Indexing & Abstracting
Full Text
Original ArticleDOI Number : 10.36811/jcshd.2021.110022Article Views : 55Article Downloads : 24
Stress testing-guided assessment of coronary artery bypass grafts patency, at 6 months of follow up. A prospective study
Boukhmis Abdelkader1* and Nouar Mohamed El-Amin2
1Department of cardiac surgery, University Hospital Center MUSTAPHA BACHA. Faculty of Medicine. University Benyoucef Benkhedda of Algiers, Algeria
2Department of cardiac surgery, University Hospital Center MUSTAPHA BACHA. Faculty of Medicine, University Benyoucef Benkhedda of Algiers, Algeria
*Corresponding Author: Boukhmis Abdelkader, Department Of Cardiac Surgery, University Hospital Center Mustapha Bacha, Faculty of Medicine. University Benyoucef Benkhedda of Algiers, Algeria, Phone: 00213661435583; Email: kaderboukhmis@yahoo.fr
Article Information
Aritcle Type: Original Article
Citation: Boukhmis Abdelkader, Nouar Mohamed El-Amin. 2021. Stress testing-guided assessment of coronary artery bypass grafts patency, at 6 months of follow up. A prospective study. J Cardiovasc Surg Heart Dis. 3: 21-29.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2021; Boukhmis Abdelkader
Publication history:
Received date: 20 September, 2021Accepted date: 16 October, 2021
Published date: 19 October, 2021
Abstract
Purpose: To assess the coronary bypass grafts patency and the repeat revascularization rate, six months after coronary artery bypass grafting (CABG).
Methods: We prospectively enrolled 145 consecutive patients undergoing isolated CABG between June 2014 and June 2016. We performed at 6 months of follow up a coronary computed tomography angiography (CTA) in patients whose stress tests were negative and an invasive coronary angiography (ICA) in the opposite case.
Results: A total of 134 CTA and 11 ICA were performed, allowing the analysis of 321 grafts, including 143 left internal thoracic arteries (LITA), 89 right internal thoracic arteries (RITA) and 89 saphenous veins grafts (SVG). The average graft patency was 95.1% for LITA, 84.3% for RITA and 64% for SVG. The best patencies were obtained when these grafts were anastomosed to the left anterior descending artery (LAD): 96.3% for LITA, and 87.5% for RITA. SVG patency was homogeneous whether between the main right coronary artery and its branches (63.4% versus 65% respectively. p = 1), or between circumflex and RCA (72.7% versus. 63.9% respectively. p=0.6). On the right and circumflex coronary arteries, the patency of the SVG was significantly lower than that of RITA (66.26% versus 83.95% respectively, p = 0.011). At 6 months of follow up, the repeat revascularization rate was 2.07% (n=3/145).
Conclusions: 6 months after CABG, RITA and LITA had good patencies especially on LAD, while SVG was occluded in almost a third of cases. On the circumflex and right coronary arteries, SVG patency was significantly lower than that of RITA.
Keywords: Coronary Artery Bypass; Exercise Testing; Coronary Angiography; Computed Tomography Angiograph
Background
Coronary bypass graft failure reduces long-term survival and increases recurrence of angina as well as iterative revascularizations [1]. This multifactorial phenomenon amplifies over time due to thrombosis, intimal hyperplasia and atherosclerosis [2]. In the 1980's, it was recognized that long-term survival was enhanced in patients undergoing coronary surgery when the left anterior descending (LAD) was grafted with a left internal thoracic artery (LITA) rather than a saphenous vein graft (SVG) [3]. This difference was predicated, at least in part, due to greater and more durable patency of the LITA compared to an increased early occlusion rate and later progressive atherosclerosis of SVG [4]. Nowadays, widespread use of the SVG continues despite its disappointing long-term patency and the right internal thoracic artery (RITA) is still marginalized although being biologically identical to LITA. This is due to the fact that the harvesting of both internal thoracic arteries (ITAs) exposes to the risk of deep sternal wound infections and requires a longer learning curve. The evaluation of the different grafts patency, according to their target coronary arteries and their configurations, will guide the choice of grafts and thus improve coronary artery bypass grafting (CABG) outcomes. Until now, the gold standard of the coronary imaging remains the invasive coronary angiography (ICA). However, current coronary computed tomography angiographies (CTA) have emerged as an accurate diagnostic tool of coronary artery bypass grafts occlusion and stenosis, with greater patient acceptance than the conventional ICA [5].
