogenesis with US: imaging, Doppler and contrast agents. Acad Radiol 7: 824. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/11048880
12. Czuczwar P, Wozniak S, Szokodziak P, et al. 2015. Influence of ulipristal acetate therapy compared with uterine artery embolization on fibroid volume and vascularity indices assessed by three-dimensional ultrasound: prospective observational study. 45: 744-750. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/25251811
13. Spanish Agency for Medicines and Healthcare Products AEMPS. ESMYA®: MONITOR LIVER FUNCTION AND DO NOT START NEW TREATMENTS AS PRECAUTIONARY MEASURES. Recommendations of the Committee for Risk Assessment in European Pharmacovigilance (PRAC). Publication date: 9 February 2018.




















