Indexing & Abstracting
Full Text
Mini ReviewDOI Number : 10.36811/jvsr.2019.110004Article Views : 27Article Downloads : 34
Role of BoLA-DRB3 genetic diversity against resistance to mastitis in cattle: Review
Namita Kumari, Shubham Loat, Shallu Saini, Nitika Dhilor, Anurag Kumar and R.S. Kataria*
ICAR-National Bureau of Animal Genetic Resources, GT Road By-Pass, Karnal-132 001 (Haryana), India
*Corresponding Author: Kataria RS, ICAR-National Bureau of Animal Genetic Resources, GT Road By-Pass, Karnal-132 001 (Haryana), India, Email: katariaranji@yahoo.co.in
Article Information
Aritcle Type: Mini Review
Citation: Namita Kumari, Shubham Loat, Shallu Saini, et al. 2019. Role of BoLA-DRB3 genetic diversity against resistance to mastitis in cattle: Review. J Veterina Sci Res. 1: 30-36.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Namita Kumari
Publication history:
Received date: 28 August, 2019Accepted date: 10 September, 2019
Published date: 12 September, 2019
The major histocompatibility complex (MHC) is an organized cluster of tightly linked genes, present in all vertebrates, playing an important role in the immune system, except the jawless fish [1]. MHC was first identified during tissue transplantation studies in mice [2] and was first known for its role in histocompatibility. Consequently, the role of MHC was discovered in immune regulation [3] and several other functions [4,5]. The important function of the MHC is to code for specialized antigen-presenting receptor glycoproteins, also called as MHC molecules. The products of these genes are involved in the induction and regulation of immune response. These molecules bind processed peptide antigens and present them to T-lymphocytes, thereby triggering immune response.
The high levels of polymorphism observed in MHC region has been associated with the effectiveness of its immunological non-self or self-recognition function [6]. Cattle MHC molecules are, known as the bovine leukocyte antigen (BoLA) complex. It is located on chromosome 23 and spans approximately 2.5Mb of the cattle genome [7]. It has been estimated that the mammalian MHC contains over 200 genes. The genes are organized into three distinct classes (class I, II, and III). Each of these classes is divided into regions and sub regions, containing pseudogenes. The BoLA complex is found in two separate regions of the chromosome rather than a single cluster of genes as seen in most mammals. The larger gene cluster is located at BTA23 band 22 and apparently contains all of the bovine class I and class II genes encoding both subunits of the classical class II proteins DQ and DR. The remaining BoLA class II loci (DIB, DNA, DOB, DYA, DYB, TCP1, LMP2, LMP7, and TAP2) are located in a cluster near the centromere at BTA23 band 12-13 [7]. A major rearrangement within the class II region has led to the division of the MHC into two distinct sub-regions, such as class IIa and class IIb, on chromosome 23. The class IIa sub-region contains the functionally expressed DR and DQ genes.
Among the three DRB genes, DRB3 is believed to be functionally important [8]. The DRB3 gene is the most polymorphic class II locus in cattle and influences both the magnitude and epitope specificity of antigen-specific T-cell responses to infectious diseases. BoLADRB3.2, which is the second exon of the third DRB bovine gene, is responsible for the β1 domain of the only widely expressed DRB gene in cattle. There is an increasing efforts to characterize and document the bovine MHC allele frequencies by breed and location, because of the role of BoLA-DRB3 alleles in resistance and/or susceptibility to infectious diseases and immune response [9,10].
Structure and function of BoLA-DRB3 gene
Molecules of Class II are heterodimer glycoproteins consisting of two polypeptide chains (α and β), both anchored in the membrane. The sub designation α1, α2, etc. refers to separate domains within the MHC gene; each domain is usually encoded by a different exon within the gene, and some genes have further domains that encode leader sequences, transmembrane sequences, etc. These molecules have both extracellular regions as well as a transmembrane sequence and a cytoplasmic tail (Figure 1). The α1 and β1 regions of the chains come together to make a membrane-distal peptide-binding domain, while the α2 and β2 regions, the remaining extracellular parts of the chains, form a membrane-proximal immunoglobulin-like domain. The antigen binding groove, where the antigen or peptide binds, is made up of two α-helixes walls and β-sheet.
Figure 1: MHC class II molecule structure on antigen presenting cell membrane.
