Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/jvsr.2019.110007Article Views : 14Article Downloads : 20
Antioxidant role and immunological function of Excoecaria agallocha in Amphiprion sebae against Vibrio alginolyticus
Nagarajan Balachandran Dhayanithi1, Thipramalai Thankappan Ajithkumar1, Gunapathy Devi2, Chellam Balasundaram3 and Harikrishnan Ramasamy4,*
1Centre of Advanced Study in Marine Biology, Faculty of Marine Sciences, Annamalai University, Parangipettai - 608 502, Tamil Nadu, India
2Department of Herbal and Environmental Science, Tamil University, Thanjavur 613 005, Tamil Nadu, India
3Department of Zoology, Nehru Memorial College, Puthanampatti 621 007, Tamil Nadu, India
4Department of Zoology, Pachaiyappa’s College for Men, Kanchipuram - 631 501, Tamil Nadu, India
*Corresponding Author: Harikrishnan Ramasamy, Department of Zoology, Pachaiyappa’s College for Men, Kanchipuram - 631 501, Tamil Nadu, India, Tel: +91 4362 227937; Fax: +91 4362 227185; Email: rhari123@yahoo.com
Article Information
Aritcle Type: Research Article
Citation: Nagarajan Balachandran Dhayanithi, Thipramalai Thankappan Ajithkumar, et al. 2019. Antioxidant role and immunological function of Excoecaria agallocha in Amphiprion sebae against Vibrio alginolyticus. J Veterina Sci Res. 1: 50-64.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Nagarajan Balachandran Dhayanithi
Publication history:
Received date: 18 November, 2019Accepted date: 27 November, 2019
Published date: 29 November, 2019
Abstract
The efficacy of supplementation diet with 0, 2.5, 5, and 10 g kg-1 of Excoecaria agallocha aqueous leaf extract on innate immune response, survival, and disease resistance was reported in clownfish, Amphiprion sebae against Vibrio alginolyticus. The mean weight gain (MWG), specific growth rate (SGR), and protein efficiency ratio (PER) did not significantly vary with any supplementation diet except with 5 g kg-1 diet on weeks 6 and 8. The white blood cell (WBC) level significantly increased with any supplementation diets on weeks 6 and 8. The phagocytic activity, alternate complement activity, and lysozyme activity increased significantly with 5 and 10 g kg-1 diet on weeks 6 and 8 whereas the respiratory burst activity significantly increased with any supplementation diets on weeks 6 and 8. The cumulative mortality with the 2.5 and 10 g kg-1 supplementation diets were 10% against V. alginolyticus whereas with 5 g kg-1 supplementation diet only 5%. V. alginolyticus count was high in infected fish fed with control diet as well as the infected fish fed with 2.5 g kg-1 diet had high bacterial count (cfu/g) in kidney, blood, and spleen (1.4 x 102, 1.1 x 103, and 1.2 x 103) whereas it was low with 10 g kg-1 diet in blood, spleen, and kidney (0.6 x 101, 0.2 x 101, and 0.2 x 101). The present results indicate that infected fish fed diet containing 5 and 10 g kg-1 of E. agallocha positively enhance the innate immune response and reduce the mortality in A. sebae against V. alginolyticus infection.
Keywords: Disease resistance; Excoecaria agallocha; Innate immune parameter; Mortality; Vibrio alginolyticus
Introduction
Emerging trends in intensive aquaculture has led to increasing disease outbreaks and associated problems in production losses which remain a primary challenge to sustain the yield [1-3]. Traditional disease control and prevention strategies employ vaccines, antibiotics, and chemotherapeutics. The application of antibiotics is often questioned due to the development of resistant bacterial strains that also cause many other problems such as environmental hazards, food safety and resistance of human pathogens. Further it can also adversely affect the health status and gut microbial flora in fish [4,5]. A similar situation has been reported with use of chemotherapeutics as a prophylactic measure [6,7]. The great strides achieved in aquaculture in recent decades have increased the interest in studies of the fish immune system and defence against diseases. In intensive fish farming, animals crowding leads to stress conditions that weaken fish immune system, leading to increased susceptibility to pathogens which in turn paves the way for the emergence of diseases. These infectious diseases contribute to economic loss and constitute at present, one of the major constraints hampering intensive fish culture. This situation and the recent restrictions on the use of antibiotics and chemotherapeutics have triggered the use of herbal immunostimulants as better alternatives to antibiotics and chemotherapeutics, and there is an increased interest in aquaculture in using these additives to prevent and/or control fish diseases [8-19].
Excoecaria agallocha is commonly known as milky mangrove (Vernacular: Thillai) species belongs to the family Euphorbiaceae. The plant enjoys a wide distribute in a number of other countries of temperate and tropical Asia, Australasia, and Southwestern Pacific in the intertidal zones. Different parts of E. agallocha extracts contain alkaloid, tannin, saponin, flavonoid, glycoside, phenolic etc. compounds [20]. E. agallocha has been used as folk medicine and has biologically active anti-HIV, anti-cancer, anti-bacterial, and anti-viral compounds [21]. It is used as purgative and in the treatment of leprosy, rheumatism, epilepsy, flatulence, dermatitis, and toothache [22,23]. Further it is reported to possess anti-ulcer, anti-cancer, and anti-oxidant activities [24-26].
Recently the demand for marine ornamental fishes has gained a thrust among aquarium hobbyists due to their multitudinal color and beauty. The annual worldwide market for ornamental marine reef fish has shown a steady increase over the past few years. This trade is almost dependent on, ornamental fishes captured from the wild. This has resulted in a considerable pressure that may lead to over-exploitation of natural population, damage to coral reef, and leading to environmental degradation. Therefore, research on the commercial rearing of these fishes is an imminent necessity to save this fragile ecosystem. However, till date efforts in this direction have been extremely limited to very few coral fishes like damsels, neon gobies etc. and that too only in temperate conditions. Besides the artificial rearing conditions cause many diseases leading to heavy economic loss. Clown fish, Amphiprion sebae is very popular among fish hobbyists and a prized fish due to its aesthetic appeal and its easy adaptability to captive conditions. It enjoys a wide distribution in Northern Indian Ocean, which includes India, Sri Lanka, and the Maldive Islands. To our knowledge there is no information on effect of this plant with reference to fish diseases. Therefore, the present study was undertaken to analyze the immunomodulatory effects and disease resistance of E. agallocha supplementation diet for the first time in clownfish fish, A. sebae against Vibrio alginolyticus infection.
