Indexing & Abstracting
Full Text
Review ArticleDOI Number : 10.36811/jvsr.2021.110013Article Views : 92Article Downloads : 57
Review on Common Infectious Diseases of Neonatal Calves
Galma Boneya Arero
Department of Veterinary Laboratory Technology, Ambo University, School of Veterinary Medicine, Ethiopia
*Corresponding Author: Galma Boneya Arero, Department of Veterinary Laboratory Technology, Ambo University, School of Veterinary Medicine, Ambo, P.O. Box 19, Ethiopia, Phone: +251965051574; Email: galmabonaya66@gmail.com; galma.boneya@ambou.edu.et
Article Information
Aritcle Type: Review Article
Citation: Galma Boneya Arero. 2021. Review on Common Infectious Diseases of Neonatal Calves. J Veterina Sci Res. 3: 14-24.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2021; Galma Boneya Arero
Publication history:
Received date: 02 July, 2021Accepted date: 15 July, 2021
Published date: 17 July, 2021
Abstract
Neonatal calf mortality is one of the important problems of calf rearing in dairy farms worldwide. A successful dairy farm operation requires that a large percentage of cows wean a live healthy calf every year. However, in many developing countries, a large number of calves die during the early neonatal life, this causing heavy economic loss. This is due to several infectious (bacterial, viral, and protozoal) and non-infectious factors (management around birth, colostrum management, calf housing, feeding system, hygiene, and pathogens) play an important role in calf rearing. This paper aims to review major infectious causes of neonatal calf mortality. Numerous studies have been conducted in the past from many parts of the world using both retrospective and prospective data sources to document the major causes of calf mortality. Of the infectious diseases of calves, neonatal diarrhea is a matter of major concern, and multiple etiological agents from viruses (Bovine rotavirus, Bovine coronavirus, Bovine viral diarrhea virus) from bacteria (Salmonella spp, Escherichia-coli, and Clostridium perfringens) from protozoal (Cryptosporidium-parvum) have been identified as major causes of neonatal calf mortality. Among the infectious agents, rotavirus and E. coli are mainly involved in the causation of neonatal calf diarrhea which leads to high mortality and morbidity in young calves. E. coli mainly plays its role up to the second week of life whereas, rotavirus up to the third week. Generally, early calf mortality leading to economic losses due to the cost of treatment, prophylaxis, increased susceptibility to other infections, reduced growth rates, and death of calves.
Keywords: Calf Mortality; Infectious Diseases; Diarrhea
Introduction
Dairying is becoming one of the most important parts of the livestock sector where calves are a future herd of a dairy farm in many developing countries to achieve better living standards. A successful dairy farm operation requires that a large percentage of cows wean a live healthy calf every year. The survival of calves and their rapid growth is the most important factor responsible to enhance the sustainability and propagation of the dairy herd [1]. Moreover, in dairy enterprises survivability of the calf is an important trait both for breeding and economic issue. However, in many developing countries, a large number of calves die during the early neonatal life, this causing heavy economic loss. Diarrhea is one of the most important causes of morbidity and mortality, leading to economic losses due to the cost of treatment, prophylaxis, increased susceptibility to other infections, reduced growth rates, and cause death of calves [2]. The success of any commercial dairy farm mainly depends on the survival rate of calf crops produced by the dairy farms, but most of the dairy farms are confronted with acute problems of neonatal calf mortality [3].
Neonatal mortality (NM) is one of the most serious diseases worldwide among new-born calves (<1-month-old). It is roughly estimated that calf mortality of 20% can reduce net profit to 38% [4]. Numerous studies have been conducted in the past from many parts of the world using both retrospective and prospective data sources to document the major causes of calf mortality. A study reported that the rate of neonatal calf mortality between 1.5% and 50% in Sululta Ethiopia [5], 15.2% in Uruguay [6], between 5.4% and 18.9% in India [7], between 10% and 70% in Wolaita [8], between 7% and 29% in northern Mexico [9,10]. Reported the overall mortality rate was 31.22%, with the maximum percentage (15.12%) of calves died within one month of age after birth in Jersey x Sahiwal crosses. However, neonatal calf mortality varies from 8.7 to 64 % throughout the world. Neonatal calf mortality in the first month of age is accounted to be 80-85% of calf mortality and is particularly high in the third week of life [11]. A high rate of calf mortality indicates an irrefutable and irrevocable economic loss to the dairy farm due to loss of the present value of the calf and loss of genetic potential for herd improvement, loss of females for further replacement, and increase treatment cost [12].
