Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ojdoh.2019.110003Article Views : 969Article Downloads : 28
Feasibility Controlling Hemoglobin A1c by Oral Hygiene Improvement: A Pilot Study
Ayako Okada1,2, Yoshiaki Nomura1*, Mayu Miyanohara1, Masahide Uraguchi1,3, Hisanori Tadokoro4, Tetsuya Nagai5, Yoshihito Fujii6, Masahiro Miura7, Ryo Kawachi8, Takanori Matsui3, Kunio Takayanagi3, Masashi Yamamoto3, Takatsugu Yamamoto2and Nobuhiro Hanada1
1Department of Translational Research, Tsurumi University School of Dental Medicine, Yokohama, Japan
2Department of Operative Dentistry, Tsurumi University School of Dental Medicine, Yokohama, Japan
3Medical Group Seiwa, Tokyo, Japan
4Kyodo Dental Clinic, Tokyo, Japan
5Nagayama Center Dental Clinic, Tokyo, Japan
6Station Dental Clinic, Sagamihara, Japan
7Seiwa Dental Clinic, Tokyo, Japan
8Machida-Kitaguchi Dental Clinic, Tokyo, Japan
*Corresponding author: Yoshiaki Nomura, Department of Translational Research, Tsurumi University School of Dental Medicine, 2-1-3 Tsurumi, Tsurumi-ku, Yokohama 230-8501, Japan, Tel: (+81)45-580-8462; Fax: (+81)45-573-2473; Email: nomura-y@tsurumi-u.ac.jp
Article Information
Aritcle Type: Research Article
Citation: Ayako Okada, Yoshiaki Nomura, Mayu Miyanohara, et al. 2019. Feasibility Controlling Hemoglobin A1c by Oral Hygiene Improvement: A Pilot Study. Open J Dent Oral Health. 1: 08-15.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Ayako Okada
Publication history:
Received date: 14 April, 2019Accepted date: 02 May, 2019
Published date: 04 May, 2019
Abstract
Purpose: Patients with diabetes mellitus are prone to be bacteremia. The major side effect of diabetes mellitus is caused by bacteremia. However, conventional periodontal treatment does not focus mainly on the elimination of pathogenic bacteria. Therefore, the aim of this study was to the management of oral bacteria improve some symptoms of diabetes mellitus.
Material and Method: We monitored Hemoglobin A1c (HbA1c) of five subjects after removed oral bacteria on the tooth surface by antibiotics using custom made tray. By using the results, simulation model was constructed.
Result: By the simulation, HbA1c levels declined over time and decreased to 6.0%. However, it took at least 600 days to come up to 6.0%. The higher the level of HbA1c at baseline, the more time takes to improve HbA1c level. Subjects with 7.3% HbA1c at baseline required 2,400 days to achieve 6.0% HbA1c.
Conclusion: The results of this study suggest that HbA1c may be improved by oral bacterial control. Clinical studies suggest that a long observational period is required to obtain high quality evidence.?
Keywords: Oral hygiene; Hemoglobin A1c; Mixed effect model
Introduction
The relationship between diabetes mellitus and periodontal disease has been intensively investigated [1-5]. These two diseases are cross-correlated. Evidence that diabetes is associated with an increased risk of periodontitis has been demonstrated by many epidemiological studies [6-8]. In contrast, efficacy of treatment of periodontitis for glycemic control of patients with diabetes mellitus is still elusive [9,10]. Systematic reviews and meta-analyses of the effect of periodontal treatment on glycemic control results in the consistent finding that periodontal treatment has been associated with a decrease of about 0.4% in Hemoglobin A1c (HbA1c) [11-17]. One of the review papers conducted by Cochrane Database of Systematic Review concluded that glycemic control improves in HbA1c with diabetes mellitus by the treatment of periodontal disease; with a mean percentage reduction rate of HbA1c is 0.29% after 3 to 4 months. The contents of periodontal treatment were scaling and root planning. However, quality evidence was low. In addition, this review paper concluded that evidence to prove that observational period for the improvement and maintenance of HbA1c after 4 months is insufficient [13]. In another systematic review concluded that auxiliary use of systemic antibiotics does not result in a statistically significant benefit in terms of HbA1c improvement in periodontal treatment of patients with diabetes mellitus [18]. However, most of the observational periods of the study used for the review was insufficient. The majority of the observation period of the study used for the review was three months. Diabetes mellitus is a chronic disease and duration of this disease is long term.