Aims of this study
The purpose of this angiographic study was to assess the coronary artery bypass grafts patency, at 6 months of follow up.
Methods
This mono-centric, observational and prospective study, of patients undergoing CABG surgery, was conducted between June 2014 and June 2016. It was approved by the institutional review board of the Faculty of Medicine of Algiers. Written and informed consent for stress testing and coronary angiography was obtained from all participants.
a. Sample size and power of the study:
The overall rate of graft patency in the on-pump group of Randomized On/Off Bypass trial (ROOBY) was 87.8% at 1 year of follow-up.We estimated that a sample size of 233 arterial and venous grafts was needed for 90% power, 5% margin of error and an anticipated overall rate of graft patency of 94% at 6 months of follow up.
b. Inclusion and exclusion criteria:
Patients ≥ 18 years of age undergoing isolated CABG surgery were eligible for enrollment. Exclusion criteria included: (1) prior cardiac surgery; (2) CABG associated to another heart disease, (3) allergy to radio contrast; (4) renal insufficiency with a glomerular filtration rate < 30 mL min−1; (5) pregnant (6) prior chest irradiation; and (7) co-morbid illness likely to reduce life expectancy to < 6 months.
c. Primary and Secondary endpoints:
The primary endpoint of this study was the proportion of patent LITAs, RITAs and SVGs 6 months after CABG. Secondary endpoint was repeat revascularization rate, 6 months after surgery.
d. Surgical technique:
A standard median sternotomy was performed in all patients. All ITAs were harvested as skeletonized grafts. Radial and gastro-epiploic arterial grafts were not used in our daily practice. SVG grafts were classically harvested without their fat tissue. We have used a single aortic cross-clamp with antegrade blood cardioplegia to construct all distal and proximal anastomoses. On pump CABG was our procedure of choice except for heavily calcified aortas, patients with hemopathy or severe left ventricular dysfunction for which off pump CABG was performed.
e. Stress testing and coronary imaging at 6 months of follow up:
Asymptomatic patients benefited from an exercise electrocardiogram while myocardial perfusion scintigraphy was performed in patients with angina or in asymptomatic patients with intermittent claudication or with positive or inconclusive exercise electrocardiogram. The coronary bypass grafts patency has been assessed by a CTA in patients whose stress tests were negative and by an ICA in the opposite case. Coronary grafts were evaluated according to the Fitzgibbon classification [1], which classifies the grafts as: A (Excellent), B (Fair: graft with a size ?50% of that of the bypassed coronary artery) or 0 (occluded).
f. Statistical analysis:
The qualitative variables were expressed in absolute and relative frequencies, while the quantitative variables were expressed with means and standard deviations. Differences between grafts potencies were compared with Fischer’s exact tests. The p value was considered statistically significant if <0.05.
Results
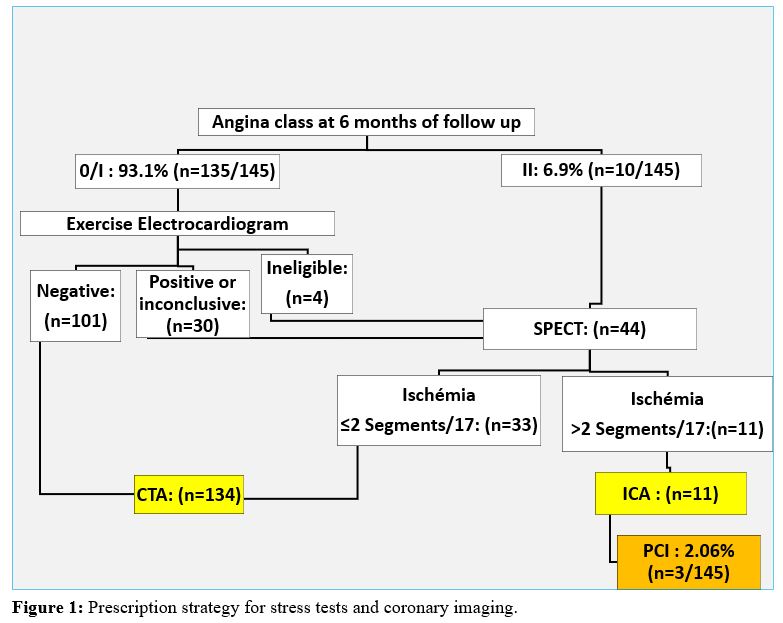