Several factors influence the health and vigor of the calves in the neonatal period. Major calf diseases that cause mortality are the results of either alone or complex interaction of the infectious and non-infectious. The important infectious causes are due to viral agents (Rotavirus type A, bovine coronavirus, and bovine viral diarrhea), bacterial agents (Salmonella spp., Escherichia-coli (E.coli) K99+, and Clostridium perfringens, and protozoan (Cryptosporidium parvum and coccidiosis). Some of the non-infectious causes are due to management problems, inadequate nutrition, exposure to severe environmental conditions, insufficient attention to the newborn calf, and failure of passive transfer of immunity [13,14]. Economic losses due to calf mortality are the first and foremost cores of dairy farm management. For this reason, the identification of factors that are responsible for the death of calves is an important prerequisite for reducing excessive mortality. Therefore, with this background information, this seminar paper aims to review the major infectious diseases of neonatal calves.
Infectious Causes of calf mortality
Viruses, bacteria, and protozoans are known to cause infectious diarrhea, this may be associated with a single infection or mixed infections and increased damage. Diarrhea in neonatal calves is one of the most challenging clinical syndromes encountered by practicing large animal veterinarian’s worldwide [15]. High mortality of neonatal calf is due to gastroenteritis, pneumonia, and septicemia. Among these, up to 60% of total calf mortality is attributed to gastroenteric conditions. Various infectious agents have been implicated in calf diarrhea. Bovine practitioners and cattle producers are aware of many enteric pathogens because these primary agents have been known to be involved in calf diarrhea for several decades and still greatly influence current cow-calf operations. Among the infectious diseases of calves, neonatal diarrhea is a matter of major concern, and multiple etiological agents like bovine rotavirus (BoRV), bovine coronavirus (BCoV), Bovine viral diarrhea virus (BVDV), Salmonella spp, Escherichia-coli, Clostridium-perfringens, and Cryptosporidium- parvum) are considered as a bottleneck in calf rearing.
Bovine Rotavirus (BRV)
Bovine rotaviruses (BRV) of the family Reoviridae are the most common causes of diarrhea in farm animals. It is contributing significantly to enteritis and diarrhea in extensive and majorly intensively reared neonatal calves among infectious agents. The agent multiplies in the intestine and spreads with the infected animal's feces [16]. In worldwide, Bovine rotavirus is the most recognized pathogen causing acute diarrhea in cattle and buffalo calves under one month of age [17]. On other hand, it has been recognized as the major pathogen of acute diarrhea in both humans and animals. Infection can produce a complete range of clinical signs from no observed abnormality through to severe diarrhea and dehydration with a high mortality rate. Newborn calves are most prone to this disease at 8-14 days old when there is an acute onset of diarrhea with the passage of very watery yellow/green feces with the infection spreading rapidly among young calves in the group. The disease is usually seen in young calves of 2-8 weeks of age and the susceptibility decreases as the age increases [18]. Typical signs include a reluctance to stand and suck, mild depression, and salivation at an earlier stage. This virus causes significant economic losses in neonates of many domestic animals. Besides affecting cattle and buffalo calves, rotaviruses also affect piglets, foals, lambs, kids, and young ones of pet animals and poultry [19]. Mortality in the first month of age calves is accounted to be 80 to 85% of the total mortality and is particularly high in the third week of life [20]. The severity of the disease varies, depending on age, nutritional conditions, and immunological status of the individuals affected [21].

Figure 1: Severe Diarrhea due to Rotavirus in calves.
