Indexing & Abstracting
Full Text
Review ArticleDOI Number : 10.36811/ojgor.2019.110003Article Views : 1997Article Downloads : 21
The under appreciated need for maternal and newborn immunization: call to action
Lawrence D FRENKEL
Clinical Professor, Departments of Pediatrics and Biological Sciences, University of Illinois, Somerset
*Corresponding author: Lawrence D. FRENKEL, M.D, Clinical Professor, Departments of Pediatrics and Biological Sciences, University of Illinois, 108 Stone Manor Drive; Somerset, NJ 08873, Tel: 732-271-4648; 908-616-8650; Email: lfrenkel@uic.edu
Article Information
Aritcle Type: Review Article
Citation: Lawrence D FRENKEL. 2019. The under appreciated need for maternal and newborn immunization: call to action. O J Gyencol Obset Res. 1: 13-18.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Lawrence D FRENKEL
Publication history:
Received date: 21 January, 2019Accepted date: 07 February, 2019
Published date: 08 February, 2019
Abstract:
In spite of clear evidence regarding the safety and efficacy of maternal and neonatal immunization, the accetance of maternal influenza and pertussis immunization and neonatal Hepatitis B vaccination has been disappointingly slow to reach optimal levels in the United States. Maternal influenza disease is associated with increased birth defects, premature delivery, and the delivery of small for gestational age infants. Influenza immunization has been recommended for all women who are or will be pregnant, regardless of trimester, during influenza season (October through May), but only about half receive them. Concerns about influenza immunization clearly overlook the solid data regarding the overall association between influenza immunization and decreased mortality and morbidity for both mothers and infants. Pertissis disease in infants under 2 months of age is associated with significantly increased morbidity and mortality. Pertussis incidence in the US has seen a significant increase in the past two decades in most age groups including that of parents and siblings. Recommended attempts to decrease infant pertussis infection include both maternal immunization and immunization of individuals who are expected to come in contact with these newborn infants (cocooning). Transmission of maternal Hepatitis B infection to their infants is associated with a significant incidence of chronic disease with subsequent cirrhosis and death. The administration of both Hepatitis B immune globulin and a “birth dose” of Hepatitis B vaccine have been demonstrated to be very safe and effective in preventing infant acquisition of Hepatitis B infection from infected mothers. A birth dose of Hepatitis B vaccine should be administered to every newborn.
Keywords: Maternal immunization; Neonatal immunization; Influenza; Pertussis, Hepatitis b; Maternal morbidity and mortality; Infant morbidity and mortality; Cocooning; Chronic hepatitis in infants; Birth dose of hepatitis b vaccine
Introduction
Safe and generally effective immunizations are the single major advance in medicine and public health world wide in the last 100 years, literally saving millions of lives. Maternal and newborn immunization has been disappointingly slow to reach optimal numbers in the United States. Maternal immunization elicits either a primary or animistic immune response in the maternal circulation, including the production of specific IgG antibodies against the immunizing agent. Transplacental transfer of IgG antibodies from mother to fetus takes place between 27 and 36 weeks gestation and provides protection for up to the first 6 months of the infant’s life. Vaccinations given to infants less than 6 months of age are not optimally effective because of their immature immune responses. The most consequential immunizations in this venue include those against influenza and pertussis. Newborn immunization does elicit a protective immune response against Hepatitis B even when this pathogen is transmitted to the infant, usually around the time of birth. The reasons for the suboptimal administration of these vital immunizations in pregnant women and newborns are not completely clear but certainly include lack of awareness on the part of both the pregnant woman and health care providers. Perhaps more vigorous efforts in both lay and professional education and a supportive perspective on the part of social and mass media would go far in improving this situation.
Influenza
Since 2004, Influenza immunization has been recommended for all women who are or will be pregnant, regardless of trimester, for each pregnancy, during influenza season (October through May) [1]. Estimates indicate that almost half of pregnant women in the United States do not receive recommended influenza immunization during pregnancy [2]. Reasons include concerns about: vaccine effectiveness, safety risk to the fetus and fear that the vaccination would give the mother the “flu”.
