Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/jvsr.2019.110009Article Views : 20Article Downloads : 42
Survey of Gastrointestinal Parasitic infection in Captive Wild Animals of a Central Zoological Garden in Iran
Vahid Nasiri1,*, Farnoosh Jameie1, Habibollah Paykari1, Nahideh Mazhari2, Saba Soltani3 and Mahdieh Pashaei3
1Protozoology Laboratory, Parasitology Department, Razi Vaccine and Serum Research Institute, Agricultural Research, Education and Extension Organization (AREEO), Karaj, Alborz, Iran
2Parasitology Department, Tabriz University of Medical Sciences, Tabriz, Iran
3Faculty of Basic Science, Karaj Branch, Islamic Azad University, Karaj, Alborz, Iran
*Corresponding Author: Vahid Nasiri, Ph.D., Assistant professor of Medical Parasitology, Protozoology Laboratory, Parasitology Department, Razi Vaccine and Serum Research Institute, Agricultural Research, Education and Extension Organization (AREEO), Karaj, Alborz, Iran. Email: v.nasiri@rvsri.ac.ir
Article Information
Aritcle Type: Research Article
Citation: Vahid Nasiri, Farnoosh Jameie, Habibollah Paykari, et al. 2019. Survey of Gastrointestinal Parasitic infection in Captive Wild Animals of a Central Zoological Garden in Iran. J Veterina Sci Res. 1: 78-91.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Vahid Nasiri
Publication history:
Received date: 25 November, 2019Accepted date: 10 December, 2019
Published date: 13 December, 2019
Abstract
Zoos are places where a great number of valuable animal species are put together taken out of their natural habitats .This survey was carried out to survey the gastrointestinal parasites in animals at a zoological garden in Tehran, Iran. A total of 143 fecal samples from various captive wild animals, consisting of 40 different species were collected randomly and analysed for the presence of the different stages of parasites by direct smear preparation and zinc sulfate flotation followed by Ziel-Neelsen staining method. Data showed that the examined animals were consist of 12 species of carnivores (36 samples), 17 species of herbivores (75), 3 species of non-human primates (11 samples), and 8 species of different species of birds (21 samples). 23(16.08%) of animals, that belonging to 14 animal species, were infected with different intestinal parasites. Among 143 samples from captive wild animals 23 samples (16.08%) were positive for gastrointestinal parasites that 5 samples (3.49%) belong to Carnivores, 13 samples (9.09%) to Herbivores, 4 samples (2.8%) to Non-human primates and 1 sample (0.7%) to Aves. The prevalence of parasites was higher in Non-human primates (36.36%) followed by Herbivores (17.33%), Carnivores (13.88%) and Aves (4.76%).Some animals infected with more than one parasite species and have mixed infection, thus, out of 23 animal samples that parasites were encountered, 12 (8.39%) were infected with helminths and 13 (9.09%) were infected with protozoa. The high prevalence of gastrointestinal parasites found in zoo animals examined in this study emphasizes the importance of controlling these parasitic diseases in order to keep animals, especially in the case of endangered species, in healthy conditions and prevent probable infection of humans working with these animals to zoonotic parasites.
Keywords: Wild animals; Zoological park; Parasite, Iran
Introduction
Although wild animals are usually infected with several species of parasites, but, natural resistance against parasitic diseases and a state of equilibrium between host and parasite generally prevent the development of clinical disease, unless in stress conditions [1]. Zoos are places where a great number of valuable animal species are put together taken out of their natural habitats [2] and these Zoological collections are represented with exotic animal species which would never or rarely meet certain parasites amongst natural circumstances. Keepers may play the role of mechanical vector of parasites and improper feeding systems can encourage the parasite infection [3].
Browsing animals forced to graze or pick up food from the ground are at a greater risk of infection with geohelminths. Serious cases of parasite infection may then arise if a parasite is introduced in a new environment where fully susceptible suitable hosts are available [4].The same situation applies to wild animals in captivity, which are normally kept in the same enclosure for prolonged periods of time, with space limitations and under constant stress, leading to immunosuppression and consequent higher susceptibility to parasitic infection [1]. In addition, as zoos are institutions which are opened to the public, close contact with humans, which would not happen in the natural environment of the captive animals, rises the risk of development of anthropozoonosis [2]. This significantly augments the risk of spreading the parasitic zoonoses posing a threat to the health of the animals themselves, the personal of the zoos and of course to the visitors [2]. Some studies have revealed that gastrointestinal parasites of wild animals in captivity include zoonotic species to humans and raise public health concerns [5-10]. By using a system of preventive and therapeutic means, parasitic infections in zoos are reduced to a minimum ,but, the absence of the natural biological balance due to the artificial amassment of various animals in one and the same location can also result in development of parasites in such animals which normally are not specific host to them [2]. To have a better understanding about the prevalence of the endoparasites those affecting zoo animals, the present study was carried out to establish the gastrointestinal parasite profile of the captive wild animals of a central zoological garden in Tehran, Iran.
Materials and Methods
Study site and animals sample collection
The Zoological Garden of this study is one of the central zoological garden in Tehran, Iran with a large number of exotic animal species. Between March and June 2018, freshly faecal samples were collected from 143 zoo animals representing 40 different species. Animals were classified into herbivorous, carnivores, non-human primates and aves. Information about the examined animals was obtained from zoo labels on the cages of each species. All samples were labeled with related animal species and were collected in 50 ml clean vials and then transported to the Parasitology laboratory of Razi Vaccine and Serum Research Institute and were stored at +4?C immediately upon arrival.
Examination techniques and the laboratory procedures
Samples were examined macroscopically, to verify the presence of nematodes, cestodes, and/or fragments of parasites, and then were processed by qualitative methods of faecal sample examination. All samples were examined by direct wet mount preparation, formalin ethyl acetate concentration, zinc sulfate flotation and Ziehl Neelsen stain technique within 24 hours of collection. Slides were microscopically screened at 100x , 400x and 1000x magnification and detected parasites were identified by their morphometric characteristics as mentioned in the references [11-14]. Collected parasites were deposited in the Museum of Parasitology Department, Razi Vaccine and Serum Research Institute, Karaj, Alborz, Iran.
Ethics Statement
This research was carried out accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Razi Vaccine and Serum Research Institute and all animals experiments were approved by Institutional Animal Care and Research Advisory Committee of the Razi Vaccine and Serum Research Institute based on the Specific National Ethical Guidelines for Biomedical Research issued by the Research and Technology Deputy of Ministry of Health and Medicinal Education of Iran.
