Indexing & Abstracting
Full Text
Case ReportDOI Number : 10.36811/ojgor.2019.110002Article Views : 4599Article Downloads : 39
Demons meigs’ syndrome secondary to begnin brenner tumor with high ca125 plasmatic level: first case described in kara teaching hospital
Aboubakari AS1, Dossouvi T2, Logbo-Akey KE1, Ajavon DR1, Dagbé M4, Darré T5* and Akpadza K4
1Department of Gynecology, Kara Teaching hospital, Togo
2Department of General surgery, Kara Teaching hospital, Togo
3Department of Radiology, Kara Teaching hospital, Togo
4Department of Gynecology, Lomé Teaching hospital, Togo
5Department of Pathology, Lomé Teaching hospital, Togo
*Corresponding author: Dr. Tchin DARRE, University of Lomé. BP 1515, Lomé, Togo, Email: paolodarre@yahoo.fr
Article Information
Aritcle Type: Case Report
Citation: Aboubakari AS, Dossouvi T, Logbo-Akey KE, et al. 2019. Demons meigs’ syndrome secondary to begnin brenner tumor with high ca125 plasmatic level: first case described in kara teaching hospital. O J Gyencol Obset Res. 1: 08-12.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Aboubakari AS
Publication history:
Received date: 26 December, 2018Accepted date: 09 January, 2019
Published date: 10 January, 2019
Abstract:
To report the first case of Demons-Meigs’ syndrome secondary to benign Brenner tumor with high CA125 plasmatic level managed in kara teaching hospital. A 40-year-old female patient was admitted with a 6-month history of abdominal distension. Clinical examination found abdomino-pelvic mass and declive dullness. Abdominal ultrasound found heterogeneous abdomino-pelvic mass of 180 mm in length and a large free ascites. Pelvic computorized tomography scan found heterogeneous extra-uterine mass of 180 mm in length, large ascites without pelvic or lombo-aortic lymphadenopathy or tumoral extension. Front thorax X-ray found bilateral pleural effusion; CA125 plasmatic level was 1138 IU/ml. Median laparotomy allow to aspire 2 liters of ascitic fluid and to perform left adnexectomy. Histological examination of surgery sample diagnosed begnin ovary Brenner tumor. Outcome after surgery was without complications with disappearance of ascites and pleural effusion, and CA125 plasmatic level back to normal value. Demons-Meigs’ syndrome is a rare benign ovarian tumor whose symptoms looks like ovarian cancer. Its fundamental characteristic is the disappearance of symptoms after ovarian tumor removal.
Keywords: Brenner tumor; Ascites; Pleural effusion; CA125; Teaching hospital of kara
Introduction
Demons-Meigs’ syndrome is a clinical trial including benign ovarian tumor, ascites and pleural effusion that both resolved after ovarian tumor removal [1,2]. Usually, cases of Demons-Meigs’ syndrome include fibrothecoma tumors, ascites and pleural effusion [3]. Increase in carbohydrate antigen 125 (CA125) in Demons-Meigs’ syndrome is rare [4]. Demons-Meigs’ syndrome secondary to benign Brenner tumor with increased CA 125 plasmatic level is also still rare [5]. Authors report the first case of Demons-Meigs’ syndrome secondary to benign Brenner tumor with increased CA125 plasmatic level managed at Kara teaching hospital in the north of Togo.
Case Report
A 40-year-old female patient, mother of 2 alive children, consulted in the gynecology department for progressive abdominal distension and pelvic heaviness since 6 months. There was no medical history of chronic disease or surgical operation. There was no weight decrease. Conjunctiva were colored, blood pressure at 120/80 mmHg. Her height was 177 cm and her weight 79 kg. Heart rate was regular at 72 beats by minute. Respiratory rate was 18 cycles by minute. There was neither lower limbs oedema nor dyspnea.
Physical digestive examination found bloating and reluctant abdomen, no hepatomegaly nor splenomegaly, and an abdomino-pelvic mass that was firm, regular, mobile, painless, with declive dullness with positive signs of fluid wave and ascites ice. At gynecological examination vulva and cervix were without findings. Uterus was without findings, separated from the abdomino-pelvic mass. Pulmonary examination found dullness on percussion, no vesicular sound of lung basis.