Bovine Coronavirus (BCoV)
Bovine Coronavirus (BCoV) is a member of coronaviridae family. This family belongs to the microbes that are etiologically associated with the enteric and respiratory disease across a wide range of mammalian and avian species and the most common causes of diarrhea in calves (Maclachlan and Dubovi, 2010). It usually affects calves that are between 4 and 30 days old. Generally, this virus affects cattle in three different ways and causes clinical syndrome. This appears as diarrhea in calves, winter dysentery in mature cattle, and respiratory system infections in various categories of cattle. According to [22], coronavirus infection in newborn calves with symptoms of enterocolitis diagnosed mainly in 1-3 weeks of age. The main symptoms are diarrhea with the discharge of yellow or greenish liquid feces with a lot of mucus, sometimes mucus mixed with blood. When ulcers form on the oral cavity and oral mucosa, there is profuse salivation. The disease lasts 5-6 days. In the case of secondary microflora complications, the lethal outcome recorded on days 2-3. Also, several phenotype and genotype indicators make it possible to distinguish between respiratory and diarrheal virus isolates. In previous studies, BCV isolation from fecal samples and nasal swabs of healthy-looking calves showed that these calves were excreted the virus in low titers and played an important role in creating clinical infections within the herd. Several epidemiologic studies have reported that high antibody titers to BCoV, most likely resulting from exposure before weaning, can have a respiratory disease-sparing effect [23]. Coronavirus infection of the intestine in calves causes profuse watery diarrhea. Affected calves become dehydrated severely, very quickly developed fever and loss of appetite. Relatively, the incidence of diarrhea is slightly lower in neonatal calf suffered of coronavirus than rotavirus [23].

Figure 2: Signs of bovine Coronavirus infection in calves.
Bovine viral diarrhea virus (BVDV)
Bovine viral diarrhea virus (BVDV) is a member of the family Flaviviridae and is an economically very important pathogen of cattle that is present at high prevalence in many countries around the world [24]. Although, BVDV infects various domestic and wild ruminants, cattle are the natural host developing the clinical sign of disease in the ranges of inapparent to severe, with high mortality rate and potential involvement of one or more organ systems [25]. It is one of the most economically important diseases of cattle worldwide causing direct economic losses through mortality, morbidity, premature culling, and reproductive losses including reduced conception rates, early embryonic death, abortions, congenital defects, and weak calves [26]. The most common clinical signs of acute transient postnatal infection of animals during the era when BVDV infection was emerging were reported to be temperatures as high as 42°C, diarrhea, ulceration of the muzzle and oral cavity, and leucopenia. Few or absence of clinical signs were detected in other infected animals. Highly virulent strains of BVDV can produce lesions like those seen in cases of Mucosal disease (MD), such as severe and widespread ulceration of the oropharynx, larynx, esophagus, and hemorrhagic enteritis. Observed clinical signs consisted of inappetence, lethargy, and reduced milk yield, with a spectrum of severity. It also causes indirect economic impacts through expenditures on vaccination, individual animal testing, and other control measures [26].
The birth of persistently infected (PI) calves with BVDV resulting from in utero-fetal exposure to the virus is extremely important in the perpetuation of the virus in an infected herd and transmission of BVDV to susceptible herds through the introduction of a PI calf [27].
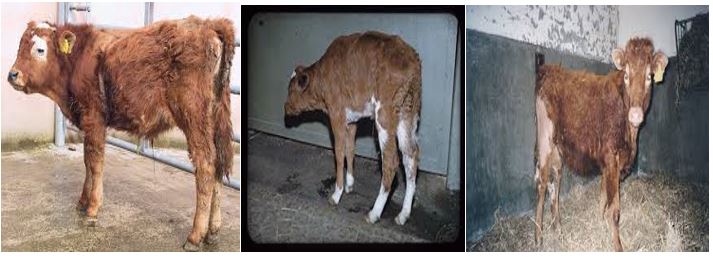
Figure 3: Signs of bovine viral diarrhea in calves.
Escherichia-coli (E. coli)
E. coli is a bacterial species that are a facultative habitant of the gastrointestinal tract, and also found in the environment. However, the infection is present in cattle and other animals due to a break of the protection barrier, extreme pathogenic bacteria type- or immunosuppression. Disease due to E. coli in calves may be present as enteric or septicaemic illness, being one of the most important causes of neonatal mortality in dairy calves [28]. E. coli can be classified into six pathogroups based on their virulence scheme. Of the E. coli pathogroups, the most common cause of neonatal calf mortality is ETEC (Enterotoxin Escherichia- coli) stains that produce the K99 (F5) adhesion antigen (E- coli K99+) and heat-stable (STa or STb) and/or heat-labile (LT1 or LT2) enterotoxins.