Materials and methods
Collection and extraction of mangrove plants
Healthy leaves of Excoecaria agallocha collected from the Vellar estuary mangroves, were washed, shade dried, and powdered. Ten grams of the dried powder was extracted in 100 ml of double distilled water using a rotary shaker for 30 min at 100 rpm. The supernatant was separated by centrifugation for 20 min at 10,000 rpm and the supernatant portion was evaporated and crude extract stored in refrigerator for further studies.
Vibrio alginolyticus
The pathogenic bacteria, V. alginolyticus (AUMOFP2) was previously isolated and reported from infected marine ornamental fish maintained in the laboratory under standard conditions [27]. Subcultures were maintained on marine zobell agar (Himedia, Mumbai) in slopes at 5 ºC and routinely tested for pathogenesis by inoculation into marine ornamental fish. Stock culture in marine zobell broth (Himedia, Mumbai) was stored at 70 ºC in 0.85% NaCl with 20% glycerol (v/v) to provide stable inoculate throughout the experiment. The culture was centrifuged at 1000 g for 10 min at 4 ºC. The supernatant was discarded and the bacterial pellet was washed three times and resuspended in phosphate-buffered saline (PBS) at pH 7.4. The OD of the solution was adjusted to 0.5 at 456 nm which corresponded to 1 x 107 cells ml-1.
Feed preparation
E. agallocha leaf extract was incorporated separately into four experimental diets, containing 0, 2.5, 5, and 10 g kg-1. Diets were prepared in the basal ratio and the proximate composition of the basal diets was calculated by the appropriate standard protocols. The basal diet contained 45.1% protein, 7.2% lipid, 14.6% ash, 7.1% moisture, and 3% fibre. To prepare the herbal supplemented feeds, ingredients were mixed thoroughly with gelatin solution (1%, w/v) containing the active principles at the appropriate concentration (Table 1). The mixture was then cold extruded, cut into pellets, air dried, and stored at room temperature (RT).
|
Table 1: Composition of supplementation for fish. |
||||
|
Ingredients (g kg-1) |
Herbal extract (g kg-1) |
|||
|
0 g |
1 g |
2 g |
4 g |
|
|
Acetes |
500 |
500 |
500 |
500 |
|
Mytha |
100 |
100 |
100 |
100 |
|
Ground net oil cake |
270 |
270 |
270 |
270 |
|
Rice bran |
100 |
90 |
80 |
60 |
|
Cod liver oil |
20 |
17.5 |
15 |
10 |
|
Vitamina & Mineralb premix |
10 |
10 |
10 |
10 |
|
Herbal extract |
0 |
2.5 |
5 |
10 |
|
aVitamin: Retinol palmitate (Vitamin A): 1400000 UI; Cholecalciferol, (Vitamin D): 200000 UI; Alpha tocopherol acetate, (Vitamin E): 14000 mg; Niacin: 8000 mg; Pantothenic acid: 3000 mg; Riboflavin (Vitamin B2): 1400 mg; Pyridoxine (Vitamin B6): 1200 mg; Thiamine (Vitamin B1): 1200 mg; Ascorbic acid (Vitamin C): 200 mg. bMinerals: FeSO4?H4O: 10000 mg; ZnO: 6000 mg; MnO: 4000 mg; CuSO4?5H2O: 600 mg, CaCO3 as carrier. |
||||
Fish
Healthy sub-adult clownfish, Amphiprion sebae (20.2±1.4 g in weight) was collected from the marine ornamental fish hatchery, Centre of Advanced Study in Marine Biology, Annamalai University, Parangipettai, Tamil Nadu. The health status of the fish was check immediately up on arrival. The fishes were acclimated in 5000 l cement tank filled with 2500 l of UV treated seawater and continuous aeration was provided. Fish were acclimated for 2 weeks prior to initiating the experiment and the fish were provided with boiled oyster meat twice a day. During the experiment, water temperature was 28.2 oC±1.4, pH 8.2±0.3, salinity 28±2.2 ppt, dissolved oxygen concentration 5.8 ± 0.6 mg l-1, and 14 h light: 10 h dark photoperiod was measured. The ammonia and nitrite contents in the water were maintained below detectable levels.
Experimental setup and challenge experiment
A total number of 25 healthy A. sebae were divided into 5 groups each in three replicate (5x25x3=375 fish) as following: 1) healthy fish fed with control diet (0 g kg-1), 2) pathogen challenged fish fed with control diet (0 g kg-1), 3) pathogen challenged fish fed with 2.5 g kg-1 diet, 4) pathogen challenged fish fed with 5 g kg-1, and 5) pathogen challenged fish fed with 10 g kg-1 of E. agallocha leaf extract supplementation feed. The experimental fishes were fed with the respective diet twice (10.00 a.m and 1.00 p.m) in a day at 5% of their body weight until end of the experiment. After 30 days of supplementation feeding, the fish were injected 0.1 ml intramuscularly with V. alginolyticus (1 x 107 cells ml-1) except control group.
Supplementation feed action on gut bacteria
Total gut bacteria of the experimental and control group fishes were assessed by the modified protocol of Velmurugan and Citarasu [28] to find out the effects of supplementation diet containing E. agallocha on the normal gut bacterial flora. The gut of the fish from each group was removed aseptically, weighed, washed with 85% sterile saline, and homogenized. The homogenate was diluted in sterile seawater and plated in nutrient agar (Hi-media, India) and TCBS (Hi-media, India). The plates were incubated at 30 ºC for 48 h and the colonies were counted quantitatively.
Growth performance, nutrient utilization, and survival rate
At 2, 4, 6, and 8-week post-challenge, the fish were fasted for 24 h before than anaesthetized with MS 222 and weighed each group. Mean weight gain (MWG), protein efficiency ratio (PER), specific growth rate (SGR), protein intake (PI), feed conversion ratio (FCR), and fish survival rates (SR %) were calculated [16] using the following equations: MWG = [initial body weight] – [final body weight]; PER = [% protein in diet] x [weight of diet consumed] / 100; SGR = [in final body weight - in initial body weight] / number of days x 100; FCR = [feed consumed] / [weight gain]; PI = [fish wet weight gain] / [fish consumed protein] x 100; Survival (%) = [final number of fish / initial number of fish] x 100.