Statistical Analysis
Results of faecal examination were analyzed using Chi-square analysis methods.
Results
A total of 143 fecal samples from various captive wild animals, consisting of 40 different species were collected randomly and analysed for the presence of the different kinds and stages of parasites. Scientific and common names of zoo animals that were sampled are listed in tables 1-3. Results showed that the examined animals were consist of 12 species of carnivores (36 samples), 17 species of herbivores (75), 3 species of non-human primates (11 samples), and 8 species of different species of birds (21 samples) that were listed in table 4. Examination of fecal samples revealed that 23(16.08%) of animals, that belonging to 14 animal species, were infected with different intestinal parasites. Table 5 presents the list of detected gastrointestinal parasites according to the captive wild animals’ species in this research. Among gastrointestinal parasites positive captive wild animals 23 samples (3.49 %) belong to carnivores (Figures 1,2), 9.09% to herbivores (Figures 3-6), 2.79% to non-human primates (Figures 7,8) and 0.69% to Aves (Figures 9,10). Types, numbers and percentages of different species of parasites indicated in table 6. Among captive wild animals the prevalence of parasites was higher in Non-human primates (36.36%) followed by herbivores (17.33%), carnivores (13.88%) and Aves (4.76).The results indicated that some animals infected with more than one parasite and have mixed infection, thus, out of 23 animal samples that parasites were encountered, 12 (8.39%) were infected with helminths and 13 (9.09%) were infected with protozoa (Table 6).
|
Table 1: The taxonomic characterization of 12 species of examined carnivores. |
||
|
scientific name |
Common name |
Number of examined carnivores |
|
Panthera pardus saxicolor |
Persian Leopard |
2 |
|
Panthera tigris tigris |
Bengal Tiger |
5 |
|
Panthera leo |
African Lion |
5 |
|
Canis lupus |
Wolf |
5 |
|
Hyaena hyaena |
Striped hyena |
2 |
|
Hystrix indica |
Indian crested porcupine |
3 |
|
Procyon lotor |
Raccoon |
2 |
|
Martes foina |
Stone marten |
1 |
|
Vulpes vulpes |
Common fox |
3 |
|
Ursus arctos |
Brown bear |
5 |
|
Suricata suricatta |
Meerkat |
2 |
|
Felis chaus |
Jungle cat |
1 |
|
Total |
12 species |
36 |
|
Table 2: The taxonomic characterization of 20 species of examined herbivores and non-human primates. |
||
|
scientific name |
Common name |
Number of examined animals |
|
Elephas maximus maximus |
Asian elephant |
11 |
|
Lama glama |
Llama |
3 |
|
Equus hemionus onager |
Asiatic wild ass(onager) |
2 |
|
Camelus ferus |
Wild Bactrian camel |
5 |
|
Cervus elaphus maral |
Maral or Red deer |
7 |
|
Lophocebus aterrimus |
Black crested mangabey |
3 |
|
Dama dama |
Fallow deer |
4 |
|
Equus ferus caballus |
Caspian horse |
5 |
|
Cervus elaphus |
Red deer |
5 |
|
Cervus dama mesopotamica |
Persian Fallow deer |
5 |
|
Marcopus rufus |
Red kangaroo |
5 |
|
Macaca mulatta |
Rhesus monkey |
5 |
|
Papio anubis |
Olive Baboon |
1 |
|
Ovis orientalis |
Wild sheep |
5 |
|
Capra aegagrus |
wild goat |
2 |
|
Gazella subgutturosa |
Goitered gazelle or Persian gazelle |
3 |
|
Pan troglodytes |
Chimpanzee |
5 |
|
Macropus rufogriseus |
