Indexing & Abstracting
Full Text
Case ReportDOI Number : 10.36811/jcshd.2019.110013Article Views : 57Article Downloads : 34
ECMO usefulness in refractory shock secondary to Phenylephrine poisoning: About a case in a Pediatric subject
Luis Raúl Meza-López1*, Silvia Hernández-Meneses1, Luis Efrén Santos-Martínez2, Juan de Dios Victoria-Zúñiga3, Samantha Toledo-García4, Paola Fernández-Domínguez5, Angel Celorio-Alcántara6 and Carlos de Jesús López-Morales7
1Department of Cardiovascular Surgery, Regional Hospital of High Specialty "Bicentenario de la Independencia", ISSSTE. Tultitlán, State of Mexico
2Head of the Department of Pulmonary Hypertension, UMAE Hospital of Cardiology, “Siglo XXI” Medical Center. IMSS, Mexico City
3Medical Subdirector, Regional Hospital of High Specialty "Bicentenario de la Independencia", ISSSTE, Tultitlan, State of Mexico
4Resident of General Surgery, Balbuena General Hospital. Mexico City
5Department of Anesthesiology, Regional Hospital of High Specialty "Bicentenario de la Independencia", ISSSTE. Tultitlán, State of Mexico
6Paediatric Intensive Care Unit, Regional Hospital of High Specialty "Bicentenario de la Independencia", ISSSTE, Tultitlán, State of Mexico
7Managing Director, Regional Hospital of High Specialty "Bicentenario de la Independencia", ISSSTE, Tultitlán, State of Mexico
*Corresponding Author: Luis Raúl Meza-López, Ciruelos No. 4, Lázaro Cárdenas, Tultitlán de Mariano Escobedo. State of Mexico, Mexico. Zip Code: 54916, Tel: (52) 1 55 13 09 01 10; Email: luisraulml@hotmail.com
Article Information
Aritcle Type: Case Report
Citation: Luis Raúl Meza-López, Silvia Hernández-Meneses, Luis Efrén Santos-Martínez, et al. 2019. ECMO usefulness in refractory shock secondary to phenylephrine poisoning: About a case in a pediatric subject. J Cardiovasc Surg Heart Dis. 1: 76-82.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Luis Raúl Meza-López
Publication history:
Received date: 04 October, 2019Accepted date: 11 October, 2019
Published date: 14 October, 2019
Abstract
Introduction: Phenylephrine is an alpha-agonist with vasoconstrictor effect. Systemic absorption or adverse reaction can cause complications that can be lethal. In those cases of adverse drug reactions with failure to conventional management, the use of ECMO has been reported as a treatment modality.
Clinical case: We report the case of a 13 years old teenager, who presented acute pulmonary edema and acute heart failure subsequent to the application of topical phenylephrine during the a rhino-septoplasty procedure, and that warranted placement of ECMO due to failure of conventional treatment.
Discussion: Venoarterial ECMO was placed by central cannulation, with ventilatory and hemodynamic improvement, performing the explant successful 4th postoperative day.
Conclusions: ECMO as a "bridge to recovery" may be an alternative in those cases of pulmonary or cardiac failure and refractory with a poor response to conventional treatment, as well as an option to save the life of these patients.
Keywords: ECMO; Acute pulmonary edema; Refractory cardiogenic shock; Phenylephrine
Introduction
Phenylephrine is a predominantly selective alpha-1-adrenergic agonist, which in high doses can cause the activation of beta receptors. His topical presentation is used commonly in ophthalmology and otolaryngology procedures. Some reports suggest that significant systemic absorption may cause adverse effects such as hypertensive crisis, tachycardia, reflex bradycardia, ventricular arrhythmias, myocardial ischemia and in some cases, death [1].
In pediatric patients, adverse reactions and secondary complications related to the use of topical phenylephrine have been reported [1] with a greater hemodynamic impact on this group due to systemic absorption through the highly vascularized nasal mucosa, lower body mass and a relatively higher pharmacological concentration during an extended period. Absorption by this route is faster and avoid "first step metabolism". On the other hand, toxicity in children is composed of an altered metabolic capacity as well as an immature blood-brain barrier [2].
Globally, drug poisoning or drug overdose is a problem that occurs in a variety of scenarios. Typically, pharmacological management and the administration of specific antidotes are effective, but in cases of life-threatening cardiovascular collapse may not be sufficient [2]. Extracorporeal membrane oxygenation (ECMO) has been successfully used in children with heart or lung failure, and its use has increased over the past decade. ECMO provides cardiopulmonary support in cases where there is no adequate response to conventional management, or when the expected treatment goals are not met despite optimal management. It is considered a "bridge therapy", by extending the time for a definitive treatment option [2]. We present the case of a pediatric patient who had acute pulmonary edema and refractory cardiac failure because of phenylephrine overdose during septoplasty, requiring ECMO.