Bleeding and separation of serum
Fishes were bled immediately after capture to eliminate possible effects of stress on analyzed parameters. The fish were anesthetized during the bleeding. The blood (0.5 ml) was collected from the caudal vasculature using a 1 ml syringe fitted 24-gauge needles. A part of blood was allowed to clot at RT for 3 h, centrifugation at 2000 rpm for 10 min than the serum was separated and stored along with PBS at -20ºC until used for the experiment.
Total white blood cell (WBC) counts
Remaining blood was mixed with diluting fluid and incubated for 5 min for complete haemolysis of RBCs. The blood mixture was loaded on the haemocytometer by holding the pipette at 45o angle and the cover slip was placed. After 5 min, the cells were counted and expressed as cells ml-1.
Immunological assay
Serum lysozyme activity was measured according to the modified method described by Ellis [29]. Respiratory burst activity was detected by the Nitro Blue Tetrazolium chloride (NBT) assay [30]. Alternative complement (ACH50) activity was determined by Sunyer and Tort [31] with sheep red blood cells (SRBCs) were used as target cells in the presence of gelatin veronal buffer (GVB). Phagocytic activity was measured from head kidney (HK) leucocytes according to Matsuyama et al. [32].
Disease resistance and survival experiment
The disease resistant experiment was conducted by the method of Harikrishnan et al. [33]. The bacteria culture, experimental setup, challenge with dose study as mentioned previously. Once the fishes were exposed with pathogen, they were observed for the clinical symptoms and mortality. The cause of death was confirmed by re-isolating the pathogen from dead fish using TCBS medium.
Reisolation of bacteria from challenged fishes
Pathogenic bacteria were re-isolated from the dead fishes which were collected from the challenged groups. Immune organs (kidney and spleen) and blood of the challenged fishes were collected, homogenized, serially diluted, and plated on TCBS agar. The plates were incubated at 30 °C for 24 to 48 h and the colonies were counted and characterized based on the color and morphology on the TCBS plates.
Statistics
Data were analyzed using one-way analysis of variance (ANOVA) to find out the significant difference at the 5% (P < 0.05) level.
|
Table 2: Growth parameter, nutritional utilization, and survival rate of A. sebae fed with 0, 2.5, 5, and 10 g kg-1 E. agallocha supplementation diets. |
||||||
|
Groups |
||||||
|
Studie |
Weeks |
C |
I |
2.5 g kg-1 |
5 g kg-1 |
10 g kg-1 |
|
MWG |
2 |
20.2 ± 1.4 |
18.8 ± 1.1 |
21.4 ± 1.5 |
22.6± 1.3 |
21.9 ± 1.8 |
|
4 |
20.8 ± 1.2 |
18.1 ± 1.3 |
21.8 ± 1.3 |
23.4± 1.0 |
22.3 ± 1.2 |
|
|
6 |
21.6 ± 1.1 |
17.7 ± 1.2 |
22.2 ± 1.2 |
23.9± 1.2* |
23.6 ± 1.6 |
|
|
8 |
21.9 ± 1.5 |
17.1 ± 1.4 |
22.7 ± 1.7 |
24.4± 1.4* |
23.8 ± 1.4 |
|
|
SGR |
2 |
0.62±0.04 |
0.60±0.03 |
0.63±0.03 |
0.65±0.04 |
0.64±0.03 |
|
4 |
0.63±0.03 |
0.59±0.02 |
0.65±0.02 |
0.69±0.03 |
0.66±0.04 |
|
|
6 |
0.63±0.03 |
0.58±0.04 |
0.67±0.04 |
0.81±0.03* |
0.69±0.03 |
|
|
8 |
0.64±0.05 |
0.57±0.03 |
0.69±0.04 |
0.87±0.05* |
0.75±0.03 |
|
|
PER |
2 |
0.77±0.11 |
0.77±0.10 |
0.78±0.11 |
0.80±0.12 |
0.79±0.11 |
|
4 |
0.78±0.10 |
0.76±0.11 |
0.80±0.10 |
0.86±0.10 |
0.83±0.10 |
|
|
6 |
0.79±0.13 |
0.75±0.11 |
0.84±0.12 |
0.94±0.12* |
0.86±0.12 |
|
|
8 |
0.81±0.12 |
0.73±0.12 |
0.86±0.10 |
0.98±0.11* |
0.89±0.10 |
|
|
FCR |
2 |
1.23±0.03 |
1.22±0.02 |
1.26±0.03 |
1.28±0.03 |
1.26±0.02 |
|
4 |
1.25±0.02 |
1.20±0.01 |
1.28±0.02 |
1.36±0.02 |
1.31±0.03 |
|
|
6 |
1.26±0.03 |
1.20±0.02 |
1.30±0.03 |
1.41±0.02* |
1.37±0.02 |
|
|
8 |
1.28±0.02 |
1.17±0.03 |
1.32±0.02 |
1.47±0.03* |
1.42±0.03* |
|
|
PI (g) |
2 |
23.2±1.1 |
23.1±1.2 |
23.5±1.3 |
23.7±1.2 |
23.5±1.3 |
|
4 |
23.5±1.0 |
23.0±1.2 |
23.9±1.0 |
24.5±1.1 |
23.8±1.2 |
|
|
6 |
23.7±1.2 |
22.6±1.2 |
24.4±1.2 |
25.6±1.0* |
24.0±1.1 |
|
|
8 |
23.9±1.3 |
23.3±1.2 |
24.9±1.1 |
26.1±1.1* |
25.8±1.2* |
|
|
SR (%) |
2 |
98.3 |
85.4 |
95.1 |
97.4 |
96.2 |
|
4 |
98.3 |
83.2 |
96.2 |
97.8 |
96.8 |
|
|
6 |
98.8 |
80.5 |
96.8 |
98.6 |
97.4 |
|
|
8 |
99.2 |
78.6 |
97.4 |
98.8 |
97.8 |
|
|
MWG: mean weight gain, SGR: specific growth rate, PER: protein efficiency ratio, FCR: feed conversion ratio, PI: protein intake (PI), and fish survival rates (SR %). |
||||||
Results
Growth
The MWG, SGR, and PER are did not significantly vary with any supplementation diet from week 2 and 4. However, these parameter was significantly increased the infected fish fed with 5 g kg-1 diet on weeks 6 and 8. The FCR and PI did not vary significantly with any diet but it was found significantly increased when infected fish fed with 5 g kg-1 diet on weeks 6 and 8 as well as 10 g kg-1 diet on 8. The better survival rate was found the infected fish fed with 5 and 10 g kg-1 diet when compared to control (Table 2).