Wallaby |
3 |
|
Cavia porcellus |
Guinea pig |
2 |
|
Sus scrofa |
Wild boar |
5 |
|
Total |
20 species |
86 |
|
Table 3. The taxonomic characterization of 8 species of different groups of examined birds. |
||
|
scientific name |
Common name |
Number of examined b |
|
Neophron percnopterus |
Egyptian vulture |
5 |
|
Falco naumanni |
Lesser kestrel |
3 |
|
Struthio camelus |
Ostrich |
3 |
|
Asio otus |
Long – eared owl |
2 |
|
Mix of different species of birds |
different species of birds |
3 |
|
Corvus cornix |
Crow |
2 |
|
Psittacula eupatria |
Parrot |
2 |
|
Coturnix coturnix |
Quail |
1 |
|
Total |
8 species |
21 |
|
Table 4: The type, species and number of examined and positive animals. |
||||
|
Number of species |
Number of individuals |
Number of |
Percentage of |
|
|
positive animals |
positive animals |
|||
|
Carnivores |
12 |
36 |
5 |
13.88% |
|
Herbivores |
17 |
75 |
13 |
17.33% |
|
Non-human primates |
3 |
11 |
4 |
36.36% |
|
Aves |
8 |
21 |
1 |
4.76% |
|
total |
40 |
143 |
23 |
16.08% |
|
Table 5: Positive number and percentage of different species of examined animals. |
|||||
|
Scientific name of animals |
Number of examined |
Number of positive animals |
Detected parasite with number of infected animals |
Percentage of positive animals |
|
|
animals |
(in species/in all) |
||||
|
Cervus elaphus maral |
7 |
3 |
Cryptosporidium sp. (3) |
42.85(2.09) |
|
|
Ovis orientalis |
5 |
3 |
coccidian oocysts (2) |
60(2.09) |
|
|
Cryptosporidium sp. (1) |
|||||
|
Capra aegagrus |
2 |
2 |
Nematode eggs (1) |
100(1.39) |
|
|
Eimeria sp. (1) |
|||||
|
|
|||||
|
Gazella subgutturosa |
3 |
2 |
Nematodirus sp. Egg (1) |
66.66 (1.39) |
|
|
Trichuris spp. Egg (2) |
|||||
|
unknown nematode eggs (1) |
|||||
|
|
|||||
|
Lama glama |
3 |
1 |
Nematodirus sp. Egg (1) |
33.33(0.69) |
|
|
Elephas maximus maximus |
11 |
2 |
unknown nematode eggs (2) |
18.18(1.39) |
|
|
Pan troglodytes |
5 |
1 |
Cryptosporidium sp. (1) |
20(0.69) |
|
|
Macaca mulatta |
5 |
2 |
Trichuris sp. Egg (2) |
40(1.39) |
|
|
Papio anubis |
1 |
1 |
Trichuris sp. Egg (1) |
100(0.69) |
|
|
Suricata suricatta |
2 |
1 |
Coccidian oocysts (1) |
50(0.69) |
|
|
Ursus arctos |
5 |
1 |
Cryptosporidium sp. (1) |
20(0.69) |
|
|
Panthera pardus saxicolor |
2 |
2 |
Coccidian oocysts (2) |
100(1.39) |
|
|
Coturnix coturnix |
1 |
1 |
Trichuris sp. Eggs (1) |
100(0.69) |
|
|
Mix of different species of birds |
3 |
1 |
Coccidian oocysts (1) |
33.33(0.69) |
|
|
Total |
55 |
23 |
|
41.81(16.08) |
|
|
Table 6: Types and numbers of different species of parasites. |
|||
|
Kinds of parasites |
Type of Detected parasites |
Number(percentage) of positive animals (n: 143) |
|
|
Protozoa |
Cryptosporidium spp. |
6(4.19) |
|
|
Coccidian spp. oocysts |
6(4.19) |
||
|
Eimeria spp. |
1(0.69) |
||
|
Helminthes |
Nematodirus spp. egg |
2(1.39) |
|
|
Trichuris spp. Eggs |
6(4.19) |
||
|
unknown nematode eggs spp. |
4(2.79) |
||
|
All parasites |
Total |
25(17.48) |
|
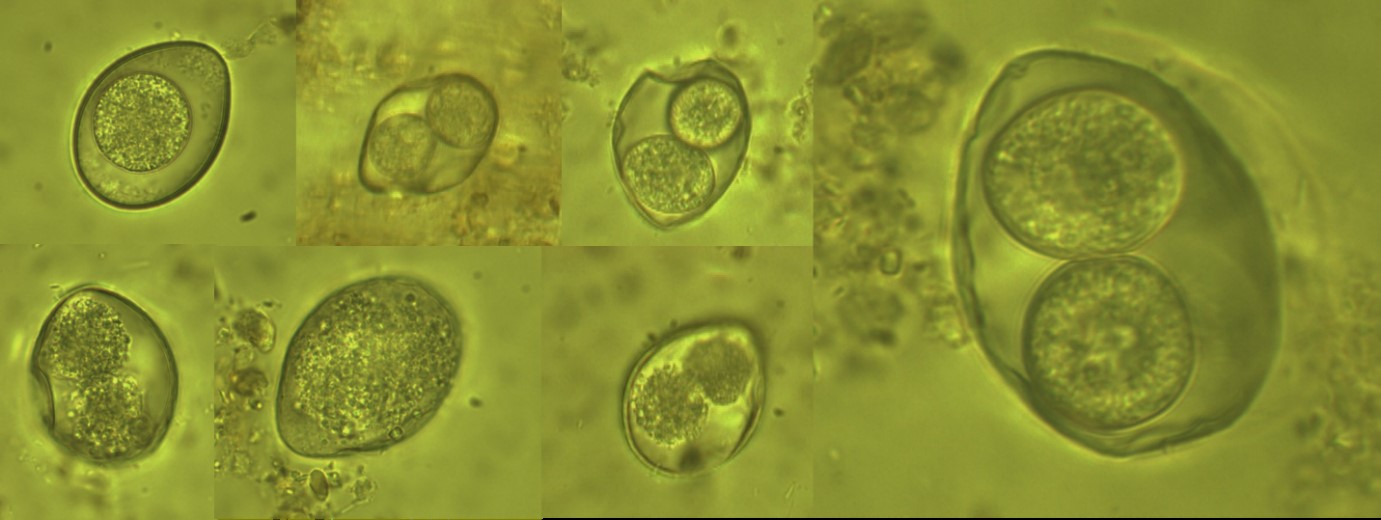
Figure 1: The detected oocysts from Panthera pardus saxicolor (Persian Leopard) (× 1000 magnification).
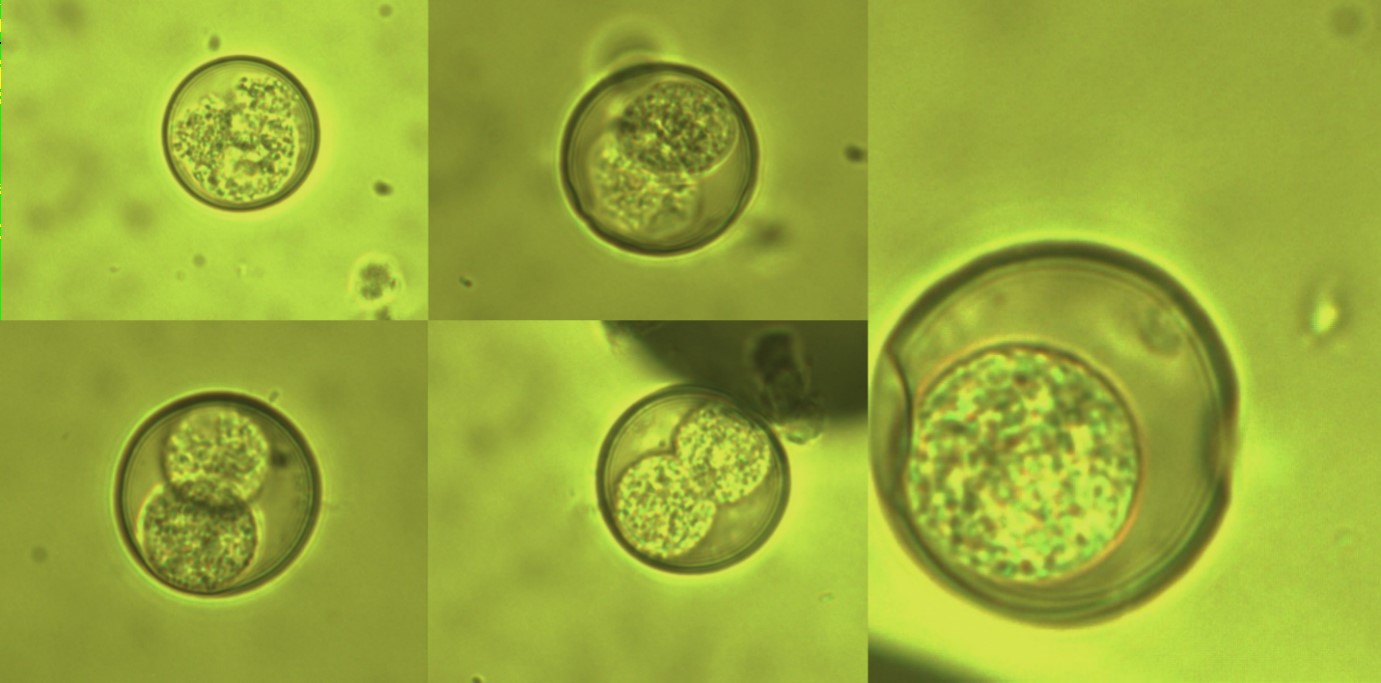
Figure 2: The detected coccidian oocysts from Suricata suricatta (Meerkat) (× 1000 magnification).