According to this clinical presentation, an ovarian tumor was suspected, leading to perform abdomino-pelvic ultrasound and plasma dosage of CA125. Abdomino-pelvic ultrasound found heterogeneous abdomino-pelvic mass both cystic and tissue of 180 mm length, large free ascites, and normal aspect of uterus, liver, spleen, kidneys and pancreas. CA125 plasmatic level was 1138 IU/mL (normal value inferior to 30 IU/mL).
Abdomino-pelvic tumor associated to ascites and CA125 high plasmatic level led to suspect malignant ovarian tumor, and thus to perform extension work-up with front thorax X-ray, and abdomino-pelvic computorized tomogaphy (CT) scan. Thorax X-ray found small bilateral pleural effusion (figure 1). CT scan found left heterogeneous extra uterine tumor of 180 mm length associated to large free ascites without peritoneal carcinosis or pelvic/lombo-aortic lymphadenopathy (figure 2).
Exploratory laparotomy under general anaesthesia by median incision was performed. There was left ovary tumor and a large ascites. Peritoneal area and other organs (right ovary, uterus, bladder, rectum, liver, omentum, mesentery, intestinal loop) aspect was normal. There were no adhesion, pelvic or lombo-aortic lymphadenopathy palpable. Two liters of colorless fluid (no sampling for peritoneal cytology) were aspired, a left adnexectomy was performed, and abdominal cavity was rinsed and aspired before closing, without drainage. Adnexectomy sample was sent to anatomopathology laboratory and the diagnosis was benign Brenner tumor. Outcome after surgery was without complications and the patient was discharged at day 5 after surgery. At the follow-up after 3 months, there was no recurrence of ascites, bilateral pleural effusion recovered and CA125 plasmatic level was normal. At the follow-up after 6 months, the patient was doing well and there was no recurrence.
Figure 1: Front thorax X-Ray showing bilateral pleural effusion.
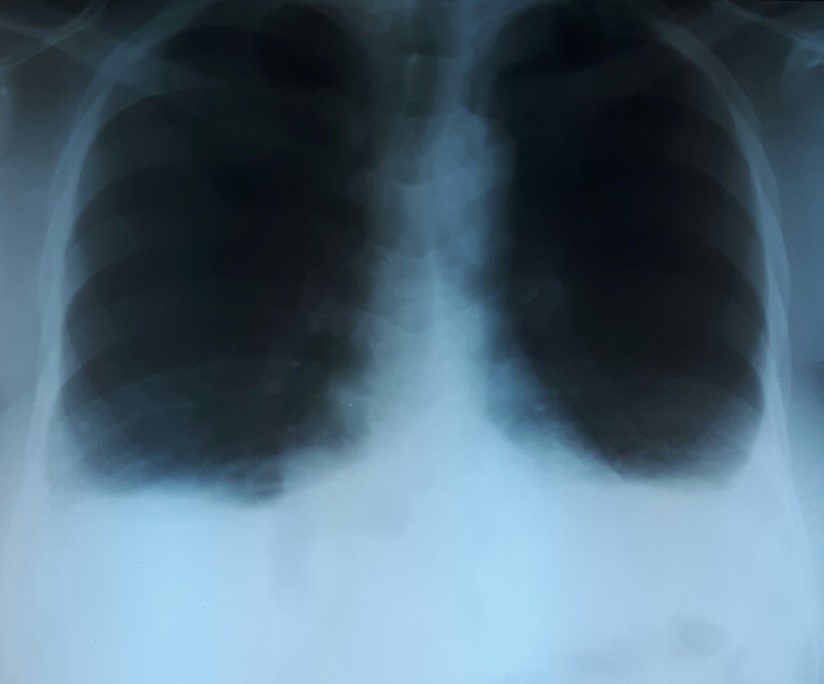
Figure 2: CT scan showing abdomino-pelvic tumor and ascites.