The prevalence of Escherichia Coli varies from 5.4 to 100%, and it is roughly estimated that calf mortality associated with E. coli of 20% may reduce net profit to 40%. Foster and Smith, (2009) studied those neonatal calves are most susceptible to ETEC infection during the first 4 days after birth and develop “-watery”- diarrhea if infected. E. coli causes infection through ingestion. Following ingestion, ETEC infects the intestinal epithelium and multiplies in enterocytes in intestinal villi. This causes watery diarrhea and weakness in 1–4-day old newborn calves. Death usually occurred within 24 hours due to severe dehydration. The fimbrial adhesion F5 (K99) promotes the adhesion of bacterial cells to glycoproteins on the epithelial surface of the jejunum and/or ileum. Bacterial enterotoxins also cause damage to the epithelial cells, resulting in fluid secretion and diarrhea (Acres, 1985).
Two of the more prominent virulence factors identified for ETEC strains are expression of fimbrial (pili) antigens that enables the bacteria to adhere to and to colonize the luminal surface of the small bowel. Secondly, elaboration of one or more enterotoxins that influence the intestinal secretion of fluids (Holland 1990). The F5 positive strains are typically isolated from calves between one and five days of age with diarrhea and it is believed that this observation is due to the decline of the expression of the F5 receptor on the bovine epithelial cells within days [29]. Other virulence factors such a sheat-stable enterotoxin (STa) and F41 may also be related to diarrhea in newborn calves. Although fimbria and enterotoxins are two known groups of the classic virulence factors. Proteomics studies have recently introduced several new proteins such as autotransporters involving in E. coli pathogenesis [30].

Figure 4: Signs of Escherichia coli infection with severe diarrhea in calves.
Salmonella species
Salmonellosis is a bacterial disease responsible for causing severe economic losses due to high mortality, especially in cases of the septicaemic form. The genus of Salmonella is divided into two species (S. enterica and S. bongori) and more than 2500 serovars. Salmonella enterica colonizes the digestive of both adult cattle and calves, but the infection is often recorded in the first 3 months of age and often causing severe symptoms. S. enterica serovar typhimurium (S. typhimurium) and serovar dublin (S. dublin) are known as the most common causative agents of salmonellosis in cattle causing acute and systemic diarrheal diseases, respectively [31]. The different clinical manifestations of salmonellosis include diarrhea, pneumonia, septic arthritis, meningitis, gangrene of distal extremities, tenesmus, rectal prolapse, and others, which are caused by the virulence of the serovars, infectious dose, and host immunity status. Serovars can be either host-adapted, such as bovine host-adapted S. dublin, or not host-adapted, such as S. typhimurium. Diarrhea caused by Salmonella infection is characterized by watery and mucoid diarrhea with the presence of fibrin and blood. Even though Salmonella can cause diarrhea in both adult cattle and calves, infection is more common and often causes severe symptoms in 10-day to 3-month-old calves. On the other hand, E. coli K99+ causes watery diarrhea, dehydration, and weakness in 1- to 4-day-old newborn calves [32]. Diarrhea due to Salmonella infection is watery and mucoid with the presence of blood and fibrin. Calves can shed Salmonella for variable periods and intermittently depending on the degree of infection [31]. Furthermore, after the disappearance of the organism from the intestinal tract, up to 5% of recovered animals may become carriers shedding the organism in their feces. Infected cattle and carriers can serve as a source of infection for other animals or even humans through food-borne routes or direct contact and so the determination of Salmonella strains in fecal samples is not only important for the diagnosis of salmonellosis but also essential to identify the carriers.

Figure 5: Signs of salmonellosis in calves with tenesmus, rectal prolapse, and severe dehydration.