Total white blood cell (WBC) counts
The WBC level slightly increased with all supplementation diets as compared to control on weeks 2 and 4. However, it significantly increased with any supplementation diets on weeks 6 and 8 as compared to the control (Figure 1).
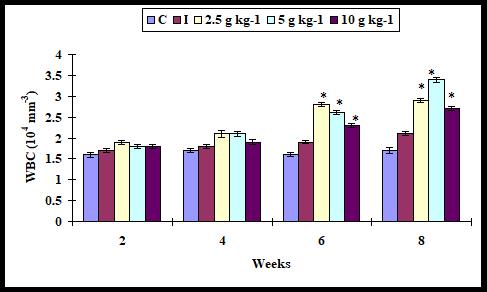
Figure 1: Total white blood cells in A. sebae fed with 0, 2.5, 5, and 10 g kg-1 E. agallocha supplementation diets against V. alginolyticus. Statistical significant difference at P < 0.05 level compared with control.
Phagocytic activity
The phagocytic activity slightly increased with all diets on weeks 2 and 4. The phagocytic activity increased significantly with 5 and 10 g kg-1 diet on weeks 6 and 8 when compared with control. However, it did not significantly increase with 2.5 g kg-1 diet (Figure 2).
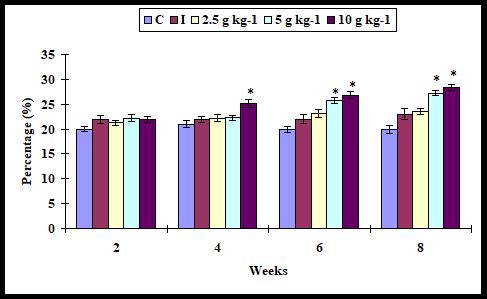
Figure 2: Phagocytic activity in A. sebae fed with 0, 2.5, 5, and 10 g kg-1 E. agallocha supplementation diets against V. alginolyticus. Statistical significant difference at P < 0.05 level compared with control.
Respiratory burst activity
The respiratory burst activity did not significantly vary in control and all supplementation diets on weeks 2 and 4. It significantly increased with all supplementation diets on weeks 6 and 8 (Figure 3).
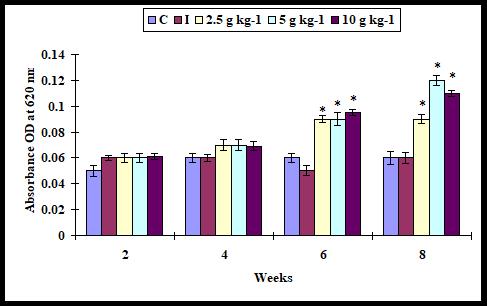
Figure 3: Respiratory burst activity in A. sebae fed with 0, 2.5, 5, and 10 g kg-1 E. agallocha supplementation diets against V. alginolyticus. Statistical significant difference at P < 0.05 level compared with control.
Alternative complement (ACH50) activity
The ACH50 activity did not increase (P > 0.05) significantly with any supplementation diet on weeks 2 and 4 whereas it was increased significantly when the infected fish fed with 5 and 10 g kg-1 diet on weeks 6 and 8. However, it was not found with 2.5 diet (Figure 4).
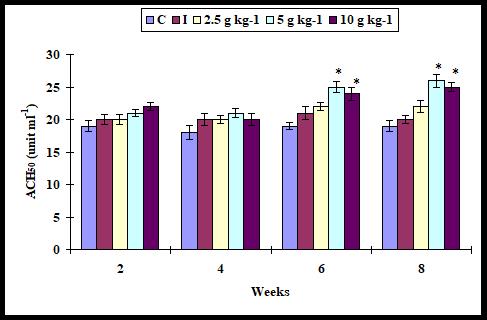
Figure 4: Alternate complement activity in A. sebae fed with 0, 2.5, 5, and 10 g kg-1 E. agallocha supplementation diets against V. alginolyticus. Statistical significant difference at P < 0.05 level compared with control.
Serum lysozyme activity
The serum lysozyme activity did not statistically increase (P > 0.05) with all supplementation diets except with 10 g kg-1 diet on weeks 2 and 4 as compared to control. It was found significant increase with 5 and 10 g kg-1 diet on weeks 6 and 8 but did not with 2.5 g kg-1 diet (Figure 5).
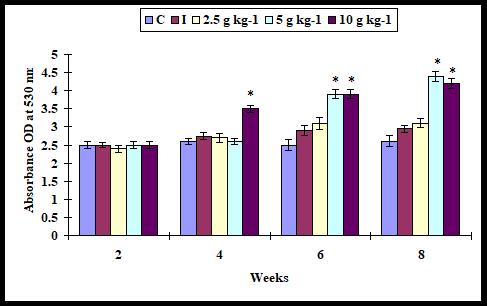
Figure 5: Serum lysozyme activity in A. sebae fed with 0, 2.5, 5, and 10 g kg-1 E. agallocha supplementation diets against V. alginolyticus. Statistical significant difference at P < 0.05 level compared with control.
Feed action on gut flora
The bacterial flora of the gut was analyzed in the experimental and control groups. The gut bacterial flora was altered due to the action of mangrove plant extract. The maximum total viable counts (TVCs) were recorded in groups fed with 2.5 and 5 g kg-1 supplementation diets with 2.2 x 105 and 1.7 x 104 (cfu/g). The TVC was 2.8 x 103 (cfu/g) in infected fish fed with 10 g kg-1 supplementation diet (Table 3).
|
Table 3: Total viable counts (cfu/g) of gut bacterial flora and Vibrio in A. sebae fed with 0, 2.5, 5, and 10 g kg-1 E. agallocha supplementation diets on week 8. |
||||||
|
Bacteria |
Sample |
C |
I |
2.5 g kg-1 |
5 g kg-1 |
10 g kg-1 |
|
Total viable gut bacterial flora counts |
|
3.4x103 |
2.2x102 |
1.8x105 |
1.0x104 |
1.2x104 |
|
Total viable Vibrio counts |
B |
0 |
1.1x103 |
0.9x102 |
0.6x101 |
0.6x101 |
|
|
S |
0 |
1.2x103 |
0.5x101 |
0.4x101 |
0.2x101 |
|
|
K |
0 |
1.4x102 |
0.6x101 |
0.4x101 |
0.2x101 |
|
B: blood, S: spleen, K: kidney. |
||||||
Disease resistance and survival
The cumulative mortality was 5% in fish fed with 5 g kg-1 diet whereas the mortality was 10% with 2.5 and 10 g kg-1 diet supplementation diets. However, the mortality rose to 90% the infected fish fed with control diet. However, there was no mortality the health fish fed with control diet (Figure 6).