Discussion
Demons Meigs’ syndrome, for which epidemioloy is not very well known, would represent 1% of ovarain tumors [6]. It may happen at any age, but is more frequent after menopause [7,8]. There is no clinical pathognomonic sign of the Demons-Meigs’ syndrome. Usual clinical presentation includes benign ovarian tumor, ascites and pleural effusion [3]. Circunstances of diagnosis are multiple and variable [9]. Usual clinical presentation is an increase in abdominal volume, pelvic heaviness, dyspnoea and lower limbs oedema [10]. Dysnoea was missing in our patient, but this can be explained by a small pleural effusion. The increase in abdominal volume is dependent of the tumor and ascites volume. Ovarian tumor, often firm, may be uni or bilateral [11]. However, the localization is mainly at the left side, like in our patient [12]. Abdominopelvic ultrasound is the first lign medical imaging examination and allows characterizing ovarian tumor, identifying sign of malignity and estimating the size of ascites [11]. The pathophysiology of ascites remains unclear [10]. There are several hypothesis that may explain its onset. According to Meigs, ascites is the consequence of tumor transudation that overlaps the capacity of resorption of peritoneum. Ascites production is explained by an increased pressure in intratumor lymphatics. Onset, more than amount, of ascites seems correlated to the size of benign ovarian tumor and the presence of myxoid composition [13]. The pathophysiology of pleural effusion is not so well known [10]. Pleural effusion is secondary to ascites. Its pathophysiology is not well resolved. According to Meigs, pleural effusion is the result of passage of ascitic fluid to the pleural space through diaphragmatic lymph vessels which are more developed on the right side than in the left side [13].
Pleural effusion is often unilateral and on the right size (75% of cases) [11]. Front thorax X-ray led to diagnosis of pleural effusion. Bilateral pleural effusion, like observed in our patient is unusual. Demons-Meigs’ syndrome clinical triad led to strongly suspect ovarian cancer [10]. Thoraco-abdomino-pelvic scan is performed to find local and distant tumor extension [11]. In the picture of Demons-Meigs’ syndrome, CT scan does not provide information about malignity of the ovarian tumor. Demons-Meigs’s syndrome may be associated with increase in CA125 plasmatic level, like it was observed in our patient [14]. Increase in CA125 plasmatic level in benign diseases is not a marker for screening or diagnosis, but is used as marker of post-surgery follow-up [11]. Its pathophysiology is still unclear [10]. It may be due to peritoneal surface irritation secondary to intra-peritoneal irritation [15]. It seems that the increase in CA125 plasmatic level is linked to the size of ascites [16]. CA125 is a marker of coelomic epithelium and peritoneal lesions. It may be increase in many diseases without being a sign of malignity. It is useful in post-surgery monitoring; its normalization is the sign of complete recovery [17-18].
Clinical diagnosis of Demons-Meigs’ syndrome is difficult. Diagnosis confirmation is based on histology. It requires histological analysis of surgery sample. Surgery includes median laparatomy with total hysterectomy and bilateral adnexectomy, often after menopause [19]. Given the wish of conception of the patient, only a left adnexectomy was performed in our case. Typical Demons-Meigs’ syndrome is secondary to fibroma or thecoma [3]. Benign granulosa and Brenner tumors were lately added to the criteria of diagnosis [20]. The Demons-Meigs’ syndrome, further to clinical triad and benign aspect of the ovarian tumor, is characterized by post-surgery outcome. Symptoms should improve and then completely recover after removal of ovarian tumor [3]. Indeed, ascites and pleural effusion completely recovered along with the normalization of CA125 plasmatic level. However, with the fear of recurrence, post-surgery follow-up is maintained [18]. Six months after surgery of ovarian tumor, the patient was asymptomatic and healthy [20].
Conclusion
Ovarian tumor, ascites and pleural effusion triad is suspected to be ovarian cancer until proof of the contrary. Increase in CA125 plasmatic level is a further diagnosis argument. This sign is observed in Demons-Meigs’ syndrome, a disease of good prognosis with a good management. Only histological diagnosis of benignity of ovarian tumor and recovery of associated symptoms after surgery confirmed the diagnosis of Demons-Meigs’ syndrome.
Declarations
Ethics approval and consent to participate
This case report was approved by the Department of Pathology of CHU of Lomé, University of Lomé.