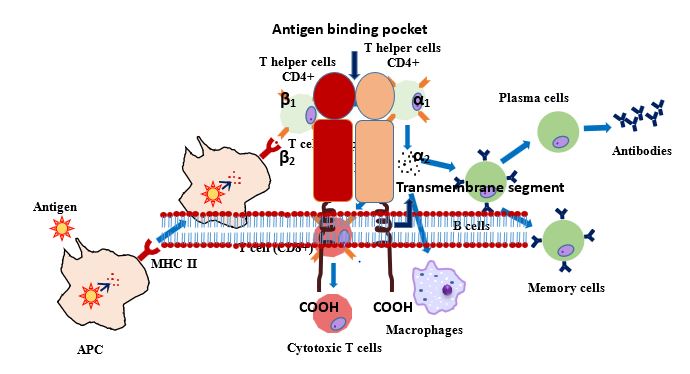
Figure 2: Schematic presentation of antigen presentation by MHC II molecules.
Under the DR region of the MHC complex DRA gene encodes α-chain, whereas highly polymorphic β chain encoded by DRB genes [11]. Three BoLA (bovine leukocyte antigen) DRB genes (DRB1, 2 and 3) are reported in bovines but only DRB3 is functional. DRB1 is a pseudogene, DRB2 is expressed at lower level and DRB3 gene is highly expressed and polymorphic as well [12]. DRB3 plays a central role in the immune system by presenting peptides derived from extracellular proteins (Figure 2). DRB3-encoded molecules share the DR alpha chain and identical DRB3 amino acid sequences in regions important for T-cell receptor interaction. However, the few differences between DRB3*0101, DRB3*0202, and DRB3*0301 change the nature of the peptide-binding pockets and account for their differences in peptide selection and presentation [13]. Class II molecules are expressed in antigen presenting cells (APC: B lymphocytes, dendritic cells, macrophages). DRB3 being a member of MHC class II genes and being polymorphic, plays significant role in disease resistance and in immune responsiveness variability [14].
Alellic diversity at BoLA-DRB3 gene locus
Numerous studies have shown that polymorphism in the genes for BoLA class II molecules determines the specificity of the immune response and plays a significant role in conferring resistance or susceptibility to various diseases. Among BoLA class II genes, BoLA-DRB3 highly polymorphic functional genes, have been found to have a stronger association with resistance/susceptibility to various diseases. Behl et al. [15], have concluded that high polymorphism in DRB3.2 locus could assist in identification of superior haplotypes for disease resistance. Takeshima et al. [16], reported high levels of polymorphism in BoLA-DRB3 and identified 5 novel BoLADRB3 alleles in Philippine native cattle. While the study provided evidence for evolutionary relationships between the cattle breeds, based on BoLA-DRB3 alleles, it was concluded that allelic information discovered would be essential to understand the correlation between MHC and diseases in East Asian cattle. However, Das et al. [17], also documented a highly polymorphic BoLA-DRB3 exon2 with significant breed-specific genetic diversities in three Bos indicus cattle breeds of South Indian, hence recommended conservation to maintain native cattle genetic diversity. In Latin American Creole cattle breeds, Giovambattista et al. [18], reported high genetic diversity of MHC DRB3 which could be as a result of multiple sources of germplasm and could be maintained by balancing selection. Takeshima et al. [19], investigated bovine MHC class II DRB3 in South American Holstein cattle populations and reported high degree of genetic diversity with a low population genetic structure.
Sun et al. [10], investigated the effect of BoLADRB3.2 polymorphisms on lameness of Chinese Holstein cows and concluded that BoLA-DRB3 exon2 might be a candidate gene for lameness susceptibility in Chinese Holstein cows. It is important to note that genotype effect at the BoLA-DRB3 could not explain variation in somatic cell count and milk yield at an extent expected of a genetic marker as reported by Oprzadek et al. [20]. Based on their study the workers concluded that the locus is just an ordinary position of the polygene, when interpreting genetic variance of somatic cell count and milk. More than 100 alleles of DRB3 gene have been identified by DNA sequencing [15], data available in the IPD database (www.ebi.ac.uk/ipd/mhc). The allele frequencies of BoLA class II genes appear to vary between breeds of cattle, such as Jersey, Holstein, Argentine Creole, Japanese Shorthorn, Japanese Black, and Brazilian dairy Gir. Thus, estimating the frequency of BoLA class II alleles can help to identify the influence of selective forces such as symmetric balancing selection and positive selection, and differentiate between various populations of cattle [21,22].
Association of BoLA- DRB3 with mastitis
The proteins that are important for the functioning of immune system are encoded by MHC [23] and DRB locus is the most polymorphic among the MHC genes [24]. The DRB3 locus exists in the antigen presenting site and any variability in this region may lead to variability in the immune responsiveness of different individuals to particular pathogens. Due to this reason, the importance of the study of polymorphism of this locus has increased [25].