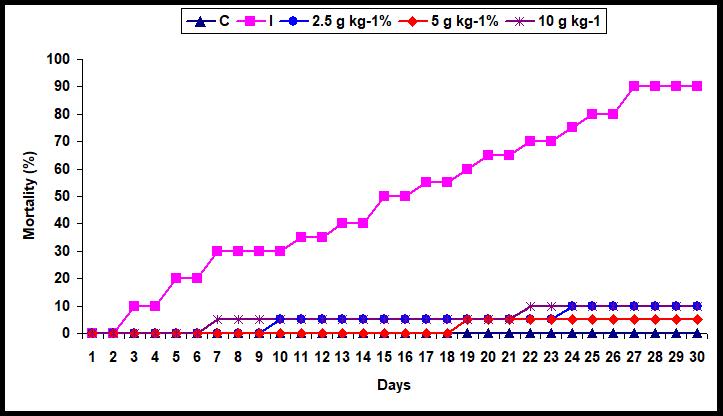
Figure 6: Mortality (%) in A. sebae (n=20) fed with 0, 2.5, 5, and 10 g kg-1 E. agallocha supplementation diets against V. alginolyticus for 30 days.
Re-isolation of bacteria from challenged fishes
When healthy fish were challenged with live V. alginolyticus manifested symptoms were loss of appetite, energy, appearance of skin erosion, and blood bleeding on the body surface. In the re-isolation experiment, the blood, spleen, and kidney were collected from dead fish and examined for the presence of Vibrio (Table 3). The bacterial count was high in infected fish fed with control diet. Similarly, the infected fish fed with 2.5 g kg-1 diet had found high bacterial count (cfu/g) in kidney, blood, and spleen (1.4 x 102, 1.1 x 103, and 1.2 x 103). However, the viable bacterial count (cfu/g) was low with 10 g kg-1 diet in blood, spleen, and kidney (0.6 x 101, 0.2 x 101, and 0.2 x 101) (Table 2).
Discussion
In aquaculture, fish are exposed to different sources of stress such as high stocking densities or manipulation. As a result of these artificial conditions, which are very different to those in the wild, their defence system weakens, making them easy target for pathogens such as viruses, bacteria, fungi, and parasites, all of which may be easily transmitted and spread rapidly leading to outbreaks of diseases. Traditional treatments such as antibiotics and chemotherapeutics create both resistant bacteria and paves the way for immunosuppression in fish [34]. Therefore, to improve aquatic animal health and to prevent diseases alternative strategies must be developed. In this regard, the traditional herbs offer a new opening in disease management since they contain natural compounds that not only boost the immunity but also have microbicidal properties. E. agallocha is a mangrove species commonly known as ‘milky mangrove’ (Vernacular: Thillai) contain a number of active constituents like alkaloid, tannin, saponin, flavonoid, glycoside, phenolic etc. [20] that possesses several biological properties [21,24-26].
Application of herbal immunostimulants is a promising area because they are biodegradable, biocompatible, and safe both for the environment and human as well as animal health [35]. Numerous studies have reported that oral administration of immunostimulants can effectively improve resistance of fishes to pathogenic bacterial, fungal, viral, and parasitic infection [8-19,36-39]. However, some researches indicate that feeding high doses of immunostimulants result in immunosuppression or feedback regulation of fish [40,41]. Hence determination of the dose which is host and pathogen specific is direly needed. These functional feed ingredients can increase disease resistance by causing up regulation of the nonspecific or specific immune system and disease resistance by, improving the host defense against pathogens.
The MWG, SGR, PER, FCR, and PI did not significantly vary with any supplementation diet but it was significantly increased with 5 g kg-1 diet on weeks 6 and 8. The better survival rate was found the infected fish fed with 5 and 10 g kg-1 diet. WBCs afford protection against microbial infections and chemical factors [42]. The WBC level slightly increased with any supplementation diets on weeks 2 and 4 while it was significantly increased with all diets on weeks 6 and 8. Monitoring these values and gathering information on the profile of leucocytes can reflect on the general immune status in fish. The differential compositions of leucocytes and increase in the neutrophiles percentage in fishes are associated with bacteria, viral or other infections [43]. Phagocytic activity is a key aspect of innate immunity, and is a part of first-line cellular nonspecific defense in vertebrates against infectious pathogens [44]. Several immunostimulants have been shown to increase the phagocytic activity in fish against bacterial, fungal, viral, and parasitic pathogens [8-19,36-39]. The phagocytic activity slightly increased any supplementation diets on weeks 2 and 4 whereas it increased significantly with 5 and 10 g kg-1 diet on weeks 6 and 8. This results represented by phagocytic ability was stimulated by herbal administration through feed. The present results after herbal administration via feed to A. sebae are in agreement with related studies in marine and freshwater fishes against viral, bacterial, fungal and parasitic infection [8-19,36-39]. Respiratory burst is considered to be an important indicator of innate defense mechanism in fish, and has been widely used to evaluate the defence ability against pathogens [45,46]. The increase in respiratory burst activity observed in the present study following administration of any supplementation diet is in accordance with previous research demonstrating that respiratory burst in different fish is stimulated after feeding with a number of herbal extracts [8-19,36-39]. On the other hand, any supplementation diet did not alter the respiratory burst on weeks and, which is in accordance with results reported in kelp grouper and olive flounder [12,13].
The alternate complement activity did not significantly get enhanced in any supplementation diet on weeks 2 and 4; however, it significantly increased with 5 and 10 g kg-1 diet on weeks 4 and 8. This is in contrast to the findings of Harikrishnan et al. [10] where oral administration of traditional Korean medicinal (TKM) supplementation diets, did not result in any change on first week in the alternate complement activity. The present result is in agreement reported in marine olive flounder in which a significant enhancement of serum complement activity was noticed after feeding with mixed herb enriched diet [11]. In addition, enhancing effect of immunostimulants depends on dosage, molecular weight, duration of feeding, environmental temperature, and the route of administration and species [9]. The bactericidal activity of the alternate complement has been well recognized as one of the key killing mechanisms of clearing bacteria in fish [47].