There are several reports that diabetes mellitus affected on oral microbiome composition. The impact diabetes mellitus on the oral microbiome composition has been studied. As a consensus report from the European Federation of Periodontology and the American Academy of Periodontology, concluded that diabetes has significant impact on the oral microbiome composition [19]. However, one report suggested that Porphyromonas gingivalis and Tannerella forsythia increased in patients with diabetes mellitus [20].
Oral bacteria invade into blood vessels via destroyed periodontal tissue. This phenomenon is called dental bacteremia and this often occurs in healthy subjects [21-23]. The major side effect of diabetes mellitus is caused by bacteremia. Patients with diabetes mellitus are prone to be bacteremia [24]. Inhibiting bacteremia can improve some symptoms of bacteremia. If dental bacteremia can be suppressed by oral hygiene improvement including periodontal treatment, some symptoms diabetes mellitus may be improved.
However, conventional periodontal treatment does not focus mainly on the elimination of pathogenic bacteria. Therefore, management of oral bacteria may improve some symptoms of diabetes mellitus. In this pilot study, we monitored HbA1c after controlled oral bacteria by antibiotics applicate only on the tooth surface by an individual tray.
Materials and Methods
Subjects
Male and four subjects with prediabetes participated in this study. Subjects were not appropriately controlled by the treatment and health instruction. Their demographic data such as gender, age, number of remaining teeth were Case 1: Female, 72 years old, 20 tooth, Case 2: Male, 68 years old, 18 tooth, Case 3: Male, 54 years old, 24 tooth, and Case 4: Male, 58 years old, 19 tooth. Inclusion criteria was as follows: without wearing removable dentures, without periodontal disease symptoms and diabetes mellitus status was diagnosed by physician as prediabetes according to the Guideline of the committee of the Japan diabetes society on the diagnostic criteria of diabetes mellitus [25].
Oral hygiene control
Dental plaque was removed by professional mechanical tooth cleaning (PMTC). After PMTC, gel containing cetylpyridinium chloride were applied by individually fabricated try for 5 minutes as professional care. The subjects were instructed to control dental plaque by drug application using individual tray after tooth brush every day as a home care [26-29].
HbA1c Measurements
Blood level HbA1c was measures by ADAMUS A1c HA-8182 (ARKREY, Osaka, Japan).
Statistical analysis
As HbA1c levels were tested in each anticipant and measured repeatedly. We applied multilevel analysis [30]. The model used in this study were specified as follows:
Data Structure: subject
Probability distribution: γ
Link function: Log
In what follows, fixed effects (resp. random effects or error terms) are denoted by Greek letters (resp. Alphabet)

Covariance Type: AR1
These analyses were carried out by IBM SPSS Statistics ver 24.0 (IBM, Tokyo, Japan). The simulation was carried out by IBM SPSS Modeler ver 18.0 (IBM, Tokyo, Japan).
Ethics
This study was approved by the Ethical committee of Tsurumi University School of Dental Medicine (Approval Number: 1304).
Results
Figure 1 shows the transition of HbA1c levels after oral hygiene control of four subjects. HbA1c levels fluctuated, however, the data seemed to be improved. Using these data, the mixed effect model specified by Method was constructed. The constructed model was shown in Table 1. Baseline HbA1c level was an important predictor of changes in HbA1c levels following oral hygiene treatment. Figure 2 shows a scatter plot of predicted values of HbA1c levels against observed HbA1c levels. Linearity was observed for 6.0 - 7.5% of HbA1c at baseline. Finally, we simulated the transition of HbA1c after oral hygiene treatment. Using the simulation data, predicted values were calculated by the mixed effect model shown in Table 1. The response surface is illustrated in Figure 3. HbA1c levels declined over time and decreased to 6.0%. However, it takes at least 600 days to come up to 6.0%. The higher the level of HbA1c at baseline, the more time takes to improve HbA1c level. Subjects with 7.3% HbA1c at baseline required 2,400 days to achieve 6.0% HbA1c by simulation.
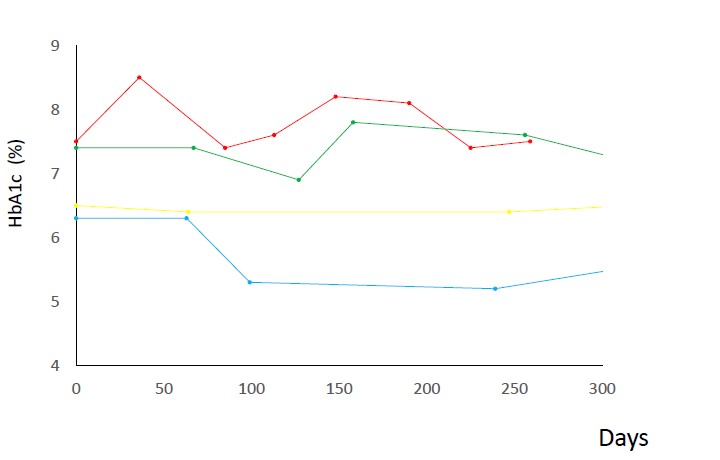
Figure 1: Transition of HbA1c levels after oral hygiene treatment. HbA1c level was improved for the two subjects with more than 6.5% HbA1c level at baseline.