The MHC has been acknowledged to regulate the progression of many infectious diseases, so it is implied that development of markers for these loci may prove helpful in pinpointing superior haplotypes for disease resistance if the association between the trait and these markers can be established. The upstream regulatory region (URR) of the DRB3 gene lies approximately 200 bp upstream of the transcriptional start site and it has strong promoter/enhancer activity. This URR of the DRB3 gene consists of a series of sequence motifs like W, X, Y, CCAAT and TATA boxes. Since these motifs are highly conserved in all MHC Class II genes, their positions as well as spacings are crucial for precise transcription of BoLA genes [15]. DRB3 exon 2 is highly polymorphic with >100 identified alleles and encode the antigen recognition site of the DR [26]. The ability of DRB3 to exhibit high polymorphism makes it a strong candidate to be used as a marker in molecular genetics and phylogenetic studies [27].
A number of studies have reported the association of one or more of the BoLADRB3.2 alleles with susceptibility/resistance to the infectious diseases in cattle. Kulberg et al., [28], studied the association of BoLA-DRB3.2 alleles with clinical mastitis in Norwegian Red cows. Genotyping of bovine leucocyte antigen DRB3.2 (BoLA-DRB3.2) in a total of 523 Norwegian Red (NR) cows from two groups selected for high protein yield and low clinical mastitis, respectively, identified 27 previously reported BoLA-DRB3.2 alleles across the groups. Significant differences in BoLADRB3.2 allele frequencies were found between the selection groups. Contradictory results from different studies investigating associations between BoLADRB3.2 alleles and mastitis indicate that future studies should focus on associations of mastitis with BoLA haplotypes rather than with single BoLA genes.
Characterization of different allelic variants of the MHC class-II gene of DRB3.2 has been investigated in mastitis and healthy cattle using PCR- RFLP by Nandedkar et al. [29]. They identified specific genotype for mastitis and healthy animals. Their study gives evidences of usefulness of DRB3 locus allelic diversity in marker assisted selection of animals on the basis of mastitis susceptible and resistant genotypes. Association analysis of BoLADRB3 alleles with mastitis resistance and susceptibility in Japanese Holstein cows have been carried out by Yoshida et al. [30]. On the basis of somatic cell count they classify the animal into healthy and mastitis affected cow and they reported specific alleles associated with resistance to mastitis. Oprzadek et al. [31], evaluated the suitability of the BoLA-DRB3 gene polymorphism to define the phenotypic value of somatic cell count of 808 Polish Holstein cows. The cows were fathered by 190 sires. Significant relationship between the occurrence of the BoLA-DRB3 gene alleles and somatic cell count were reported. Recently, Suprovych et al., [32], explored the BoLADRB3 gene polymorphism in the two cattle breeds having commercial importance: Ukrainian black-pied dairy (UBPD) and the Ukrainian red-pied dairy (URPD) and its association with mastitis. For genotyping, PCRRFLP technique was applied using RsaI, HaeIII and XhoII restriction enzymes by the workers. They observed that in 276 UBPD cows total 32 alleles were present, out of which six alleles (*03, *08, *10, *22, *24 and *28) were identified having a frequency of more than 5% (total amount of 50.4%). The most frequently occurring allele was BoLA-DRB3.2*24 with a frequency of 19.2% and they reported that four BoLA-DRB3.2 were truly associated with mastitis namely *24 and *26 with susceptibility and *13 and *22 with resistance. Similarly, in 117 URPD cattle, they identified 22 alleles from which the most frequent alleles were *07, *22, *11, *24, *01, *03 and *16 (total frequency 64.5%). Allele BoLA-DRB3.2*07 (present in 25.6% of cows) was the most commonly found. In this population, four alleles truly associated with mastitis were identified. Animals susceptible to mastitis were having alleles *07 and *08, and resistant animals had alleles *22 and *24.
Karthikeyan et al., [33,34], have investigated the genetic variability of mastitis resistance and concluded that it may be attributed by the polygenic control of the many involved genes. Polygenic nature of this trait initiated the need to explore the candidate genes which are responsible for mastitis resistance which may aid in producing the herd with the animals having genetically mastitis resistance alleles.
Though lot of work has been done to understand the genetic diversity at MHC class II DRB locus in cattle, but complexity of the structure in terms of duplications and high levels of polymorphism poses a challenge and makes it difficult to work on these molecules. At least most clear about DRB3 locus is that the higher allelic diversity in a herd is definitely linked to the fitness of the animals. Intense breeding programs are probably leading to loss of genetic diversity at this locus and thus appearance of mastitis and other infectious diseases is more common now. Therefore, periodic screening of animals for allelic diversity and introduction of animals, bulls in particular having desirable alleles should implemented. For that reason, native cattle breeds of tropical regions which are not under intensive selection program have an upper hand.