A total of 145 consecutive patients (119 men and 26 women), with an average age of 59.15 +/- 9.6 (37 to 84) years; who underwent isolated CABG six months before, were enrolled prospectively. Although CABG on-pump was our procedure of choice, 6 patients were operated on off-pump due to extensive aortic calcifications (n = 5) or preoperative thrombocytopenia (n = 1). The mean number of distal anastomosis per patient was 2.4 +/- 0.8 (1 to 5). Bilateral internal thoracic artery (BITA) grafting was performed in 61.4% (n = 89/145) of patients, more frequently with insitu configuration than with a composite Y-graft (75.3 % versus 24.7%). At 6 months of follow up, 93.1% (n=135/145) patients were asymptomatic among which 131 patients underwent exercise electrocardiogram, while 03 patients with a left Bundle Branch Block and 01 patients with intermittent claudication underwent a myocardial perfusion scintigraphy. This myocardial perfusion single photon emission computed tomography (SPECT) was also performed in 10 patients with angina class II (Canadian cardiovascular. society classification) and 30 asymptomatic patients whose exercise electrocardiogram testing was positive or inconclusive. One hundred forty-five patients benefited from 134 CTA and 11 ICA; which allowed the analysis of 321 coronary grafts (143 LITA, 89 RITA and 89 SVG). (Figure 1). Baseline characteristics of patients are summarized in table 1.
Abbreviations
CTA: Coronary Computed Tomography Angiography;
ICA: Invasive Coronary Angiography;
PCI: Percutaneous Coronary Intervention;
SPECT: Single Photon Emission Computed Tomography.
After this short follow-up, we found that the SVGs were either perfectly patent (type A), or completely occluded (type O), with no sign of intermediate stenosis, whether anastomotic or truncal. Furthermore, 05 LITAs and 04 RITAs presented a “string sign”, however their sizes were at all points greater than 50% of that of the target coronary artery. Since these ITA grafts did not meet the definition of type B, they were classified as type A.
|
Table I: Baseline characteristics of the patients having benefited from coronary imaging. |
|
|
Total patients |
145 |
|
Male sex |
82.1% |
|
Age (Year) |
59.15 +/- 9.6 (37 to 84) |
|
Hypertension |
66.9% (n = 97) |
|
Medically treated diabetes |
61.4% (n = 89) |
|
Previous smoker |
59.3% (n = 86) |
|
Hyperlipidemia |
57.9% (n = 84) |
|
Body-mass index ≥ 30 Kg/m² |
27,6 % (n = 40) |
|
Previous stroke |
4.8% (n = 7) |
|
Angina |
88.3% (n = 128) |
|
LVEF?50% |
60% (n=87) |
|
LVEF=30-50% |
36.5% (n = 53) |
|
LVEF?30% |
3.4% (n = 5) |
|
Left main disease |
21.4% (n = 31) |
|
Three-vessel disease |
48.3% (n = 70) |
|
Abbreviations: BITA: Bilateral Internal Thoracic Artery; LVEF: Left Ventricular Ejection Fraction; NYHA: The New York Heart Association Functional classification. |
|
a. Primary end points
Patency rate of internal thoracic arteries (ITAs) grafts: The average ITAs patencies were 95.1% for LITA and 84.3% for RITA. The best patencies were obtained when these grafts were anastomosed to the left anterior descending artery (LAD): 96.3% for LITA, and 87.5% for RITA (Table 2).