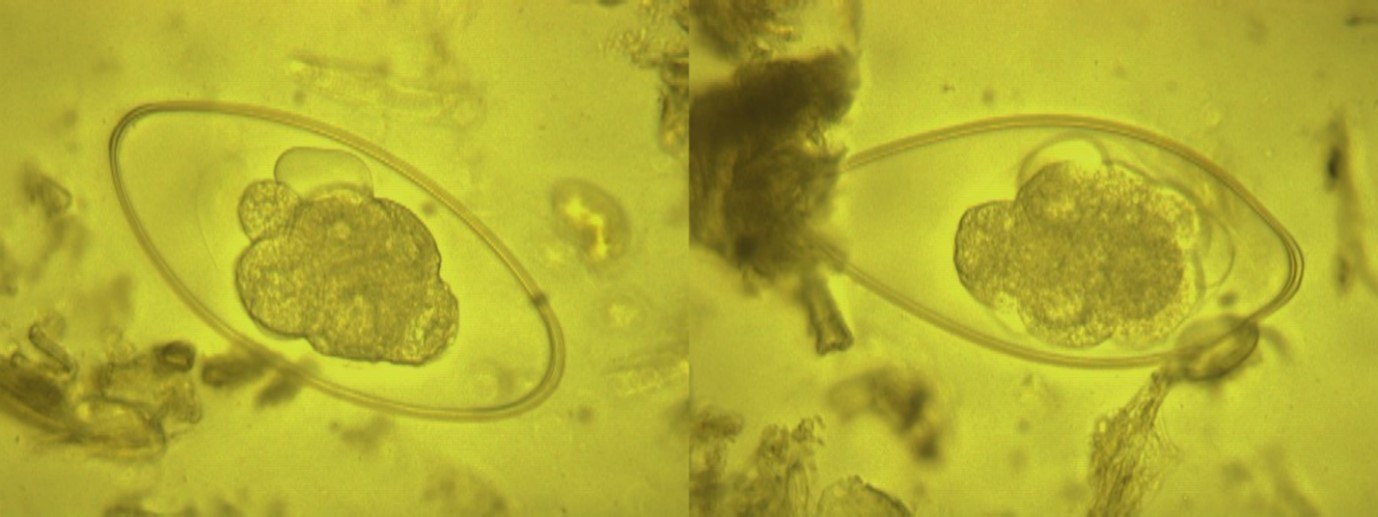
Figure 3: The detected Nematodirus spp. eggs from Lama glama(Llama) (×400 magnification).
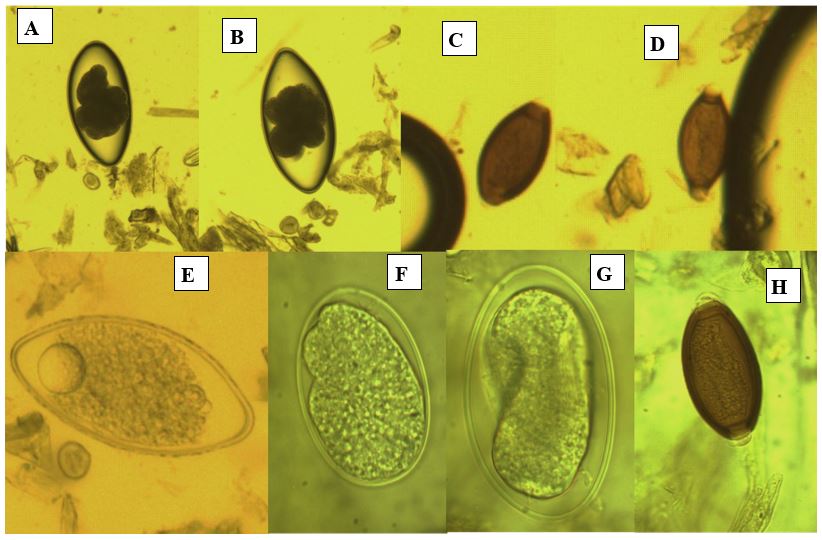
Figure 4: The detected different nematodes eggs from Gazella subgutturosa(Goitered gazelle or Persian gazelle) (×1000 magnification).A, B: Nematodirus sp.; C, D, and H:Trichurissp.; E, Fand G; unknown nematodes eggs.