References
1. Tizard IR. 2004. Acquired immunity: antigenpresenting receptors. Veterinary immunology: an introduction. Elsevier, Philadelphia, PA, USA. 153: 67-77.
2. Gorer PA. 1937. The genetic and antigenic basis of tumour transplantation. The Journal of Pathology and Bacteriology. 44: 691-697. Ref.: https://bit.ly/2k6Spnv
3. Benacerraf B, McDevitt HO. 1972. Histocompatibility-linked immune response genes. Science. 175: 273-279. Ref.: https://bit.ly/2k2iR1o
4. Bonner JJ. 1986. Major histocompatibility complex influences reproductive efficiency: evolutionary implications. Journal of craniofacial genetics and developmental biology. Supplement. 2: 5-11. Ref.: https://bit.ly/2kpuhg9
5. Penn DJ, Potts WK. 1999. The evolution of mating preferences and major histocompatibility complex genes. The American Naturalist. 153: 145-164. Ref.: https://bit.ly/2kppueC
6. Amills M, Jiménez N, Jordana J, et al. 2004. Low diversity in the major histocompatibility complex class II DRB1 gene of the Spanish ibex, Capra pyrenaica. Heredity. 93: 266. Ref.: https://go.nature.com/2kzMSWI
7. Rothschild MF, Skow L, Lamount SJ. 2000. The major histocompatibility complex and its role in disease resistance and immune responsiveness. In: Axford RFE, Bishop SC, W. Nicholas F, Owen JB, editors. Breeding for Disease Resistance in Farm Animals. Wallingord, Wash, USA: CAB International. 243-252.
8. Takeshima SN, Aida Y. 2006. Structure, function and disease susceptibility of the bovine major histocompatibility complex. Animal Science Journal. 77: 138-150. Ref.: https://bit.ly/2kzNgVa
9. Carignano HA, Beribe MJ, Caffaro ME, et al. 2017. BOLA?DRB 3 gene polymorphisms influence bovine leukaemia virus infection levels in Holstein and Holstein× Jersey crossbreed dairy cattle. Animal genetics. 48: 420-430. Ref.: https://bit.ly/2m1f70J
10. Sun L, Song Y, Riaz H, et al. 2013. Effect of BoLA-DRB3 exon2 polymorphisms on lameness of Chinese Holstein cows. Molecular biology reports. 40: 1081-1086. Ref.: https://bit.ly/2lJ4vUo
11. Zhao Y, Xu H, Shi L, et al. 2011. Polymorphisms in Exon 2 of MHC class II DRB3 gene of 10 domestic goats in southwest China. Asian-Australasian Journal of Animal Sciences. 24: 752-756. Ref.: https://bit.ly/2kBxOaZ
12. Wei K, Zhang Z, Wang X, et al. 2010. Lineage pattern, trans-species polymorphism, and selection pressure among the major lineages of feline Mhc-DRB peptide-binding region. Immunogenetics. 62: 307-317. Ref.: https://bit.ly/2kpuW16
13. Dai S, Crawford F, Marrack P, et al. 2008. The structure of HLA-DR52c: comparison to other HLA-DRB3 alleles. Proceedings of the National Academy of Sciences. 105: 11893-11897. Ref.: https://bit.ly/2kqWCmf
14. Radwan J, Biedrzycka A, Babik W. 2010. Does reduced MHC diversity decrease viability of vertebrate populations?. Biological conservation. 143: 537-544. Ref.: https://bit.ly/2lYZLKa
15. Behl JD, Verma NK, Tyagi N, et al. 2012. The major histocompatibility complex in bovines: a review. ISRN veterinary science. Ref.: https://bit.ly/2kBNBGL
16. Takeshima SN, Miyasaka T, Polat M, et al. 2014. The great diversity of major histocompatibility complex class II genes in Philippine native cattle. Meta gene. 2: 176-190. Ref.: https://bit.ly/2lFS0su
17. Das DN, Sri Hari VG, Hatkar DN, et al. 2012. Genetic diversity and population genetic analysis of bovine MHC class II DRB3. 2 locus in three Bos indicus cattle breeds of Southern India. International journal of immunogenetics. 39: 508-519. Ref.: https://bit.ly/2lJ4TCk
18. Giovambattista G, Takeshima SN, Ripoli MV, et al. 2013. Characterization of bovine MHC DRB3 diversity in Latin American Creole cattle breeds. Gene. 519: 150-158. Ref.: https://bit.ly/2k65o8P