Clostridium Perfringes
Clostridium perfringens are Gram-positive, spore-forming, anaerobic bacteria that cause a wide range of diseases in animals and commonly found in many environments including soil, water, poorly preserved feeds, contaminated or improperly thawed colostrum or milk, calf housing environments, and the normal bovine intestinal tract. Among infectious causes, C. perfringens is of importance as a cause of enteritis and enterotoxaemia in many domestic species. Because of the widespread nature of this organism, calves are mostly exposed to C. perfringens in their environment and commonly ingest the bacteria in various quantities, after which it enters the stomach and intestine. Sometimes, bacteria are ingested from the feeds in sufficient quantities to cause disease, but oftentimes small quantities are ingested, followed by rapid proliferation in the intestine. Bovine necro-hemorrhagic enteritis from Clostridium perfringens is a common cause of sudden death with necro-hemorrhagic lesions in the small intestine. The disease typically affects neonatal calves in good to excellent body condition that are fed with a large amount of milk or milk replacer, often without showing signs of illness [33]. Infection due to C. perfringens type C occurs worldwide and affects several animal species. Although several studies described the occurrence of C. perfringens type C infection in calves [33]. Enterotoxaemia due to C. perfringens is more likely to affect baby calves (within the first two months of age) than mature cattle because the calves lack a fully functioning rumen. C. perfringens feeds on starches and sugars in the small intestine. Clostridium-perfringens consists of five types of toxins, these designated A to E, which are identified based on the level of toxins they produce in animals. It is the effect of these specific toxins causing the typical clinical signs and a syndrome attributable to each type. Each toxin type of this disease is associated with specific enteric infections of various animal species [34].
The degree of causing diseases in C. perfringens is mediated by its intimidating arsenal of toxins and degradative enzymes. Among toxin-forming bacteria, C. perfringens produces at least 16 toxins and extracellular enzymes. However, none of the single strains produces this entire toxin panoply, resulting in considerable variation in the repertoire of toxins and degradative enzymes produced by different strains of this bacterium. These strain to strain differences in toxin production allow the classification of C. perfringens isolates into five toxin types (A, B, C, D, and E), based on the presence of genes encoding four so called major toxins: alpha, beta, epsilon, and iota toxin. C. perfringens type C can cause sudden death in neonatal calves less than 10 days of age [35]. Enterotoxaemia due to C. perfringens type C may result in severe bloody diarrhea, although oftentimes calves die before diarrhea develops. Varying degrees of diarrhea, and occasionally sudden onset of weakness and coma, have also been associated with type A, which generally affects a slightly older (2-4 weeks old) calf. C. perfringens type E is considered an infrequent cause of hemorrhagic enteritis and sudden death in neonatal calves; however, few reports also describe type E enterotoxaemia in adult cows [36].
Cryptosporidium
Cryptosporidium species are among the leading causative agents of diarrhea in humans and agriculturally important livestock species throughout the world, namely calves in the first month of life. Some studies revealed that Cryptosporidium is the second most common pathogen causing calf diarrhea, and slightly more common in beef sucker units than in dairy herds. Three Cryptosporidium species are most known to cause infection in cattle these are C. parvum, C. anderson, and C. bovis [37]. This disease usually occurs in calves in the first 6 weeks of life and can harm weight gain and survival in the first month of life [38]. In most calves, diarrhea starts 3–5 days post-infection and lasts from 4 to17 days. Oocyst shedding begins at4 days after birth and peaks at 7–18 days, decreasing after3 weeks [39]. Cryptosporidium parvum infection has been most extensively studied in calves, where it has been found most often; at age one to four weeks, and causes diarrhea, weight loss, and/or impaired weight gain [37]. Among the broadly diverse pathogens that are causally linked to neonatal calf diarrhea, Cryptosporidium parvum is the most ubiquitously seen in several studies. Previous studies have indicated that cryptosporidiosis may occur more frequently in dairy calves than in beef calves because the former is born throughout the year and is confined to pens or hutches, which can facilitate a high level of year-round transmission [40]. During the 10–14-day-old age period most often used, neonatal calves are functional monogastric and remain so up to 4 weeks of age [41].
Experimental infections have been carried out and it has been shown that infected animals shed oocysts with no clinical manifestations of the disease in adult cows. The importance of C. parvum as an etiological agent of the gastroenteritis-like syndrome in dairy calves during their first weeks of life has been confirmed. Therefore, diarrhea, dehydration, weakness, anorexia, abdominal pain, and mortality are the major clinical sign observed [41]. Cryptosporidiosis has been studied in many countries and its prevalence ranges from 3.4 %–96.6 % in pre-weaned calves. One of the most important techniques to control diarrhea in neonatal calves is colostrum management. The passive transfer of maternal antibodies to newborn calves may minimize neonatal diarrhea caused by C. parvum [42].

Figure 6: Sign of cryptosporidiosis in one-month-old calves.