The concern about influenza vaccine efficacy, in general, highlights the unfortunate experience with vaccine strain mismatches [2]. Higher coverage rates are associated when providers both recommended and provided immunization [1,3]. The unexplained disparity in influenza immunization coverage between black and white pregnant women continues to be noted [2].
Concerns about influenza immunization clearly overlook the solid data regarding the overall association between influenza immunization and decreased mortality and morbidity for both mothers and infants [4,5,6] specifically with decreased influenza disease in immunized infants during their first 6 months of life [7]. Maternal influenza disease is associated with significantly increased mortality and hospitalizations, ICU admissions [8] and their infants suffer increased birth defects, premature delivery, and the delivery of small for gestational age newborns [9,10]. A large study of almost 250,000 immunized pregnant women and their infants reported a 70% reduction in laboratory confirmed influenza and an 81% reduction in hospitalizations [4]. Maternal influenza immunization has been demonstrated to be safe for their infants [6].
Pertussis
Pertissis disease in infants under 2 months of age has been associated with significantly increased morbidity and mortality [11,12,13]. Half of infants younger than 1 year of age who get pertussis require treatment in the hospital and an even higher percentage in infants less than 2 months of age do so. During the past decade, as many as 3,000 infants were hospitalized for pertussis each year in the United States. In spite of a highly immunized population, Pertussis incidence in the US has seen a significant increase in the past two decades [11]. This increase seems to have occurred since the substitution of the acellular vaccine for the whole cell vaccine. This is thought to be due to more rapid waning of immunity against pertussis with the less reactogenic acellular vaccine than with the whole cell vaccine. Especially impressive increases in disease incidence have occurred in adolescent and young adult populations [12]. Pertussis is highly contagious; studies have indicated that the source of infection for infants presenting with pertussis disease is not only their mother but also fathers and siblings and other relatives and caregivers [13,14]. Therefore, recommended attempts to decrease infant infection include both maternal immunization and immunization of those individuals who are expected to come in contact with these newborn infants (cocooning) including family members, healthcare personnel, and others.
The active transfer of maternal antibodies across the placenta from mother to infant mostly occurs between 27 and 36 weeks gestation. Since antibodies against Bordetella pertussis wane after a few years, decreasing to below protective levels, maternal immunization with Tdap vaccine is recommended for every pregnant woman with each pregnancy [15]. In spite of the recommendations regarding the efficacy and safety of this vaccine only 54.4% of women report receiving pertussis immunization during their pregnancy [2]. A recent report confirmed the higher pertussis toxin antibodies resulting from third trimester maternal immunization [16] and a report from England documented its effectiveness in preventing disease [17]. A small case control study from England and Wales reported 93% disease prevention effectiveness with these maternal pertussis vaccination recommendations [18]. Data from England on over 20,000 pregnancies failed to demonstrate any significant safety risks associated with maternal pertussis immunization [19]. Finally, a large US study demonstrated the safety of maternal immunization in infants [6]. One possible problem potentially associated with maternal immunization is the subsequent suppression of infant antibody responses [20]. The implications of this issue remain unresolved.
Hepatitis B
Each year approximately 1000 infants are born in the US with Hepatitis B (HB) infection and 90% of these infants will develop chronic hepatitis. This is in contrast to 30% in 1 to 5 year old children and <5% in individuals older than 5 years who go on to develop chronic HB infection after being infected after the neonatal period. One quarter of these chronically infected infants will eventually die of cirrhosis or hepatocellular carcinoma [21]. HB serology should be performed on every pregnant woman and the results be made available to her caregiver and to the institution in which she deliveries. Some success has been achieved, with over 84% of women now being tested for HB seropositivity during pregnancy and almost 95% of infants born to infected women receive the recommended prophylaxis [22].