19. Takeshima SN, Giovambattista G, Okimoto N, et al. 2015. Characterization of bovine MHC class II DRB3 diversity in South American Holstein cattle populations. Tissue antigens. 86: 419-430. Ref.: https://bit.ly/2kzNRGo
20. Oprzadek J, Sender G, Pawlik A, et al. 2015. Locus BoLA-DRB3 is just an ordinary site of the polygene when explaining genetic variance of somatic cell count and milk yield. Journal of Dairy Research. 82: 449-452. Ref.: https://bit.ly/2m4kA73
21. Takeshima S, Saitou N, Morita M, et al. 2003. The diversity of bovine MHC class II DRB3 genes in Japanese Black, Japanese Shorthorn, Jersey and Holstein cattle in Japan. Gene. 316: 111-118. Ref.: https://bit.ly/2lD2vwT
22. Takeshima S, Chen S, Miki M, Kado M, et al. 2008. Distribution and origin of bovine major histocompatibility complex class II DQA1 genes in Japan. Tissue Antigens. 72: 195-205. Ref.: https://bit.ly/2k6XqfI
23. Othman OE, Ahmed S. 2010. Genetic Polymorphism of BoLA-DRB3 Exon 2 in Egyptian Buffalo. Genes, Genomes and Genomics. 4: 70-73. Ref.: https://bit.ly/2kzceE7
24. Maillard J C, Martinez D, Bensaid A. 1996. An Amino Acid Sequence Coded by the Exon 2 of the BoLA DRB3 Gene Associated with a BoLA Class I Specificity Constitutes a Likely Genetic Marker of Resistance to Dermatophilosis in Brahman Zebu Cattle of Martinique (FWI) a. Annals of the New York Academy of Sciences. 791: 185-197. Ref.: https://bit.ly/2lZ0vPs
25. Bot J, Karlsson LJE, Greef J, et al. 2004. Association of the MHC with production traits in Merino ewes. Livestock Production Science. 86: 85-91. Ref.: https://bit.ly/2lJFvvU
26. Schwab AE, Geary TG, Baillargeon P, et al. 2009. Association of BoLA DRB3 and DQA1 alleles with susceptibly to Neospora caninum and reproductive outcome in Quebec Holstein cattle. Veterinary parasitology. 165: 136-140. Ref.: https://bit.ly/2kzbdvG
27. Untalan PM, Pruett JH, Steelman CD. 2007. Association of the bovine leukocyte antigen major histocompatibility complex class II DRB3* 4401 allele with host resistance to the Lone Star tick, Amblyomma americanum. Veterinary parasitology. 145: 190-195. Ref.: https://bit.ly/2m4kZq5
28. Kulberg S, Heringstad B, Guttersrud OA, et al. 2007. Study on the association of BoLA?DRB3. 2 alleles with clinical mastitis in Norwegian Red cows. Journal of Animal Breeding and Genetics. 124: 201-207. Ref.: https://bit.ly/2kBOAqr
29. Nandedkar PV, Bagale SS, Shaikh GM, et al. 2017. MHC Allele Polymorphism in Cattle Mastitis. Indian Research Journal of Extension Education. 36-41. Ref.: https://bit.ly/2lDsPqG
30. Yoshida T, Furuta H, Kondo Y, et al. 2012. Association of BoLA-DRB3 alleles with mastitis resistance and susceptibility in Japanese Holstein cows. Animal science journal. 83: 359-366. Ref.: https://bit.ly/2k66Yrh
31. Oprzadek JM, Brzozowska AM, Urtnowski P, et al. 2018. Association of BoLA-DRB3 genotype with somatic cell count in milk of Polish Holstein cattle. Revista Brasileira de Zootecnia: 47. Ref.: https://bit.ly/2kz3IFb
32. Suprovych TM, Suprovych MP, Koval TV, et al. 2018. BoLA-DRB3 gene as a marker of susceptibility and resistance of the Ukrainian black-pied and red-pied dairy breeds to mastitis. Regulatory Mechanisms in Biosystems. 9: 363-368. Ref.: https://bit.ly/2kzcYJp
33. Karthikeyan A, Radhika G, Aravindaksham TV, et al. 2016. Genetic basis of mastitis resistance in cattle. International Journal of Science, Environment and Technology. 5: 2192-2199.
34. Lovegrove JA, Griffin BA, Juturu, et al. 2013.The acute and long-term effects of dietary fatty acids on vascular function in health and disease. CurrOpinClinNutrMetab Care. 16: 162-167. Ref.: https://bit.ly/2m7amTy




