Conclusion
Neonatal calf mortality is one of the major problems of dairy farms worldwide. The first three months of calves’ life are the most critical period for the survivability of calves in any dairy farm. The mortality due to infectious diseases causes heavy losses to the farmers and industry as well as the economy worldwide. Ensuring simple managemental practices such as providing adequate intake of colostrum within the first 12 hours of life, proper housing, good hygiene to minimize disease transfer, providing clean drinking water, developing appropriate feeding protocols to encourage early rumen development and proper animal healthcare can all lead to improved calf performance and can reduce calf mortality. Thus, raising high-pedigree farm-born calves scientifically is the most profitable method of reducing herd replacement costs. Greater attention might be paid to the time of gestation of the dam, colostrum feeding, proper timing and management of calves, and hygiene of calf barns. Because of the complex nature of dairy management systems, the varieties of causes are responsible for diseases and deaths of the dairy calf.
Acknowledgments
I would like to express my deepest heartfelt admiration and thanks to Dr. Golo Dabasa for his intellectual advice materials provision, constructive suggestion and devoting of his time by editing and correcting this paper.
References
1. Kochewad S, Singh J, Patil V. 2013. Calf mortality. Indian Farming. 62: 23-26.
2. Rocha T, Silva F, Gregori F. 2017. Longitudinal study of bovine rotavirus group a in newborn calves from vaccinated and unvaccinated dairy herds. Trop Anim Health Prod. 49: 783-790. Ref.: https://pubmed.ncbi.nlm.nih.gov/28321789/ DOI: https://doi.org/10.1007/s11250-017-1263-2
3. Gitau GK, Aleri JW, Mbuthia PG. 2010. Causes of calf mortality in peri-urban area of Nairobi, Kenya. Trop Anim Health Prod. 42: 1643-1647. Ref.: https://pubmed.ncbi.nlm.nih.gov/20526675/ DOI: https://doi.org/10.1007/s11250-010-9614-2
4. Khan A, Khan M. 1991. Aetiopathology of neonatal calf mortality. Med J Islam World Acad Sci. 4: 159-165.
5. Dagne K, Kassa T, Kebede N. 2018. Occurrences of Dairy Calf Mortality and Morbidity and the Associated Risk Factors in Sululta and its Environs, Central Ethiopia. J Vet Sci Anim Husb. 6: 503-514.
6. Caffarena R, Casaux M, Schild C. 2021. Causes of neonatal calf diarrhea and mortality in pasture-based dairy herds in Uruguay: a farm-matched case-control study. Brazilian J Microbiol. 1-12. Ref.: https://pubmed.ncbi.nlm.nih.gov/33575990/ DOI: https://doi.org/10.1007/s42770-021-00440-3
7. Mishra A, Rawat N, Nanawati S. 2015. Studies on the calf mortality pattern in Gir breed. Int J Livest Prod. 6: 47-51.
8. Asmare A, Kiros WA. 2016. Dairy calf morbidity and mortality and associated risk factors in Sodo town and its suburbs, Wolaita zone, Ethiopia. Slovak J Anim Sci. 49: 44-56.
9. Mellado M, Lopez E, Veliz F. 2014. Factors associated with neonatal dairy calf mortality in a hot-arid environment. Livest Sci. 159: 149-155.
10. Kharkar K, Raghuwanshi D, Lende S. 2017. Mortality Pattern in Crossbred Calves of Dairy Cattle. Sci. Joined as life Memb. Soc Krishi vigyan. 116.
11. Singh D, Kumar M, Choudhary P. 2009. Neonatal calf mortality–an overview. Intas Polivet. 10: 165-169.
12. Parvez M, Faruque M, Khatun R. 2020. Prevalence of abortion, calf mortality and proportion of cattle population in commercial dairy farms of Bangladesh. Res J Vet Pr. 8: 51-55.
13. Klein-Jöbstl D, Iwersen M, Drillich M. 2014. Farm characteristics and calf management practices on dairy farms with and without diarrhea: A case-control study to investigate risk factors for calf diarrhea. J Dairy Sci. 97: 5110-5119. Ref.: https://pubmed.ncbi.nlm.nih.gov/24881793/ DOI: https://doi.org/10.3168/jds.2013-7695
14. Monney J, Adjogoua E, Karamoko Y. 2020. Incidences of Calf Diarrhea and the Associated Risk Factors in Ivory Coast (2015-2017). Rev Ciências Agroveterinárias. 19: 454-461.