The ACIP and the AAP recommend immunization of infants, born to mothers who are HB surface antigen positive (actively carrying the virus), with a dose of HB vaccine, regardless of weight, within 12 hours of birth (“birth dose”) and a dose of Hepatitis B specific hyper immune globulin (HBIG) (see scenario A on Figure). Receipt of the birth dose of HB vaccine, even without HBIG, reduces the incidence of infantile acquisition of HB virus by 95% [21]. Newborns, born to seronegative mothers (see scenario B on Figure) and who weigh more than 2000 grams should receive the birth dose of HB vaccine within 24 hours of birth. The infants born to seronegative mothers and who weigh less than 2000 grams should receive their first dose of HB vaccine at one month of age or at the time of hospital discharge {whichever comes first}. Newborns whose maternal HB status is unknown (see scenario C), should receive the birth dose of HB vaccine within 12 hours of birth. If their birth weight is 2000 grams or more, they should receive HBIG within 7 days of birth or at hospital discharge {which ever comes first}. Infants who weigh less than 2000 grams should receive HBIG, within12 hours of birth.
Figure 1: HB Immunization of newborn infants [21].
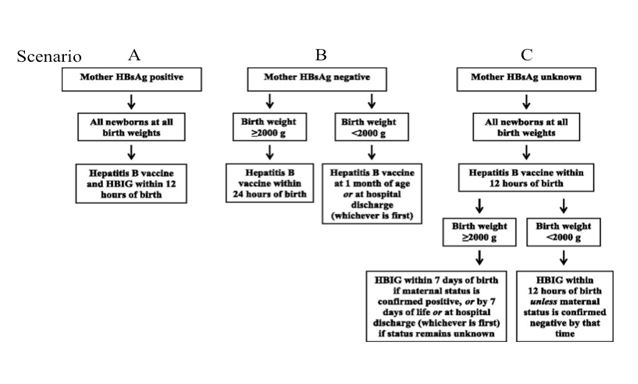
This protocol allows all infants, both HB virus exposed or not, to receive appropriate, safe, and effective preventive treatment for this serious disease. Underweight infants should subsequently receive the full course of HB immunizations because their immune systems may not have been mature enough to provide optimal responses to the first dose of HB vaccine given in the hospital. All infants born to HB seropositive mothers (who hopefully have received preventive intervention with HBIG and/or HB vaccine) should be screened for antibody to HB surface antigen and for HB surface antigen itself, at least one month after receiving HBIG and again one month after receiving the last HBV dose in the series. This will generally occur at 9 to 19 months of age, and generally occurs after hospital discharge. Note that the usual schedule for HB vaccine for normal healthy, HB unexposed infants is: the first dose at birth, the second dose is at 1 to 2 months of age, and the third between 6 and 18 months of age. Chronic HB infection is generally asymptomatic although slow liver cell destruction and fibrosis is ongoing. It is estimated that two-thirds of individuals with chronic HB are unaware of it and this includes as many as 2 million individuals in the US. Since a variety of errors can result in the report of false negative maternal HB status, the birth dose of HB vaccine given within 24 hours of birth is strongly recommended for every newborn. Completion of the HB immunization series reduces the transmission of HB from mother to her infant to less than 1% [21]. In addition, it prevents transmission from other infected family members, caregivers, and rare but potential iatrogenic medical transmission.
The presence of antibody to HB surface antigen reflects protection from this virus. The presence of HB surface antigen suggests persistent infection and requires further evaluation. Testing of HB seropositive mothers also allows for maternal antiviral therapy during pregnancy [22]. Currently the rate of receipt of the birth dose of HB vaccine in the US is clearly suboptimal! A coordinated dedication of hospitals and involved physicians is required to meet the goal of elimination of neonatal hepatitis B. Practitioners who care for pregnant women have an obligation to help educate the pregnant woman and those around her about HB infection, it’s diagnosis, treatment, and preventive immunization.
Conclusion
Health care providers have an obligation to help educate the public about important life threatening infections and their prevention with broadly available, safe, and effective recommended vaccines. A targeted and multipronged approach has been shown to be effective in increasing birth dose Hepatitis B immunization rates as well as maternal influenza and pertussis immunization but much more needs to be done to reduce neonatal morbidity and mortality from these infections. Practitioners who care for pregnant women, especially obstetricians, should provide leadership in lay and professional education and enforcement of hospital policies in this arena.