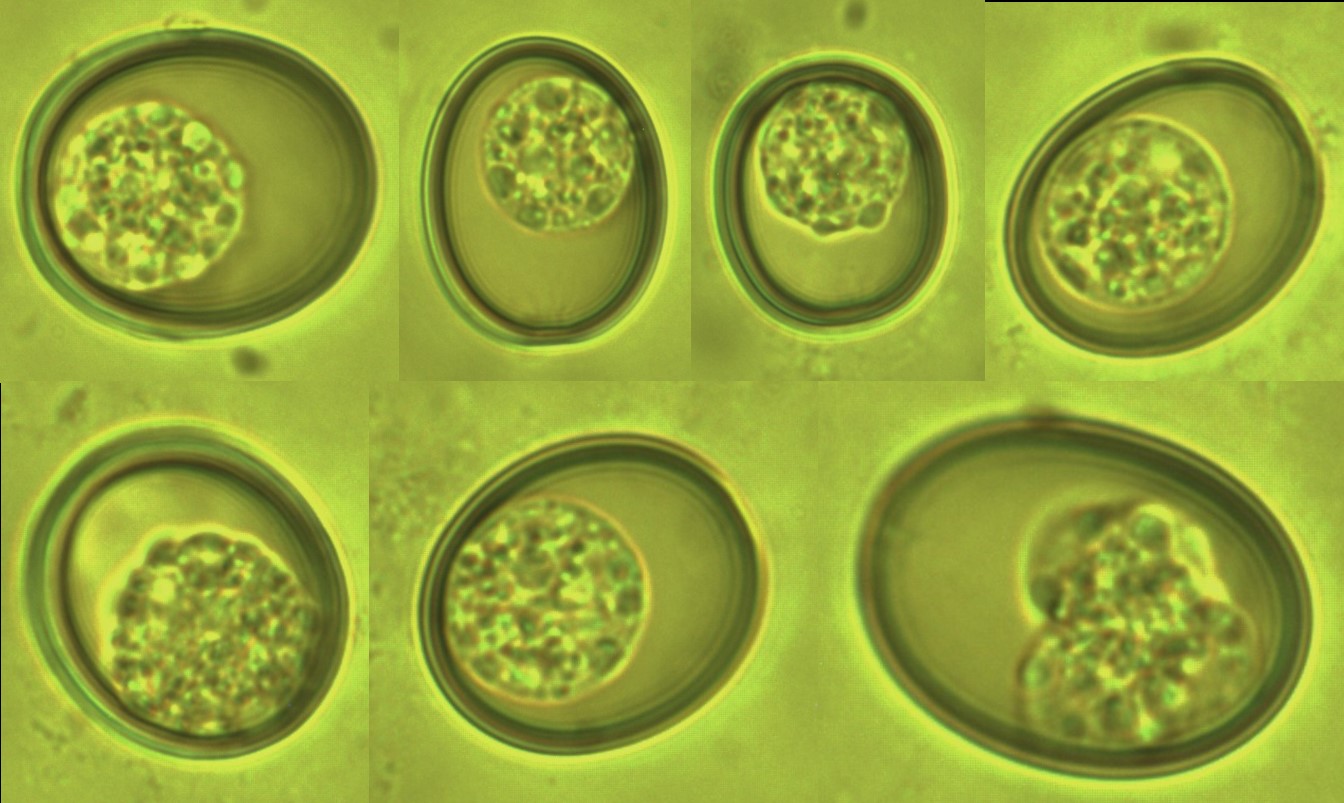
Figure 5: The Eimeria sp. from Capra aegagrus (Wild goat) (×1000 magnification).
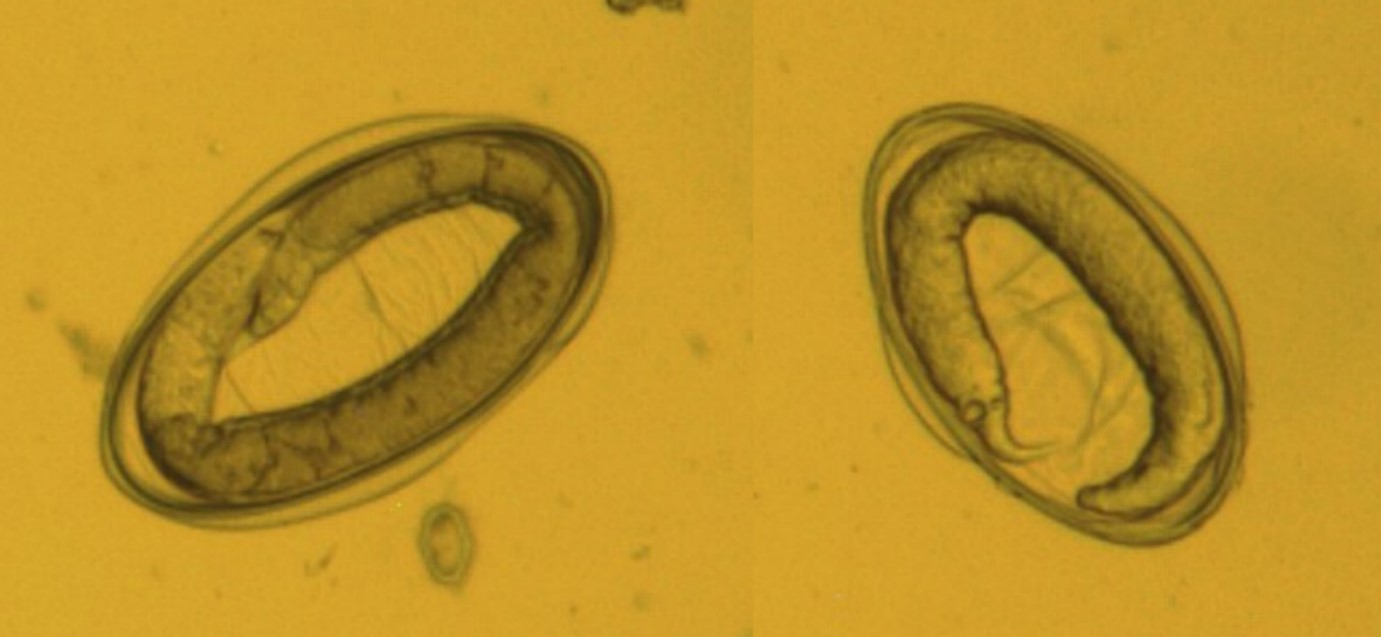
Figure 6: The nematodes eggs from Capra aegagrus (Wild goat) (× 1000 magnification).