|
Table 2: Coronary grafts patencies at the 6th postoperative month. |
||
|
Target coronary arteries |
Coronary bypass grafts |
% (Patent / Total) |
|
LAD |
LITA |
96.3 (130/135) |
|
RITA |
87.5 (7/8) |
|
|
Diagonales |
LITA |
100 (5/5) |
|
SVG |
33.3 (2/6) |
|
|
OM |
RITA |
83.9 (52/62) |
|
LITA |
75 (6/8) |
|
|
SVG |
72.7 (16/22) |
|
|
Main RCA |
SVG |
63.41 (26/41) |
|
RITA |
66.7 (2/3) |
|
|
RCA branche |
SVG |
65 (13/20) |
|
RITA |
87.5 (14/16) |
|
|
Abbreviations: LAD: Left Anterior Descending; LITA: Left Internal Thoracic Artery; OM: Obtuse Marginal Arteries; RCA: Right Coronary Artery; RITA: Right Internal Thoracic Artery; SVG: Saphenous Vein Graft. |
||
Indeed, the average patency of the 2 ITAs was significantly higher when they were dedicated to LAD compared to circumflex marginal arteries (95.8% versus 82.8% respectively, p = 0.002. Odds ratio = 0.213. 95% confidence interval (CI) [0.062; 0.649]. Fisher's exact test). However, the patency of RITA did not present a significant difference between right and circumflex coronary arteries (84.2% versus 83.9% respectively. p=1. Odds Ratio: 1.025. 95% CI [0.224; 6.496]. Fisher's exact test). RITA patency was 93.10% in BITA-Y grafting and only 80% with in situ BITA grafting. Moreover, the BITA-Y grafting allowed us to achieve significantly more distal arterial anastomosis per patient than the in situ BITA grating (2.77+/-0.84 Versus 2.06+/-0.24 respectively, p?0.0001).
Patency rate of SVGs
We noted that the average patency of SVG was significantly lower than that of all ITA grafts (64.04% versus 91.10% respectively. p<0.0001. Odds ratio= 0.174. 95% CI [0.088-0.338]. Fisher's exact test). It was also significantly lower than that of RITA on RCA and circumflex marginal arteries (66.26% versus 83.95% respectively. p = 0.011. Odds ratio = 0.377, 95% CI [0.163-0.837]. Fisher's exact test) (Table 2). SVG patency was homogeneous regardless of its target coronary artery. Thus, it was not significantly different whether between the main RCA and its branches (63.4% versus 65% respectively. p = 1. Odds ratio = 0.934. 95% CI [0.255-3.226]. Fisher's exact test), or between OM and RCA (72.7% versus. 63.9% respectively. p=0.6. Odds ratio = 1.497. 95% CI [0.467-5.374] Fisher's exact test). (Table 2). Note that all patients received only Aspirin postoperatively and that all SVGs were harvested according to the classical method without their fat tissue.
b. Secondary end point:
Among the 145 patients who underwent a coronary imaging at 6 months of follow-up, only 3 patients (2.07%) underwent a percutaneous coronary intervention (PCI) (Figure 1). The other 08 patients whose stress tests were positive and underwent an ICA did not need iterative revascularization because their grafts were patent and their native coronary arteries did not show any evolution of atherosclerosis. These patients underwent incomplete revascularization related to highly calcify coronary branches with poor run-off or less than 1 millimeter lumen. Their symptoms were controlled with an optimal medical treatment.
Discussion
This study objectified that after 6 months of follow-up, the occlusion rate of SVG is alarming, since 35.96% of patients having undergone an arterio-venous CABG had an occluded venous graft, against 33% in the multicenter study "RIGOR" [6]. We also noted that, in the short term, the SVG patency obeys the law of "All or nothing", which suggests that, in this time interval, their occlusion is due to a thrombotic mechanism. This occlusion mechanism seems to intervene early since Bassri noted that after the first postoperative week, SVG occlusion rate is around 10% [7]. Furthermore, Goldman [8] objectified that if a SVG was patent at 1 week, that graft had a 68% of being patent at 10 years. This clearly demonstrates that the early postoperative period is crucial for the patency of venous grafts. In 2016, ACC / AHA Guideline [9] suggest that dual antiplatelet therapy (Aspirine with clopidogrel initiated early postoperatively) for 12 months after CABG may be reasonable to improve vein graft patency. Our patients were operated before the publication of these guidelines and therefore received only Aspirin throughout their follow-up. This may partly explain their high rate of SVGs occlusion. Later; the SVGs which represent the overwhelming majority of conduits used worldwide for routine CABG, are also subject to midterm fibro-muscular changes and late atheromatous occlusion [2,8]. Lopes objectified in a randomized controlled trial [10] that the rates of vein-graft failure at 12 to 18 months was 46.7% after endoscopic harvesting and 38% after direct vision harvesting. The SVGs used in the population of the present study were skeletonized according to the conventional open technique. This can partly explain the occlusion of a third of them after Sousa et al [11] demonstrated that the “No Touch” harvesting significantly improves the patency of SVGs compared to the conventional technique. The excellent early patency of LITA to LAD, noted in our study and by others authors [7, 8,12], confirms its status as cornerstone of CABG surgery. The present study objectified that the patency of RITA is significantly higher than that of SVG on the right and circumflex coronary territories, thus corroborating the finding of Tatoulis in his angiographic study [12]. RITA must therefore be the graft of choice to revascularize these arteries, apart from contraindications to the simultaneous harvesting of both ITAs (Obese and diabetic women, chronic bronchitis) or in the case of uncritical stenosis of the RCA [13].