15. Seid U, Dawo F, Tesfaye A. 2020. Isolation and Characterization of Coronavirus and Rotavirus Associated with Calves in Central Part of Oromia, Ethiopia. Vet Med Int. 2020. Ref.: https://pubmed.ncbi.nlm.nih.gov/33335702/ DOI: https://doi.org/10.1155/2020/8869970
16. Gumusova SO, Yazici Z, Albayrak H. 2007. Rotavirus and coronavirus prevalence in healthy calves and calves with diarrhea. Medycnya Weterinaria. 63: 62-64.
17. Alfieri A, Parazzi M, Takiuchi E. 2006. Frequency of group a rotavirus in diarrhoeic calves in Brazilian cattle herds, 1998–2002. Trop Anim Health Prod. 38: 521-526. Ref.: https://pubmed.ncbi.nlm.nih.gov/17265766/ DOI: https://doi.org/10.1007/s11250-006-4349-9
18. Dhama K, Chauhan R, Mahendran M. 2009. Rotavirus diarrhea in bovines and other domestic animals. Vet Res Commun. 33: 1-23. Ref.: https://pubmed.ncbi.nlm.nih.gov/18622713/ DOI: https://doi.org/10.1007/s11259-008-9070-x
19. Murphy F, Gibbs E, Horzinek M. 1999. Veterinary virology. Elsevier.
20. Snodgrass D, Terzolo H, Sherwood D. 1986. Aetiology of diarrhoea in young calves. Vet Rec. 119: 31-34. Ref.: https://pubmed.ncbi.nlm.nih.gov/3750766/ DOI: https://doi.org/10.1136/vr.119.2.31
21. Chatzopoulos D, Athanasiou L, Fthenakis G. 2013. Rotavirus infections in domestic animals. J Hell Vet Med Soc. 64: 145-160.
22. Mnikova L, Ishkova T, Alekseyenkova S. 2021. Bovine coronavirus: virus isolation, laboratory diagnostics and specific prevention, in: IOP Conference Series: Earth and Environmental Science. IOP Publishing. 42058.
23. Ellis J. 2019. What is the evidence that bovine coronavirus is a biologically significant respiratory pathogen in cattle? Can Vet J. 60: 147. Ref.: https://pubmed.ncbi.nlm.nih.gov/30705449/
24. Scharnböck B, Roch F, Richter V. 2018. A meta-analysis of bovine viral diarrhoea virus (BVDV) prevalences in the global cattle population. Sci Rep. 8: 1-15. Ref.: https://pubmed.ncbi.nlm.nih.gov/30258185/ DOI: https://doi.org/10.1038/s41598-018-32831-2
25. Brodersen B. 2014. Bovine viral diarrhea virus infections: manifestations of infection and recent advances in understanding pathogenesis and control. Vet Pathol. 51: 453-464. Ref.: https://pubmed.ncbi.nlm.nih.gov/24476940/ DOI: https://doi.org/10.1177/0300985813520250
26. Richter V, Lebl K, Baumgartner W. 2017. A systematic worldwide review of the direct monetary losses in cattle due to bovine viral diarrhoea virus infection. Vet J. 220: 80-87. Ref.: https://pubmed.ncbi.nlm.nih.gov/28190502/ DOI: https://doi.org/10.1016/j.tvjl.2017.01.005
27. Yitagesu E, Jackson W, Kebede N. 2021. Prevalence of bovine abortion, calf mortality, and bovine viral diarrhea virus (BVDV) persistently infected calves among pastoral, peri-urban, and mixed-crop livestock farms in central and Northwest Ethiopia. BMC Vet Res. 17: 1-10. Ref.: https://pubmed.ncbi.nlm.nih.gov/33607976/ DOI: https://doi.org/10.1186/s12917-021-02798-w
28. Muktar Y, Mamo G, Tesfaye B. 2015. A review on major bacterial causes of calf diarrhea and its diagnostic method. J Vet Med Anim Heal. 7: 173-185.