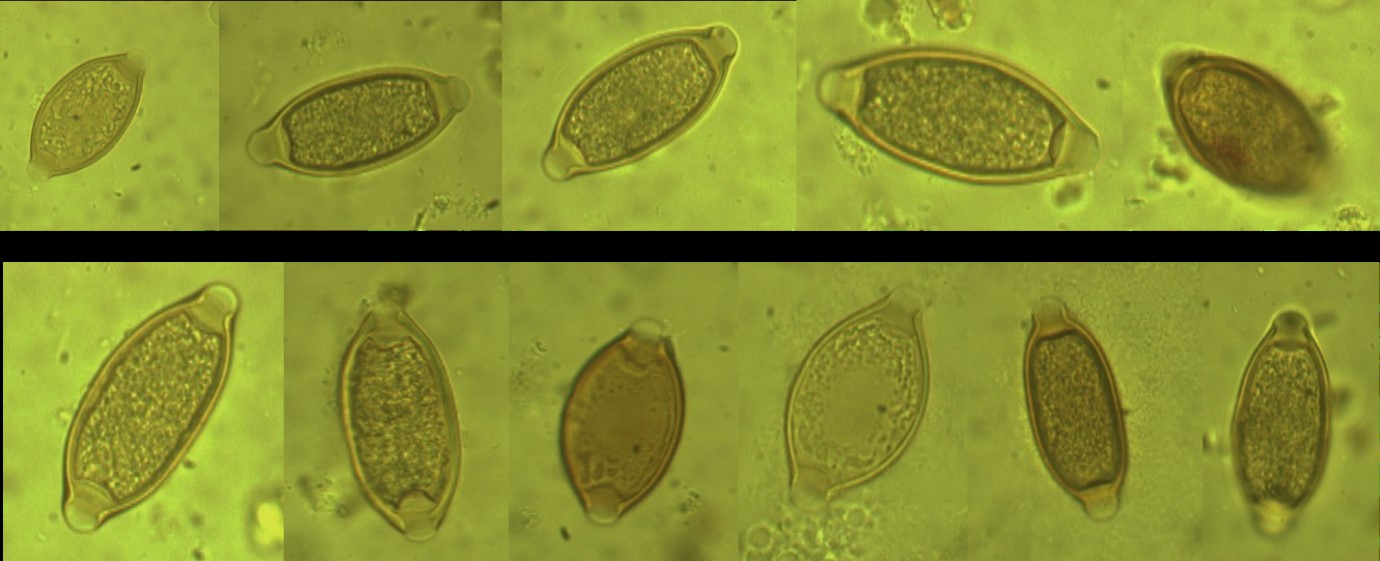
Figure 7: The Trichuris spp. eggs from Macaca mulatta (Rhesus monkey) (× 1000 magnification).
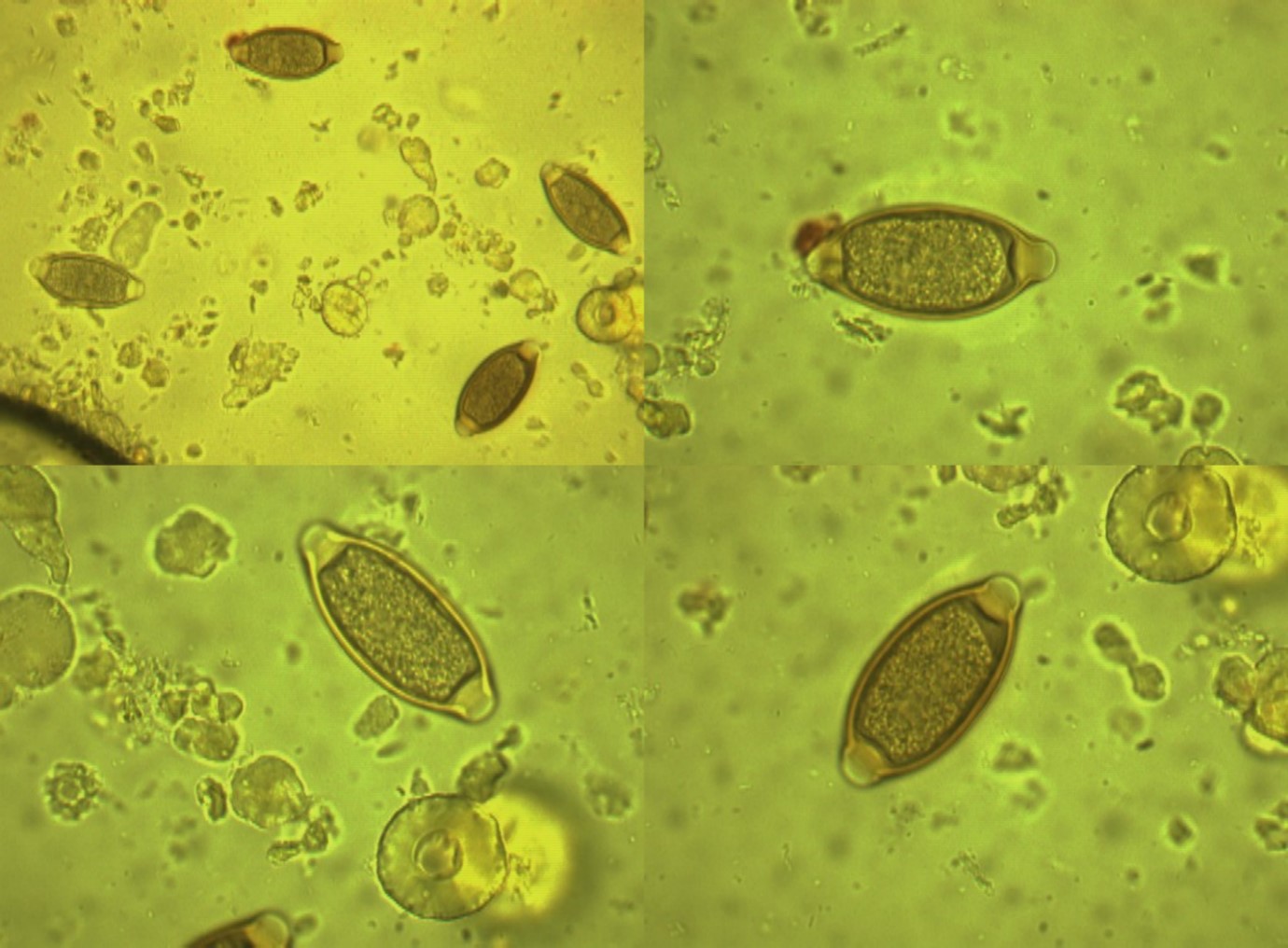
Figure 8: The Trichuris spp. eggs from Papio anubis (Olive Baboon) (× 1000 magnification).
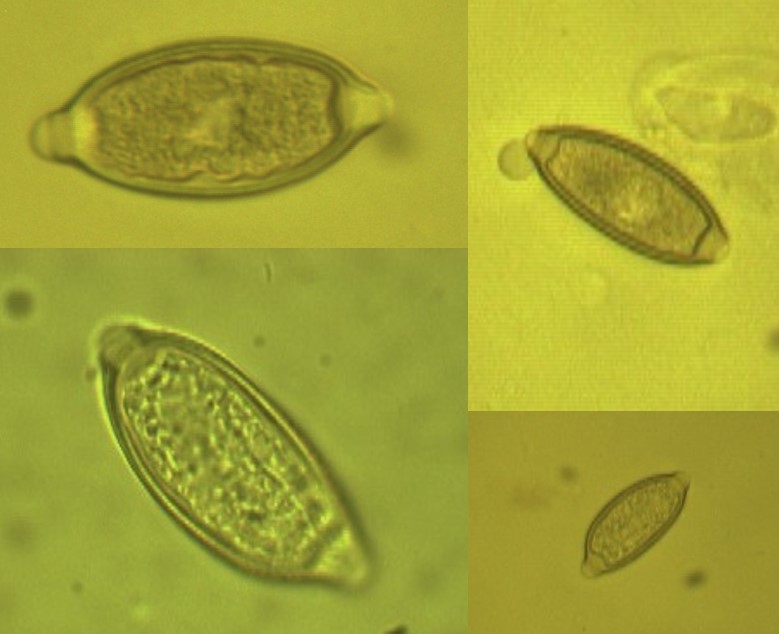
Figure 9: The Trichuris sp. eggs from Coturnix coturnix (quail) (× 1000 magnification).