References
- Ding H, Black CL, Ball S, et al. 2017. Influenza vaccination coverage among pregnant women-United States, 2016–17 Influenza Season MMWR Morb Mortal Wkly Rep. 66: 1016-1022. [Ref.]
- Kahn KE, Black C, Ding H, et al. 2018. Influenza and Tdap vaccination coverage among pregnant women-United States, April 2018 MMWR Morb Mortal Wkly Rep. 67: 1055-1059. [Ref.]
- Grohskopf LA, Sokolow LZ, Broder KR, et al. 2016. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices United States, 2016-17 influenza season. MMWR Recomm Rep. 65: 1-54. [Ref.]
- Shakib JH, KorgenskiK, Presson AP, et al. 2016. Influenza in infants born to women vaccinated during pregnancy. Pediatrics. 137.[Ref.]
- Fell DB, Sprague AE, Liu N, et al. 2012. H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. Am J Public Health. 102: 33-40. [Ref.]
- Sukumaran L, McCarthyNL, Kharbanda EO, et al. 2018. Infant hospitalization and mortality after maternal vaccination. Pediatrics. 141. [Ref.]
- Zaman K, Roy E, Arifeen SE, et al. 2008. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 359: 1555-1564. [Ref.]
- Siston AM, Rasmussan SA, Honein MA, et al. 2010. Pandemic 2009 influenza A (H1N1) virus illness among pregnant women in the United States. 303: 1517-1525. [Ref.]
- Luteijn JM, Brown MJ, Dolk H. 2014. Influenza and congenital anomalies: a systematic review and meta-analysis. Birth Defects Human Reprod. 29: 809-23. [Ref.]
- Omer SB, Goodman D, Steinhoff MC, et al. 2011. Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: a retrospective cohort study. PLoS Med. 8. [Ref.]
- Pertussis-United States, 1997-2000. 2002. MMWR Morbid Mortal Wkly Rep. 51: 73-26. [Ref.]
- Clark TA. 2014. Changing pertussis epidemiology: everything old is new again. Clin Infect Dis. 209: 978-981. [Ref.]
- Masseria C, Martin CK, Krishnanajah G, et al. 2017. Incidence and burden of pertussis among infants less than 1 year of age. Pediatr Infect Dis J. 36: 54-61. [Ref.]
- Skoff TH, Kenyon C, Cocoros N, et al. 2015. Sources of Infant pertussis infection in the United States. Pediatrics. 136: 635-641. [Ref.]
- Updated recommendations for use of tetanus toxoid, reduced diphtheria, and acellular pertussis vaccine (Tdap)in pregnant women-Adviaory Committee on Immunization Practices (ACIP), 2012. MMWR Morbid Mortal Wkly Rep. 2013; 62: 131-135. [Ref.]
- Healy CM, Rench MA, Swaim LS, et al. 2018. Association between third trimester Tdap immunization and neonatal pertussis antibody concentration. 320. [Ref.]
- Amirthalingam G, Andrews N, Campbell H, et al. 2014. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 384: 521-1527. [Ref.]
- Dabrera G, Aminthalingam G, Andrews N. et al. 2015. A case controlled study to estimate the effectiveness of maternal pertussis vaccination in preventing newborn infants in England and Wales, 2012-2013. Clin Infect Dis. 60: 333-337. [Ref.]
- Donegan K, King B, Bryan P. 2014. Safety of pertussis vaccination in pregnant women in UK: observational study. BMJ. 349:4219. [Ref.]
- Edwards KM. 2018. Protecting infants from pertussis disease. J Am MedAssoc Pediatr. 172: 1012-1013. [Ref.]
- AAP Committee on Infectious Diseases. AAP Committee on Fetus and Newborn. Elimination of perinatal Hepatitis B: providing the first vaccine dose within 24 hours of birth. Pediatrics. 2017; 140. [Ref.]
- Schally H, Velez C, Rheingold A, et al. 2018. Prevention of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recom Rep. 67: 1-31. [Ref.]




