Figure 2: Scatter plot of predictive values against observed values of HbA1c. Predictive values of HbA1c was calculated by the mixed effect model shown in Table1.
| Table 1: Mixed effect model to predict the HbA1c after oral hygiene treatment. | ||||
|---|---|---|---|---|
| Coefficient | 95%CI | P-value | ||
| HbA1c at Baseline | 0.187 | 0.124-0.249 | <0.001 | |
| Time | <0.001 | <0.001 - <0.001 | 0.423 | |
| Intercept | 0.635 | 0.195-1.075 | 0.006 | |
Coefficient of HbA1c level at baseline was statistically significant. Details of model specification was described in Method.
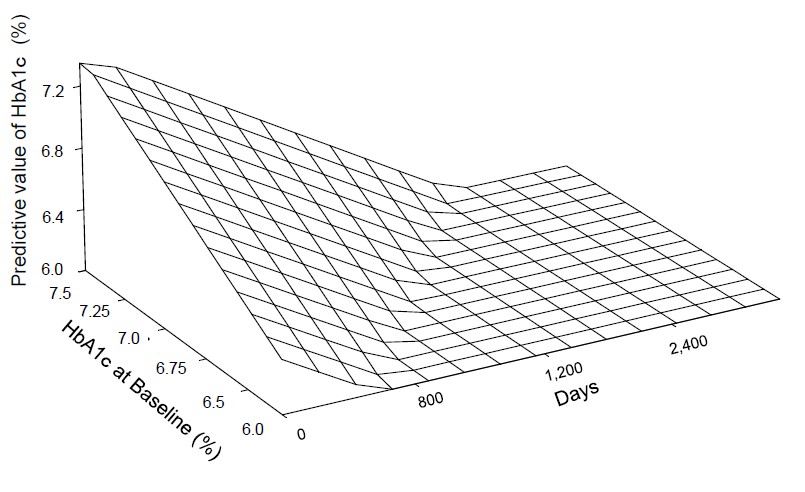
Figure 3: The response surface of the simulation of transition of HbA1c. HbA1c levels were decreased with the time passes and it come up to 6.0%. However, it takes at least 600 days. The higher levels of HbA1c at baseline, the more time is necessary to improve HbA1c levels. Subjects with 7.3% HbA1c at baseline, 2400 days were necessary to attain 6.0% HbA1c by the simulation.
Discussion and Conclusion
In this study, the statistical model and its simulation clearly showed that HbA1c was improved by controlling oral bacteria. First, we established a methodology of selective elimination mutans streptococci from the oral cavity [28]. By the fabrication of individual tray, antibiotics can selectively deliver to the tooth surface, without oral mucosal microbiome. We called this drug delivery system as dental drug delivery system abbreviated as 3DS [25-28]. Biofilm that contains pathogenic oral bacteria selectively formed on the tooth surface as a biofilm. Controlling the biofilm on the tooth surface is the key element of oral hygiene.
This methodology is applicable to periodontal pathogens. To improve the periodontal conditions and by the elimination of periodontal pathogens, dental bacteremia may be suppressed [30,31]. Dental bacteremia causes the weak but contentious damage to blood vessels. We are currently studying the effect of this method to control oral bacteremia to obtain healthy blood vessels. The relationship between periodontal disease and systemic health has been investigated. One of the important elements of this relationship is dental bacteremia. Several studies have shown that endothelial dysfunction was improved by controlling oral bacteria [32-34].
The molecular mechanisms of diabetes mellitus by dental bacteria is still inconclusive. When oral bacteria form patients with diabetes mellitus transferred to normal germ-free mice, osteoclast genesis and periodontal bone loss were induced [35]. Diabetes mellitus increases IL-17 expression, leading to altered virulence of oral microorganisms and increased periodontal inflammation [36]. However, oral pathogens directly affect diabetes mellitus. Further studies are needed to reveal the effects of oral pathogens on diabetes mellitus. The results of simulation in this study showed that oral bacterial control resulted in improved HbA1c levels. However, it may take at least 600 days and the subjects with 7.3% of HbA1c at baseline required 2,400 days to recover to 6.0% HbA1c.