Tatoulis noted that LITA and RITA, which are biologically similar, had comparable patencies for the same target coronary. This finding, confirms the safety of BITA grafting with in situ crossover RITA to LAD, and offers the surgeon more flexibility to revascularize all the left coronary arteries, including the distal marginal one, using only 02 ITAs with an insitu configuration. Although it has been shown that the patency of the RITA is better with BITA-Y configuration than with that of the insitu BITA [14], the statistical power of our study was not high enough to confirm this difference. Indeed, BITA-Y grafts can easily reach their coronary targets and allows trimming off the distal end of ITAs, known to be more spastic [15]. Glineur objectified in a recent randomized trial that this better patency of RITA with BITA-Y grafting resulted in fewer major adverse cardiovascular and cerebrovascular events, compared to the in situ BITA configuration [16]. Since LITA-RITA Y grafting makes it possible to achieve significantly more distal arterial anastomosis without affecting the patency of its grafts, total arterial revascularization is possible with this configuration, which saves the surgeon the need for another arterial graft. We also found that the two ITA grafts had better patency on LAD than that on the circumflex marginal arteries. This was previously noted by Tatoulis [4] and can be explained by the greater operative difficulty encountered during revascularization of the circumflex marginal arteries, as well as by the possibility of occurrence of tension and or torsion in the ITA grafts, especially when crossing the transverse sinus.
Limitation of this study:
The non-consent of 68 asymptomatic patients (30.36%. n=68/224) to underwent stress testing and coronary imaging may have reduced mean patency of the different coronary bypass grafts. The statistical power of this study is not sufficient enough to detect a statistically significant difference between LITA and RITA patencies for same target coronary arteries, as well as the impact of the configuration of BITA grafting (BITA-Y or in situ BITA) on the patency of RITA.
Conclusions
At 6 months of follow up, RITA and LITA had good average patencies, especially on LAD, while SVG was occluded in almost a third of cases. On the circumflex and right coronary arteries, RITA patency was significantly higher than that of SVG and may therefore be the graft of choice to revascularize these territories. These findings should encourage the use of bilateral internal thoracic artery grafting except in patients at risk of developing deep sternal wound infections.
List of abbreviations
BITA: Bilateral Internal Thoracic Artery
CABG: Coronary Artery Bypass Grafting
CTA: Computed Tomography Angiography
ICA: Invasive Coronary Angiography
ITAs: Internal Thoracic Arteries
LAD: Left Anterior Descending
LITA: Left Internal Thoracic Artery
OM: Obtuse Marginal Artery
RCA: Right Coronary Artery RITA: Right Internal Thoracic Artery
SPECT: Single Photon Emission Computed Tomography
SVG: Saphenous Vein Graft
References
1. Fitzgibbon GM, Kafka HP, Leach AJ. 1996. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 28: 616-626. Ref.: https://pubmed.ncbi.nlm.nih.gov/8772748/ DOI: https://doi.org/10.1016/0735-1097(96)00206-9
2. Motwani JG, Topol EJ. 1998. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 97: 916-931. Ref.: https://pubmed.ncbi.nlm.nih.gov/9521341/ DOI: https://doi.org/10.1161/01.cir.97.9.916