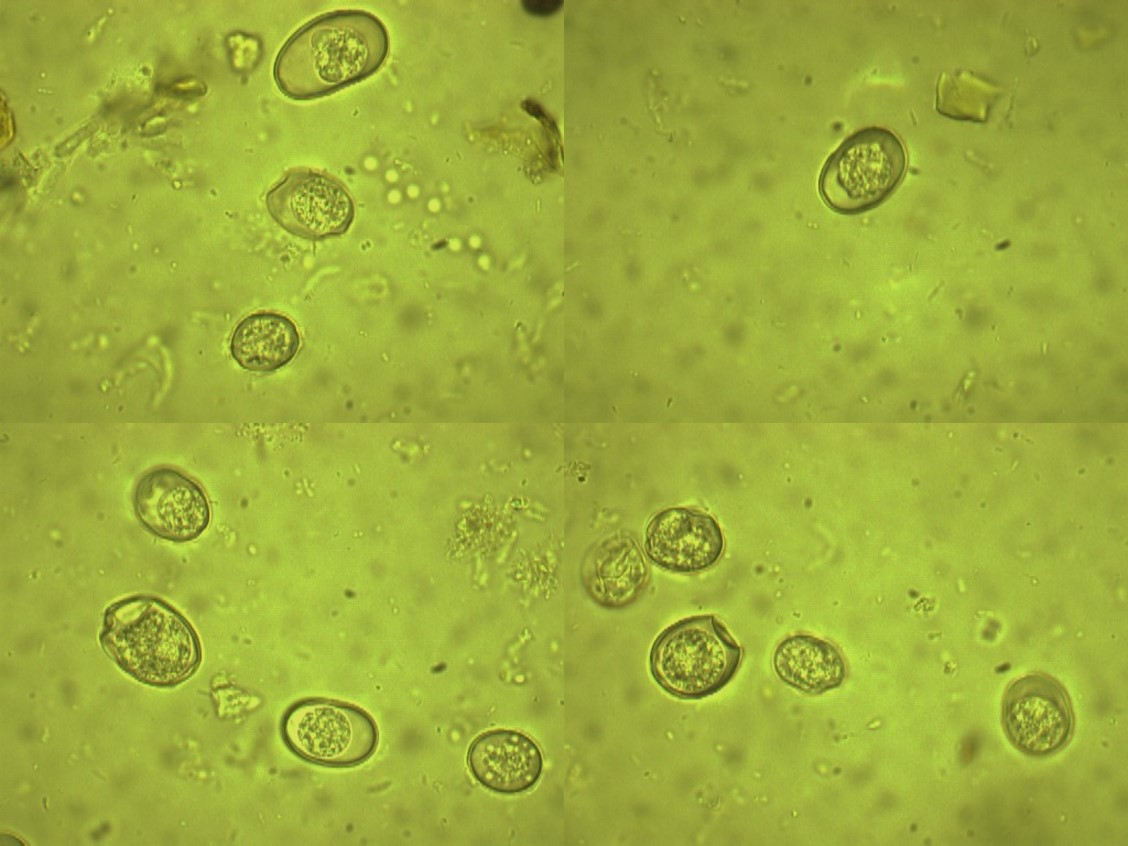
Figure 10: The Eimeria oocysts from bird’s cages(X 100 magnification).
Discussion
Regular coprological examinations seem to be an efficient tool to control the parasite burden in most of the animals, especially in wild animals that were kept in captivity conditions. Parasite control, due to the specific nature of zoological collection is one of the pillars of preventive health care of zoo animals [3]. In the present research, wild animal species in a central National Park of Tehran were investigated for gastrointestinal parasites by examination of faecal samples. The overall prevalence of these parasites in the animals at zoological garden, showed an infection rate of 16.08%. The prevalence of gastrointestinal helminths (8.39%) were almost equal to protozoans (9.09) and the gastrointestinal helminths comprised mainly of nematodes that this finding agrees with the reports of other researchers [6] that nematodes were responsible for most of the helminthic diseases of veterinary importance, because they don’t need intermediate hosts [6]. All the parasites genera identified in this research have previously been identified and described in captive wild animals by other researchers [15]. Here, we reported that among the infected non-human primates, there was a higher occurrence of helminths (36.36%) that was, according to observe in a zoological garden at Kenya, where higher occurrence of helminths (64.4%) and lower of protozoa (17.1%) were reported [16]. In a previous study, they attempt to ascertain the prevalence of species of nematodes in gazelle in Iran [17]. All gazelles studied harbored several species of nematodes in the alimentary canal. Their findings suggest that gazelles harbor a relatively small number of nematodes, the principal genera being Marshallagia, Nematodirus and Nematodirella. These data were similar to those of wild sheep and domestic ruminants in Iran [17]. In our study, we reported that among the infected Gazella subgutturosa, there was an occurrence of helminths (1.39%) including Nematodirus sp., Trichuris spp. and unknown Nematode eggs.
Previously, we carried out a survey to establish the gastrointestinal parasites profile in animals at the Eram zoological garden in Tehran, Iran, that according to our study, examination of fecal samples revealed that 24 (16.7%) of animals were infected with intestinal parasites. Out of 24 parasites encountered, 10 (41.6%) and 14 (58.4%) were helminths and protozoa, respectively. Cryptosporidium spp. infection was detected in 6 (4.1%) of samples [18]. In a recently published study [19], one hundred fresh fecal samples were collected from 35 species of animal lived in Eram park zoo, Tehran, Central Iran during Oct 2015 to Jun 2015. 65.7% (23/35) of zoo animal species were infected with intestinal parasites. The superfamily Trichostrongyloidea (6/16) and Strongylus sp. (4/16) were the most prevalent helminths, while Blastocystis sp. (6/14), Entamoeba cyst (3/14) and Eimeria sp. (3/14) were the common protozoan parasites. For the first time, Bivitellobilharzia nairi egg was identified an elephant at Iran. They indicated that Intestinal parasitic infections were apparently circulating among animals of the Eram park zoo [19].