Even through Cochrane Database of Systematic Review concluded that treatment of periodontal disease by scaling and root plaining does improve glycemic control in patients with diabetes, with a mean percentage reduction in HbA1c of 0.29% at 3 - 4 months [12]. The average decline rate of HbA1c it is 0.29% in 3 - 4 months and quality of this evidence is low. The papers reviewed in this systematic review, the observational period for the five studies was 6 months [37] and the observation period of the other studies was 3 to 4 months. Evidence suggested by clinical studies was low due to the short observational period.
Another systematic review showed that combination therapy of conventional therapy and systemic antibiotics did not produce statistically significant benefits in terms of HbA1c improvement in periodontal treatment of patients with diabetes [13]. The observational period of the studies reviewed were up to 6 months. In addition, contentious intake antibiotics for periodontal treatment and subsequent oral microbial improvement may not apply. In this concept, a specific drug delivery system is required. The methodology to control oral bacteria, by using individually fabricate tray, without disturbing mucosal microbiome can be a candidate for long-term control of oral microbiome.
In conclusion, the results of this preliminary study indicated that HbA1c levels can be controlled by improving oral hygiene conditions. To control oral bacteria, 3DS may be the best way to control bacteria in the oral cavity. Evidence suggested by clinical studies was low due to the short observational period.
Acknowledgments
This study was supported by JSPS KAKENHI Grant Numbers 17K12030.
References
- Vergnes JN, Canceill T, Vinel A, et al. 2018. The effects of periodontal treatment on diabetic patients: The DIAPERIO randomized controlled trial. J Clin Periodontol. 45: 1150-1163. [Ref.]
- Alasqah M, Mokeem S, Alrahlah A, et al. 2018. Periodontal parameters in prediabetes, type 2 diabetes mellitus, and non-diabetic patients. Braz Oral Res. 6:32. [Ref.]
- Wernicke K, Zeissler S, Mooren FC, et al. 2018. Probing depth is an independent risk factor for HbA1c levels in diabetic patients under physical training: a cross-sectional pilot-study. BMC Oral Health. 18: 46. [Ref.]
- Naiff P, Carneiro V, Guimarães MDC. 2018. Importance of Mechanical Periodontal Therapy in Patients with Diabetes Type 2 and Periodontitis. Int J Dent. [Ref.]
- Kocher T, Holtfreter B, Petersmann A, et al. 2019. Effect of Periodontal Treatment on HbA1c among Patients with Prediabetes. J Dent Res. 98:171-179. [Ref.]
- Taylor GW, Loesche WJ, Terpenning MS. 2000. Impact of oral diseases on systemic health in the elderly: diabetes mellitus and aspiration pneumonia. J Public Health Dent. 60: 313-320. [Ref.]
- Mealey BL, Oates TW. 2006. American Academy of Periodontology. Diabetes mellitus and periodontal diseases. J Periodontol. 77: 1289-1303. [Ref.]
- Taylor GW, Borgnakke WS. 2008. Periodontal disease: associations with diabetes, glycemic control and complications. Oral Dis. 14: 191-203. [Ref.]
- Darre L, Vergnes J N, Gourdy P, et al. 2008. Efficacy of periodontal treatment on glycaemic control in diabetic patients: A meta-analysis of interventional studies. Diabetes Metab. 34: 497-506. [Ref.]
- Teshome A, Yitayeh A. 2016. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: systematic review and meta-analysis. BMC Oral Health. 17: 31. [Ref.]
- Janket S J, Wightman A, Baird A E, et al. 2005. Does periodontal treatment improve glycaemic control in diabetic patients? A meta-analysis of intervention studies. J Dent Res84: 1154-1159. [Ref.]
- Simpson T C, Needleman I, Wild S H, et al. 2010. Treatment of periodontal disease for glycaemic control in people with diabetes. Cochrane Database Syst Rev. [Ref.]
- Teeuw W J, Gerdes V E A, Loos B G. 2010. Effect of periodontal treatment on glycaemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care. 33: 421-427. [Ref.]
- Engebretson S, Kocher T. 2013. Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. J Clin Periodontol. 40: 153-163. [Ref.]
- Liew A K, Punnanithinont N, Lee Y C, et al. 2013. Effect of non-surgical periodontal treatment on HbA1c: a meta-analysis of randomized controlled trials. Aust Dent J. 58: 350-357. [Ref.]