Leukocytes are the key components of immune system in fish [48]. The leukocyte numbers or the proportion of different cell types is affected by a number of factors such as sex, growth, life-stage, nutritional status, stressors, and bacterial infection [49]. The different types of leukocytes possess different the pattern recognition receptors (PRRs) which may bring about different immunological responses [50]. In fish, glucan receptors have been reported to exist on neutrophils [48,51] and macrophages [52]. This study showed that the infected fish fed with any supplementation diet, leukocyte counts progressively increased on weeks 2 and 4. It was found the infected fish fed with 5 and 10 g kg-1 diet on weeks 4 and 8. Herbs act as a positive regulator of resting neutrophils [18,19], that result in higher survival rate of the fish as observed in this study.
Gut microflora play an important role in the digestive process and disease susceptibility of marine feeders [53]. The wild and cultured aquatic animals harbor a diverse bacterial flora, including Aeromonas, Plesiomonas, Photobacterium, Pseudoalteromonas, Pseudomonas, Vibrio etc. [54]. So, an understanding the intestinal bacterial floral interactions of the host is of much significance for the development of a healthy cultivation environment and also to optimize the potential species growth. However, the intestinal bacteria, such as Aeromonas and Vibrio are often opportunistic invading pathogens to the host [55]. In this study, the maximum TVCs (cfu/g) recorded with 2.5 g kg-1 diet (1.4 x 102, 1.1 x 103, and 1.2 x 103) in kidney, blood, and spleen while it was low with 10 g kg-1 diet (0.6 x 101, 0.2 x 101, and 0.2 x 101) in blood, spleen, and kidney. The present results reveal that the gut microbial flora changed qualitatively and quantitatively possibly due to the active principles of present in the mangrove extract. Bacterial pathogens could have been retarded by the active extracts, by inhibiting the transcription and arresting the protein synthesis of the bacteria [56]. A similar trend was observed in the present study. This lends support to previous studies in Indian white shrimp [57] which reported that the methanolic extracts of Murraya koeniji, Psoralea corylifolia and Quercus infectoria effectively controlled the pathogens such as Salmonella, Vibrio, Yersinia, and Aeromonas. Herbal diets prepared from five different species viz. Adathoda vasika, Murraya koenigii, Ocimum basilicum, Psoralea corylifolia, and Qurcus infectoria effectively suppressed the growth of pathogens such as Pseudomonas aeruginosa, Staphylococcus aureus, Aeromonas hydrophila, Vibrio harveyi, and V. parahaemolyticus in the P. monodon by enhancing the immune system positively [56].
After being challenged with V. alginolyticus, all the treated groups showed a reduced mortality. The cumulative mortality was 5% the infected fish fed with 5 g kg-1 diet whereas the mortality was 10% with 2.5 and 10 g kg-1 diet supplementation diets. Compared with non- E. agallocha supplementation diet had 90% mortality. Enhancement of the non-specific immune parameters by the E. agallocha leaf extracts could possibly be an important factor in reducing the percentage mortality, thereby protecting the fish against the V. alginolyticu. Earlier studies have also revealed that dietary supplementation and intraperitoneal injection of various herbal extracts reduced the mortality and increased the survival rate against bacterial, viral, fungal, and parasitic infection [8-19,36-39]. Though, there is no positive correlation between the effect of immunostimulant and dosage, a higher dosage might enhance or inhibit the immune response [8-19,36-39]. Hence it is important to estimate the pattern of protection afforded by the extract to determine its efficacy as an immunostimulant. The present study demonstrate the potential of E. agallocha extract as an oral immunostimulant can enhance the innate immune immunity, gut microbial flora, and survival rate in A. sebae, although further detailed immunological and molecular studies against other pathogen are necessary in other fish species before recommendation of E. agallocha extract through feed additive.
Acknowledgement
Authors thank Prof. T. Balasubramanian, Former Dean, Faculty of Marine Science and the Annamalai University for providing facilities. First and second authors are thankful to the University Grants Commission (UGC), New Delhi for providing the financial assistance. Reference No. U.G.C. No. 33-384/2007 (SR).
References
1. Verschuere L, Rombaut G, Sorgeloos P, et al. 2000. Probiotic bacteria as biological control agents in aquaculture. Microbiology and Molecular Biology Reviews. 64: 655-671. Ref.: https://bit.ly/2OjZ1v8
2. Subasinghe RP. 2005. Epidemiological approach to aquatic animal health management: opportunities and challenges for developing countries to increase aquatic production through aquaculture. Preventive Veterinary Medicine. 67: 117-124. Ref.: https://bit.ly/2DjYRNH
3. Torrecillas S, Makol A, Caballero MJ, et al. 2007. Immune stimulation and improved infection resistance in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish and Shellfish Immunology. 23: 969-981. Ref.: https://bit.ly/2Olm2Oh
4. Austin B, Austin DA. 2007. Bacterial fish pathogens: diseases of farmed and wild fish. 4th ed. Chichester, UK: Springer-Praxis.
5. Hernández P. 2005. Responsible use of antibiotics in aquaculture. In: FAO, editor. FAO fisheries technical paper no 469, Rome. 469: 1-97. Ref.: https://bit.ly/2QMnGtO
6. Harikrishnan R, Balasundaram C. 2005. Modern trends in Aeromonas hydrophila disease management with fish. Review in Fisheries Science. 13: 281-320. Ref.: https://bit.ly/33eNYY3
7. Harikrishnan R, Balasundaram C, Heo MS. 2009. Effect of chemotherapy, vaccination and immunomodulation in goldfish, Carassius auratus against Aeromonas hydrophila. Disease of Aquatic Organisms. 88: 45-54.