According to previous research [1], as animal were apparently healthy during the period of examination and there was no reported mortality and clinical signs, the observed prevalence indicates probable subclinical infection, which may flare up under stress conditions and can cause pathogenicity [1]. Based on the prevalence of gastrointestinal parasites, by administration of desired anti helminthic drugs to the captive wild animals periodically that coupled with better sanitary measures, it would be able to reduce the parasitic infection in the zoos [20]. The parasitic prevalence survey is a way of monitoring the impact on the health and maintenance of wild animals’ population [21], and the prevalence of gastrointestinal parasites recorded in the wild animals in this study shows the need to design and implement a control program for parasite elimination.
In conclusion, the findings of this study reported that both protozoan and helminth gastrointestinal parasites are prevalent in the wild animals of this zoo that they can serve as potential reservoirs of some zoonotic parasite for transmission to humans. It should pay attention that among husbandry procedures and diseases preventive measures, the routine monitoring of parasitic diseases and the use of selective treatments can represent crucial measures for the control of gastrointestinal parasitic infections in zoological gardens. The high prevalence of gastrointestinal parasites found in zoo animals examined in this study emphasizes the importance of controlling these parasitic diseases in order to keep animals, especially in the case of endangered species, in healthy conditions and prevent probable infection of humans working with these animals to zoonotic parasites.
Acknowledgements
The authors would like to thank all people that contributed in the preparation of the samples.
References
1. Mir AQ, Dua K, Singla LD, et al. 2016. Prevalence of parasitic infection in captive wild animals in Bir Moti Bagh mini zoo (Deer Park). Patiala, Punjab. Vet World. 9: 540-543. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/27397973
2. Panayotova-Pencheva MS. 2013. Parasites in captive animals: a review of studies in some European zoos. Der Zool Garten. Elsevier. 82: 60-71. Ref.: https://bit.ly/2s9WYRo
3. Kvapil P, Kastelic M, Dovc A, et al. 2017. An eight-year survey of the intestinal parasites of carnivores, hoofed mammals, primates, ratites and reptiles in the Ljubljana zoo in Slovenia. Folia Parasitol (Praha). 21: 64. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/28443822
4. Borgsteede FH. 1996. The effect of parasites on wildlife. Vet Q. 138-140. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/8933697
5. Levecke B, Dorny P, Geurden T, et al. 2007. Gastrointestinal protozoa in non-human primates of four zoological gardens in Belgium. Vet Parasitol. 148: 236-246. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/17656023
6. Otegbade AC, Morenikeji OA. 2014. Gastrointestinal parasites of birds in zoological gardens in south-west Nigeria. Trop Biomed. 31: 54-62. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/24862045
7. Adejinmi OJ, Ayinmode AB. 2008. Preliminary investigation of zooanthroponosis in a Nigerian Zoological Garden. Vet Res. 2: 38-41. Ref.: https://bit.ly/2LBY1Rc
8. Akinboye DO, Ogunfetimi AA, Fawole O, et al. 2010. Control of parasitic infections among workers and inmates in a Nigerian zoo. Niger J Parasitol. 31. Ref.: https://bit.ly/2sUuITf
9. Opara M, Osuji C, Opara J. 2010. Gastrointestinal parasitism in captive animals at The Zoological Garden, Nekede Owerri, Southeast Nigeria. Ostrich. 2: 21-28. Ref.: https://bit.ly/2LyhT7x
10. Ajibade WA, Adeyemo OK, Agbede SA. 2010. Coprological survey and inventory of animals at Obafemi Awolowo University and University of Ibadan Zoological Gardens. World J Zool. 5: 266-271. Ref.: https://bit.ly/2s8CgS2
11. Soulsby EJL. 1968. Helminths, arthropods and protozoa of domesticated animals. Bailliere Tindall. Ref.: https://bit.ly/36gtbFx
12. Zajac AM, Conboy GA. 2012. Veterinary clinical parasitology. John Wiley & Sons. Ref.: https://bit.ly/2RwseET
13. Yamaguti S. 1961. Systema Helminthu: Vol. 3. The Nematodes of Vertebrates. Intersicence Publishers.
14. Bowman DD. 2014. Georgis’ Parasitology for Veterinarians-E-Book. 10th ed. Elsevier Health Sciences. 499. Ref.: https://bit.ly/36eygOE
15. Lim YAL, Ngui R, Shukri J, et al. 2008. Intestinal parasites in various animals at a zoo in Malaysia. Vet Parasitol. 157: 154-159. Ref.: https://bit.ly/33WuQi4
16. Munene E, Otsyula M, Mbaabu DAN, et al. 1998. Helminth and protozoan gastrointestinal tract parasites in captive and wild-trapped African non-human primates. Vet Parasitol. 78: 195-201. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/9760061
17. Eslami A, Rahbari S, Nikbin S. 1980. Gastro-intestinal nematodes of gazelle, Gazella subgutturosa, in Iran. Vet Parasitol. 7: 75-78. Ref.: https://bit.ly/2qEaug0
18. Nasiri V, Jameie F, Soltani S, et al. 2017. Gastrointestinal Parasitic infection in Captive Wild Animals at the Eram Zoological Garden, Tehran, Iran. 3rd International and 10th National Congress of Parasitology and Parasitic Diseases of Iran (NICOPA10). Shiraz: Iran J Parasitol. 189.
19. Kiani H, Mirzapour A, Mobedi I, et al. 2018. Frequency of Intestinal Parasites among Zoo Animal by Morphometric Criteria and First Report of the Bivitellobilharzia nairi from Elephant (Elephasmaximus maximus) in Iran. Iran J Parasitol. 13: 611-617. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/30697315
20. Thawait VK, Maiti SK, Dixit AA. 2014. Prevalence of gastro-intestinal parasites in captive wild animals of Nandan Van Zoo, Raipur, Chhattisgarh. Vet World. 7.
21. Allwin B. 2015. Prevalence of Gastrointestinal Parasites in Gaur (Bos gaurus) and Domestic Cattle at Interface Zones of the Nilgiri Hills, Tamil Nadu, India. J Vet Sci Technol. 07: 1-6. Ref.: https://bit.ly/342vo5V




