- Sgolastra F, Severino M, Pietropaoli D, et al. 2013. Effectiveness of periodontal treatment to improve metabolic control in patients with chronic periodontitis and type 2 diabetes: a meta-analysis of randomized clinical trials. J Periodontol. 84: 958-973. [Ref.]
- Lira Junior R, Santos CMM, Oliveira BH, et al. 2017. Effects on HbA1c in diabetic patients of adjunctive use of systemic antibiotics in nonsurgical periodontal treatment: A systematic review. J Dent. 66: 1-7. [Ref.]
- Chapple ILC, Genco R. 2013. Working Group 2 of Joint EFP/AAP Workshop. 2013. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol. 40: 106-112. [Ref.]
- Campus G, Salem A, Uzzau S, et al. 2005. Diabetes and periodontal disease: a case-control study. J Periodontol. 76: 418-425. [Ref.]
- Mang-de la Rosa MR, Castellanos-Cosano L, et al. 2014. The bacteremia of dental origin and its implications in the appearance of bacterial endocarditis. Med Oral Patol Oral Cir Bucal. 19: 67-74. [Ref.]
- Baltch AL, Pressman HL, Hammer MC, et al. 1982. Bacteremia following dental extractions in patients with and without penicillin prophylaxis. Am J Med Sci. 283: 129-140. [Ref.]
- Al-Saadi M, Al-Gret A. 2006. Prevalence of bacteremia in patients with diabetes mellitus in Karbala, Iraq. J Bacteriology Research. 3: 108-116. [Ref.]
- Aminoshariae A, Kulild J. 2010. Premedication of patients undergoing dental procedures causing bacteremia after total joint arthroplasty. J Endod. 36: 974-977. [Ref.]
- Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus, Seino Y, Nanjo K, Tajima N, et al. 2010. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig. 1: 212-228. [Ref.]
- Tamaki Y, Nomura Y, Takeuchi H, et al. 2006. Study of the clinical usefulness of a dental drug system for selective reduction of mutans streptococci using a case series. J Oral Sci. 48: 111-116. [Ref.]
- Nomura Y, Senpuku H, Tsuge S, et al. 2001. Controlling opportunistic pathogens in the oral cavity of preschool children by the use of 3DS. Jpn J Infect Dis. 54: 199-200. [Ref.]
- Takeuchi H, Fukushima K, Senpuku H et al. 2001. Clinical study of mutans streptococci using 3DS and monoclonal antibodies. Jpn J Infect Dis. 54: 34-36. [Ref.]
- Nomura Y, Takeuchi H, Kaneko N, et al. 2004. Feasibility of eradication of mutans streptococci from oral cavities. J Oral Sci. 46: 179-183. [Ref.]
- Nomura Y, Morozumi T, Nakagawa T, et al. 2017. Site-level progression of periodontal disease during a follow-up period. PLoS One. 12: 0188670. [Ref.]
- Arteagoitia I, Rodriguez Andrés C, Ramos E. 2018. Does chlorhexidine reduce bacteremia following tooth extraction? A systematic review and meta-analysis. PLoS One. 13. [Ref.]
- Horliana AC, Chambrone L, Foz AM, et al. 2014. Dissemination of periodontal pathogens in the bloodstream after periodontal procedures: a systematic review. PLoS One. 9. [Ref.]
- Mercanoglu F, Oflaz H, Oz O, et al. 2004. Endothelial dysfunction in patients with chronic periodontitis and its improvement after initial periodontal therapy. J Periodontol. 75: 1694-1700. [Ref.]
- Seinost G, Wimmer G, Skerget M, et al. 2005. Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. Am Heart J. 149: 1050-1054. [Ref.]
- Blum A, Kryuger K, Mashiach Eizenberg M, et al. 2007. Periodontal care may improve endothelial function. Eur J Intern Med. 18: 295-298. [Ref.]
- Graves DT, Corrêa JD, Silva TA. 2019. The Oral Microbiota Is Modified by Systemic Diseases. J Dent Res. 98:148-156. [Ref.]
- Xiao E, Mattos M, Vieira GHA, et al. 2017. Diabetes Enhances IL-17 Expression and Alters the Oral Microbiome to Increase Its Pathogenicity. Cell Host Microbe. 22: 120-128. [Ref.]
- Chen L, Luo G, Xuan D, et al. 2012. Effects of non-surgical periodontal treatment on clinical response, serum inflammatory parameters, and metabolic control in patients with type 2 diabetes: a randomized study. J Periodontol. 83: 435-443. [Ref.]




