29. Kramer S, Kietzmann M, Pankow W. 2012. Einsatz von Fluorchinolonen bei bakteriellen Harnwegsinfektionen der Katze. Tierärztliche Prax. Ausgabe K Kleintiere/Heimtiere. 40: 113-121. Ref.: https://pubmed.ncbi.nlm.nih.gov/22526815/
30. Yadegari Z, Brujeni GN, Ghorbanpour R. 2019. Molecular characterization of enterotoxigenic Escherichia coli isolated from neonatal calves diarrhea, in: Veterinary Research Forum. Faculty of Veterinary Medicine, Urmia University, Urmia, Iran. 73. Ref.: https://pubmed.ncbi.nlm.nih.gov/31183019/ DOI: https://doi.org/10.30466/vrf.2019.34313
31. Cho Y, Yoon K. 2014. An overview of calf diarrhea-infectious etiology, diagnosis, and intervention. J Vet Sci. 15: 1. Ref.: https://pubmed.ncbi.nlm.nih.gov/24378583/ DOI: https://doi.org/10.4142/jvs.2014.15.1.1
32. Fossler C, Wells S, Kaneene J. 2005. Herd-level factors associated with isolation of Salmonella in a multi-state study of conventional and organic dairy farms: I. Salmonella shedding in cows. Prev Vet Med. 70: 257-277. Ref.: https://pubmed.ncbi.nlm.nih.gov/15964089/ DOI: https://doi.org/10.1016/j.prevetmed.2005.04.003
33. Goossens E, Valgaeren B, Pardon B. 2017. Rethinking the role of alpha toxin in Clostridium perfringens-associated enteric diseases: a review on bovine necro-haemorrhagic enteritis. Vet Res. 48: 1-17. Ref.: https://pubmed.ncbi.nlm.nih.gov/28209206/ DOI: https://doi.org/10.1186/s13567-017-0413-x
34. Ohtani K, Shimizu T. 2016. Regulation of toxin production in Clostridium perfringens. Toxins (Basel). 8: 207. Ref.: https://pubmed.ncbi.nlm.nih.gov/27399773/ DOI: https://doi.org/10.3390/toxins8070207
35. Smith B, Gunn A, McGuirk S. 2015. Manifestations and management of disease in neonatal ruminants. Large Anim. Intern. Med. Fifth Ed. Elseiver. 303-306.
36. Redondo L, Farber M, Venzano A. 2013. Sudden death syndrome in adult cows associated with Clostridium perfringens type E. Anaerobe. 20: 1-4. Ref.: https://pubmed.ncbi.nlm.nih.gov/23354004/ DOI: https://doi.org/10.1016/j.anaerobe.2013.01.001
37. Abeywardena H, Gasser J. 2015. A perspective on Cryptosporidium and Giardia, with an emphasis on bovines and recent epidemiological findings. Adv Parasitol. 88: 243-301. Ref.: https://pubmed.ncbi.nlm.nih.gov/25911369/ DOI: https://doi.org/10.1016/bs.apar.2015.02.001
38. Delafosse A, Chartier C, Dupuy M. 2015. Cryptosporidium parvum infection and associated risk factors in dairy calves in western France. Prev Vet Med. 118: 406-412. Ref.: https://pubmed.ncbi.nlm.nih.gov/25623968/ DOI: https://doi.org/10.1016/j.prevetmed.2015.01.005
39. Trotz-Williams L, Martin S, Leslie K. 2007. Calf-level risk factors for neonatal diarrhea and shedding of Cryptosporidium parvum in Ontario dairy calves. Prev Vet Med. 82: 12-28. Ref.: https://pubmed.ncbi.nlm.nih.gov/17602767/ DOI: https://doi.org/10.1016/j.prevetmed.2007.05.003
40. Olson M, O’Handley R, Ralston B. 2004. Update on Cryptosporidium and Giardia infections in cattle. Trends Parasitol. 20: 185-191. Ref.: https://pubmed.ncbi.nlm.nih.gov/15099558/ DOI: https://doi.org/10.1016/j.pt.2004.01.015
41. Fayer R. 2010. Taxonomy and species delimitation in Cryptosporidium. Exp Parasitol. 124: 90-97. Ref.: https://pubmed.ncbi.nlm.nih.gov/19303009/ DOI: https://doi.org/10.1016/j.exppara.2009.03.005
42. Furman-Fratczak K, Rzasa A, Stefaniak T. 2011. The influence of colostral immunoglobulin concentration in heifer calves’ serum on their health and growth. J Dairy Sci. 94: 5536-5543. Ref.: https://pubmed.ncbi.nlm.nih.gov/22032377/ DOI: https://doi.org/10.3168/jds.2010-3253




