8. Jin CN, Harikrishnan R, Moon YG, et al. 2010. Effectiveness of chemotherapeutants against scuticociliate Philasterides dicentrarchi, a parasite of olive flounder. Veterinary Parasitology. 168: 19-24. Ref.: https://bit.ly/35xCyjY
9. Harikrishnan R, Balasundaram C, Heo MS. 2011. Impact of plant products on innate and adaptive immune system of cultured finfish. Aquaculture. 317: 1-15. Ref.: https://bit.ly/35xHuoK
10. Harikrishnan R, Jaeyun H, Balasundaram C, et al. 2010. Effect of traditional Korean medicinal (TKM) triherb extracts on the innate immune system and disease resistance of Paralichthys olivaceus against Uronema marinum. Veterinary Parasitology. 170: 1-7. Ref.: https://bit.ly/2rnouuq
11. Harikrishnan R, Balasundaram C, Kim MC, et al. 2010. Effect of a mixed herb enriched diet on the innate immune response and disease resistance of Paralichthys olivaceus against Philasterides dicentrarchi infection. Journal of Aquatic Animimal Health. 22: 235-243. Ref.: https://bit.ly/37wIHid
12. Harikrishnan R, Kim JS, Kim MC, et al. 2011. Lactuca indica extract as feed additive enhances immunological parameters and disease resistance in Epinephelus bruneus to Streptococcus iniae. Aquaculture. 318: 43-47. Ref.: https://bit.ly/2OAuh7K
13. Harikrishnan R, Kim JS, Kim MC, et al. 2011. Prunella vulgaris enhances the non-specific immune response and disease resistance of Paralichthys olivaceus against Uronema marinum. Aquaculture. 318: 61-66. Ref.: https://bit.ly/2s7DyN4
14. Harikrishnan R, Balasundaram C, Jawahar S, et al. 2011. Solanum nigrum enhancement of the immune response and disease resistance of tiger shrimp, Penaeus monodon against Vibrio harveyi. Aquaculture. 318: 67-73. Ref.: https://bit.ly/37A5h9G
15. Harikrishnan R, Balasundaram C, Jawahar S, et al. 2012. Immunomodulatory effect of Withania somnifera supplementation diet in the giant freshwater prawn Macrobrachium rosenbergii (De Man) against Aeromonas hydrophila. Fish and Shellfish Immunology. 32: 94-100. Ref.: https://bit.ly/2XIkbWI
16. Harikrishnan R, Kim MC, Kim JS, et al. 2012. Effect of Coriolus versicolor supplementation diet on innate immune response and disease resistance in kelp grouper Epinephelus bruneus against Listonella anguillarum. Fish and Shellfish Immunology. 32: 339-344. Ref.: https://bit.ly/2DcGkmz
17. Harikrishnan R, Kim JS, Balasundaram C, et al. 2012. Effect of Magnifera indica kernel enriched feed on immune response of Penaeuus indicus against white spot syndrome virus (WSSV). Aquaculture International.
18. Harikrishnan R, Balasundaram C. 2008. In vitro and in vivo studies of the use of some medicinal herbals against fish pathogen Aeromonas hydrophila in goldfish. Journal of Aquatic Animal Health. 20: 165-176. Ref.: https://bit.ly/2QK3g4J
19. Harikrishnan R, Balasundaram C, Kim MC, et al. 2009. Innate immune response and disease resistance in Carassius auratus by triherbal solvent extracts. Fish and Shellfish Immunology. 27: 508-515. Ref.: https://bit.ly/2OhYt8T
20. Brindha P, Sasikala B, Purushothaman K. 1981. Phytochemical analysis of E. alba. BMEBR. 22: 84-96.
21. Patra JK, Gouda S, Sahoo SK, et al. 2012. Chromatography separation, 1H NMR analysis and bioautography screening of methanol extract of Excoecaria agallocha L. from Bhitarkanika, Orissa, India. Asian Pacific Journal of Tropical Biomedicine. 2: 50-56. Ref.: https://bit.ly/2pMBLw7
22. Sheikh JH, Hitoshi A, El-Sayed M, et al. 2009. Antioxidative and anti-histamine-release activities of Excoecaria agallocha L. Pharmacologyonline. 2: 927-936. Ref.: https://bit.ly/35xE71k
23. Thirunavukkarasu P, Ramkumar L, Ramanathan T. 2009. Anti-ulcer activity of Excoecaria agallocha bark on NSAID-induced gastric ulcer in Albino rats. Global Journal of Pharmacology. 3: 123-126. Ref.: https://bit.ly/2Olo2WN
24. Konoshima T, Konishi T, Takasaki M, et al. 1998. Anti-tumor-promoting activity of the Diterpene from Excoecaria agallocha. II. Biological and Pharmaceutical Bulletin. 21: 993-996.
25. Subhan N, Alam MA, Ahmed F, et al. 2008. In vitro antioxidant property of the extract of Excoecaria agallocha (Euphorbiaceae). DARU. 16: 149-154. Ref.: https://bit.ly/2OfYhXC
26. Batsa AJS, Periyasamy K. 2013. Anticancer activity of Excoecaria agallocha leaf extract in cell line model. International Journal of Pharmacy and Biological Sciences. 3: 392-398. Ref.: https://bit.ly/2Daxm9o
27. Dhayanithi NB, Ajith Kumar TT, Balasubramanian T. 2012. In vitro and experimental Screening of mangrove herbal extract against Vibrio alginolyticus in marine ornamental fish. World Academic Science Engineering and Technology. 68: 1310-1324. Ref.: https://bit.ly/2XHXgLj
28. Velmurugan S, Citarasu T. 2010. Effect of herbal antibacterial extracts on the gut floral changes in Indian white shrimp Fenneropenaeus indicus. Romanian Biotechnological letters. 15: 5709-5717. Ref.: https://bit.ly/2OFkhu9
29. Ellis AE. 1990. Lysozyme assays. In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS, Van Muiswinkel WB, eds. Techniques in Fish Immunology. SOS Publications, Fair Haven NJ. 1990: 101-103.