3. Loop FD, Lytle BW, Cosgrove DM. 1986. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 314: 1-6. Ref.: https://pubmed.ncbi.nlm.nih.gov/3484393/ DOI: https://doi.org/10.1056/nejm198601023140101
4. Tatoulis J, Buxton BF, Fuller JA. 2004. Patencies of 2,127 Arterial to Coronary Conduits Over 15 Years. Ann Thorac Surg. 77: 93-101. Ref.: https://pubmed.ncbi.nlm.nih.gov/14726042/ DOI: https://doi.org/10.1016/s0003-4975(03)01331-6
5. Jones CM, Athanasiou T, Dunne N. 2007. Multi-Detector Computed Tomography in Coronary Artery Bypass Graft Assessment: A Meta-Analysis. Ann Thorac Surg. 83: 341-348. Ref.: https://pubmed.ncbi.nlm.nih.gov/17184705/ DOI: https://doi.org/10.1016/j.athoracsur.2006.08.018
6. Gluckman TJ, Segal JB, Schulman SP. 2009. Effect of antiplatelet factor-4/heparin antibody induction on early saphenous vein graft occlusion after coronary artery bypass surgery. J Thromb Haemost. 7: 1457-1464. Ref.: https://pubmed.ncbi.nlm.nih.gov/19995414/ DOI: https://doi.org/10.1186/1471-2261-9-53
7. Bassri H, Salari F, Noohi F. 2009. Evaluation of early coronary graft patency after coronary artery bypass graft surgery using multislice computed tomography angiography. BMC Cardiovasc Disord. 9: 53. Ref.: https://pubmed.ncbi.nlm.nih.gov/19995414/ DOI: https://doi.org/10.1186/1471-2261-9-53
8. Goldman S, Zadina K, Moritz T. 2004. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 44: 2149-2156. Ref.: https://pubmed.ncbi.nlm.nih.gov/15582312/ DOI: https://doi.org/10.1016/j.jacc.2004.08.064
9. Levine GN, Bates ER, Bittl JA. 2016. ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease. J Am Coll Cardiol. 68: 1082-1115. Ref.: https://pubmed.ncbi.nlm.nih.gov/27036918/ DOI: https://doi.org/10.1016/j.jacc.2016.03.513
10. Lopes RD, Hafley GE, Allen KB. 2009. Endoscopic versus open vein-graft harvesting in coronary-artery bypass surgery. N Engl J Med. 361: 235-244. Ref.: https://pubmed.ncbi.nlm.nih.gov/19605828/ DOI: https://doi.org/10.1056/nejmoa0900708
11. Samano N, Geijer H, Liden M. 2015. The no-touch saphenous vein for CABG maintains a patency of 16 years comparable to the left internal thoracic artery: a randomized trial. J Thorac Cardiovasc Surg. 150: 880-888. Ref.: https://pubmed.ncbi.nlm.nih.gov/26282605/ DOI: https://doi.org/10.1016/j.jtcvs.2015.07.027
12. Tatoulis J, Buxton BF, Fuller JA. 2011. The right internal thoracique artery: the forgotten conduit- 5,766 patients and 991 Angiograms. Ann Thorac Surg. 92: 9-15. Ref.: https://pubmed.ncbi.nlm.nih.gov/21718825/ DOI: https://doi.org/10.1016/j.athoracsur.2011.03.099
13. Windecker S, Kolh P, Alfonso F. 2014. ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 35: 2541-2619. Ref.: https://pubmed.ncbi.nlm.nih.gov/25173339/ DOI: https://doi.org/10.1093/eurheartj/ehu278
14. Glineur D, D'hoore W, Price J. 2012. Survival benefit of multiple arterial grafting in a 25-year single-institutional experience: the importance of the third arterial graft. Eur J Cardiothorac Surg. 42: 284-290. Ref.: https://pubmed.ncbi.nlm.nih.gov/22290925/ DOI: https://doi.org/10.1093/ejcts/ezr302
15. He GW. 1993. Contractility of the human internal mammary artery at the distal section increases toward the end. Emphasis on not using the end of the internal mammary artery for grafting. J Thorac Cardiovasc Surg. 106: 406-411. Ref.: https://pubmed.ncbi.nlm.nih.gov/8361180/
16. Glineur D, Boodhwani M, Hanet C. 2016. Bilateral Internal Thoracic Artery Configuration for Coronary Artery Bypass Surgery. A Prospective Randomized Trial. Circ Cardiovasc Interv. 9: e003518. Ref.: https://pubmed.ncbi.nlm.nih.gov/27406988/ DOI: https://doi.org/10.1161/circinterventions.115.003518




