Consent to publish
A copy of the written consent is available for review by the editor of this journal.
References
- Lurie S. 2000. Meigs´ syndrome: the history of the eponym. Eur J Obstet Gynecol Reprod Biol. 92: 199-204.[Ref.]
- Griffin JP. 1996. Dame Mary Page -the first recorded case of Meigs' syndrome? J R Coll Physicians Lond. 30: 465.[Ref.]
- Brun JL. 2007. Demons syndrome revisited: a review of the literature. Gynecol Oncol. 105: 796-800.[Ref.]
- Korkolis DP, Koulaxouzidis GV, Apostolikas N, et al. 2004. Ovarian fibrothecoma associated with Meigs' syndrome and elevated serum CA 125. J BUON. 9: 473-475.[Ref.]
- Buttin BM, Cohn DE, Herzog TJ. 2001. Meigs’ syndrome with an elevated CA125 from benign Brenner tumors. Obstet Gynecol. 98: 980-982.[Ref.]
- Sumi Saha, Meiri Robertson. 2012. Meigs' and Pseudo-Meigs' syndrome. Australas J Ultrasound Med. 15: 29-31.[Ref.]
- Jiang W, Lu X, Zhu ZL, et al. 2010. Struma ovarii associated with pseudo-Meigs’ syndrome and elevated serum CA 125: a case report and review of the literature. J Ovarian Research. 3: 1-5.[Ref.]
- Shiau CS, Chang MY, Hsieh CC, et al. 2005. Meigs’ Syndrome in a Young Woman with a Normal Serum CA-125 Level. Chang Gung Med J. 28: 587-591.[Ref.]
- Mui MP, Tam KF, F Tam FK, et al. 2009. Coexistence of struma ovarii withmarked ascites and elevated CA-125 levels: case report and literature review. Archives Gynecol Obstetric. 279: 753-757.[Ref.]
- Kim EK, Harold MPP. 2018. Hydrothorax, ascite and abdominal mass: not alzays signs of a malignancy-Three cases of Meigs’ syndrome. Radiology Case. 12: 17-26.[Ref.]
- Loué VA, Gbary E, Koui S, et al. 2013. Bilateral Ovarian Fibrothecoma Associated with Ascites, Bilateral Pleural Effusion, and Marked Elevated Serum CA-125. Case Rep Obstet Gynecol. 2013: 189072.[Ref.]
- Roth LM, Talerman A. 2007. The enigma of struma ovarii. Pathology. 39: 139-146.[Ref.]
- Meigs JV. 1954. Fibroma of the ovary with ascite and hydrothorax Meigs' syndrome. Am J Obstet Gynecol. 67: 962-987.[Ref.]
- Jones OW III, Surwit EA. 1989. Meigs’ syndrome and elevated CA125. Obstet Gynecol. 73: 520-521. [Ref.]
- Gerstenmaier JF, Gibson RN. 2014. Ultrasound in chronic liver disease. Insights Imaging, 5: 441-455.[Ref.]
- Liou JH, Su TC, Hsu JC. 2011. Meigs' syndrome with elevated serum cancer antigen 125 levels in a case of ovarian sclerosing stromal tumor. Taiwanese J Obstet Gynecol. 50: 196-200.[Ref.]
- Bergmann JF, Bidart JM, George M, et al. 1987. Elevation of CA125 in patients with benign and malignant ascites. Cancer. 59: 213-217.[Ref.]
- Darré T, Aboubakari AS, N'Bortche BK, et al. 2017. Primary ovarian angiosarcoma in a 12- year -old girl: a case report of an exceptional localization in a context of limited resources country. BMC Clinical Pathology. 17: 16.[Ref.]
- Buamah PK, Skillen AW. 1994. Serum CA125 concentrations in patients with benign ovarian tumours. J Surg Oncol. 56: 71-74.[Ref.]
- Cisse CT, Ngom PM, Sangare M, et al. 2004. Ovarian fibroma associated with Demons-Meigs syndrome and elevated CA 125. J Gynecol Obstet Biol Reprod. 33: 251-254.[Ref.]




