30. Tseng DY, Ho PL, Huang SY, et al. 2009. Enhancement of immunity and disease resistance in the white shrimp, Litopenaeus vannamei, by the probiotic, Bacillus subtilis E20. Fish and Shellfish Immunology. 26: 339-343. Ref.: https://bit.ly/2Deg6Ab
31. Sunyer JO, Tort L. 1995. Natural hemolytic and bactericidal activities of sea bream Sparus aurata serum are effected by the alternative complement pathway. Veterinary Immunology and Immunopathology. 45: 333-345. Ref.: https://bit.ly/2Ogabks
32. Matsuyama H, Mangindaan REP, Yano T. 1992. Protective effect of schizophyllan and scleroglucan against Streptococcus sp. infection in yellowtail (Seriola quinqueradiata). Aquaculture. 101: 197-203. Ref.: https://bit.ly/2OGmgyq
33. Harikrishnan R, Nisha Rani M, Balasundaram C. 2003. Hematological and biochemical parameters in common carp, Cyprinus carpio, following herbal treatment for Aeromonas hydrophila infection. Aquaculture. 221: 41-50. Ref.: https://bit.ly/35y84yk
34. Panigrahi A, Azad IS. 2007. Microbial intervention for better fish health in aquaculture: the Indian scenario. Fish Physiology and Biochemistry. 33: 429-440. Ref.: https://bit.ly/2DaonFl
35. Ortuno J, Cuesta A, Rodriguez A, et al. 2002. Oral administration of yeast, Saccharomyces cerevisiae, enhances the cellular innate immune response of gilthead seabream (Sparus aurata L.). Veterinary Immunology and Immunopathology. 85: 41-50. Ref.: https://bit.ly/2qHF4Fq
36. Harikrishnan R, Balasundaram C, Dharaneedharan S, et al. 2009. Effect of plant active compounds on immune response and disease resistance in Cirrhina mrigala infected with fungal fish pathogen, Aphanomyces invadans. Aquaculture Research. 40: 1170-1181. Ref.: https://bit.ly/2OkUybD
37. Harikrishnan R, Jaehyun H, Balasundaram C, et al. 2010. Effect of Punica granatum solvent extracts on immune system and disease resistance Paralichthys olivaceus against lymphocystsis disease virus (LDV). Fish and Shellfish Immunology. 29: 668-673. Ref.: https://bit.ly/35v8Lbr
38. Harikrishnan R, Balasundaram C, Heo MS. 2010. Potential use of probiotics- and triherbal extract-enriched diets to control Aeromonas hydrophila infection in carp. Disease of Aquatic Organisms. 92: 41-49. Ref.: https://bit.ly/2KQyGm8
39. Harikrishnan R, Kim JS, Kim MC, et al. 2011. Styrax japonica supplementation diet enhances the innate immune response in Epinephelus bruneus against bacterial and protozoan infections. Experimental Parasitology. 129: 260-265. Ref.: https://bit.ly/2pJF5rQ
40. Ranzani-Paiva MJT, Ishikawa CM, das Eiras AC, et al. 2004. Effects of an experimental challenge with Mycobacterium marinum on the blood parameters of Nile tilapia, Oreochromis niloticus (Linnaeus, 1757). Brazilian Archives of Biology and Technology. 6: 45-53. Ref.: https://bit.ly/2rkPYRs
41. Castro R, Couso N, Obach A, et al. 1999. Effect of different β-glucans on the respiratory burst of turbot (Psetta maxima) and gilthead seabream (Sparus aurata) phagocytes. Fish and Shellfish Immunology. 9: 529-541. Ref.: https://bit.ly/2XGNNDW
42. Harikrishnan R, Nisha Rani M, Balasundaram C. 2003. Hematological and biochemical parameters in common carp, Cyprinus carpio, following herbal treatment for Aeromonas hydrophila infection. Aquaculture. 221: 41-50. Ref.: https://bit.ly/2QKVZS9
43. Ranzani PM, Rodrigues EL, Veiga ML, et al. 2003. Differential leukocyte counts in “dourado”, Salminus maxillosus Valenciennes, 1840, from the Mogi-Guacu River, Pirassununga, SP. Brazilian Journal of Biology. 63: 665-672. Ref.: https://bit.ly/2DcA26i
44. Olivier G, Eaton CA, Campbell N. 1986. Interaction between Aeromonas salmonicida and peritoneal macrophages of brook trout (Salvelinus fontinalis). Veterinary Immunology and Immunopathology. 12: 223-234. Ref.: https://bit.ly/2XJt5Di
45. Secombes C, Fletcher T. 1992. The role of phagocytes in the protective mechanisms of fish. Annual Review in Fish Diseases. 2: 53-71. Ref.: https://bit.ly/2OF4UC1
46. Miyazaki T. 1998. A simple method to evaluate respiratory activity of blood phagocytes from Japanese flounder. Fish Pathology. 33: 141-142. Ref.: https://bit.ly/35ys0Rz
47. Ellis AE. 2001. Innate host defense mechanisms of fish against viruses and bacteria. Development and Comparative Immunology. 25: 827-839. Ref.: https://bit.ly/2KOJVeY
48. Pali? D, Andreasen CB, Herolt DM, et al. 2006. Immunomodulatory effects of β-glucan on neutrophil function in fathead minnows (Pimephales promelas Rafinesque, 1820). Development and Comparative Immunology. 30: 817-830. Ref.: https://bit.ly/2QUEzlU
49. Misra CK, Das BK, Mukherjee SC, et al. 2006. Effect of long term administration of dietary β -glucan on immunity, growth and survival of Labeo rohita fingerlings. Aquaculture. 255: 82-94. Ref.: https://bit.ly/2XKFUxp
50. Bricknell I, Dalmo RA. 2005. The use of immunostimulants in fish larval aquaculture. Fish and Shellfish Immunology. 19: 457-472. Ref.: https://bit.ly/2Ogbj7y
51. Ainsworth AJ. 1994. A beta-glucan inhibitable zymosan receptor on channel catfish neutrophils. Veterinary Immunology and Immunopathology. 41: 141-152. Ref.: https://bit.ly/2rlnlDG
52. Engstad RE, Robertsen B. 1994. Specificity of a β-glucan receptor on macrophages from Atlantic salmon (Salmo salar L.). Development and Comparative Immunology. 18: 397-408. Ref.: https://bit.ly/2DcOiwb
53. Venkatesalu V, Sundaramoorthy P, Anantharaj M, et al. 2004. Studies on the fatty acid composition of marine algae of Rameswaram coast. Seaweed Research Utilization. 26: 83-86.
54. Oxley APA, Shipton W, Owens L, et al. 2002. Bacterial flora from the gut of the wild and cultured banana prawn, Penaeus merguiensis. Journl of Applied Microbiology. 93: 214-223. Ref.: https://bit.ly/2KSHtns
55. Asfie M, Ishigaki T, Okano R, et al. 2000. Antibacterial abilities of microflora in the Japanese flounder (Paralichthys olivaceus) rearing aquaria. Suisanzoshoku. 48: 227-231. Ref.: https://bit.ly/2OgbzU4
56. Rao PS, Parekh KS. 1981. Antibacterial activity of Indian seaweed extracts. Botanica marina. 24: 577-582. Ref.: https://bit.ly/2rswJ8r
57. Ravikumar S, Inbaneson SJ, Suganthi P, et al. 2011. Mangrove plants as a source of lead compounds for the development of new antiplasmodial drugs from South East coast of India. Parasitology Research. 108: 1405-1410. Ref.: https://bit.ly/2DglVgC




