Clinical Case
A 13-year-old male teenager with a history of allergy to pollen and dust, and diagnosis of septal deviation and cornet hypertrophy, who underwent septoplasty and cauterization. The procedure was performed under general anesthesia and orotracheal intubation; as part of the surgical technique, the region was infiltrated with 2% lidocaine/epinephrine, and subsequently gauzes with phenylephrine were also placed. During transoperatory, the patient presented 40 beats/minute sinus bradycardia, requiring a single dose of 2 mg atropine, continuing surgery. After this, patient presented 160 beats/minute tachycardia, with sustained high blood pressure of 180/100mmHg, and pink frothy exudate through the endotracheal tube; as well as sudden hypotension and subsequently pulseless electrical activity, requiring 2 CPR cycles. 5 minutes later; patient again presented pulseless electrical activity and 2 more resuscitation cycles were given. Infusions with norepinephrine and adrenaline were started with partial response with hypotension and sinus tachycardia of 130 beats/minute. A computerized tomography (CT) scan was performed, documenting brain edema, as well as suggestive images of pulmonary edema and bilateral pleural effusion. Also, an echocardiogram was performed, which showed biventricular failure and infusions of vasopressin and methylene blue were also started with poor response to treatment and refractory cardiogenic shock.
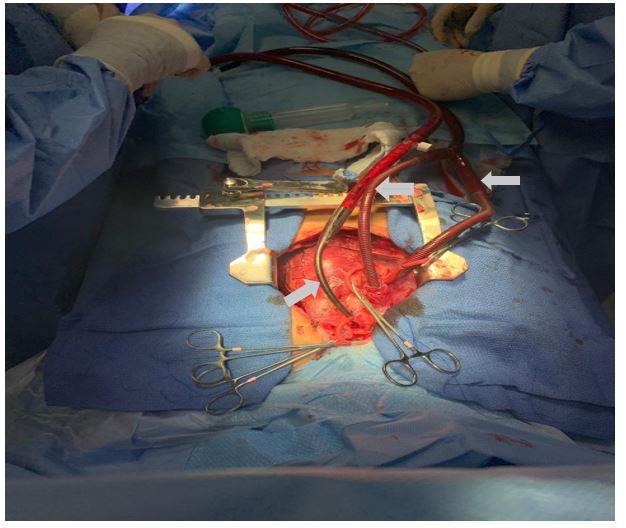
Due to poor cardiac output despite receiving multiple vasopressors and clinical deterioration, patient was cannulated for VA ECMO. For this case, we used an EOS ECMO™ device (LivaNova, Inc; MIL: SRN, Italy). Because of to the lack of adequately sized peripheral cannulas, central-type cannulation was chosen, using a 17 Fr BIO-MEDICUS™ (Medtronic Inc., Minneapolis, MN) femoral cannula, and 28 and 30 Fr venous cannulas (Sorin Group, MO, Italy) for venous return (Figure 1). No significant bleeding was observed and subsequently the chest was left open, closing only the skin. ECMO parameters, drugs used, laboratory tests and vital signs of the patient are shown in Table 1.
|
Table 1: Laboratory values, vital signs, drugs used and ECMO parameters. |
|||||
|
Parameters |
Before ECMO |
Day 1 |
Day 2 |
Day 3 |
Day 4 |
|
pH |
7.2 |
7.42 |
7.4 |
7.46 |
7.5 |
|
pCO2 (mmHg) |
27.9 |
20.5 |
43.8 |
42.3 |
35.6 |
|
pO2 (mmHg) |
211.6 |
126.7 |
94.1 |
96.8 |
83.7 |
|
Lactate(mmol/L) |
11.5 |
4.8 |
1 |
0.5 |
1 |
|
HCO3(mEq/L) |
11 |
13.6 |
28 |
31.2 |
28.3 |
|
BE (mmol/L) |
-14.1 |
-8 |
3.4 |
7.3 |
5.7 |
|
pO2/FiO2 |
226.4 |
316.8 |
130.1 |
133.7 |
119.6 |
|
Leukocytes (103/uL) |
9.25 |
19.11 |
15.68 |
13.22 |
14.22 |
|
Hb (g/dL) |
8.13 |
13.59 |
12.86 |
12.22 |
12.75 |
|
Hct (%) |
24.36 |
40.06 |
37.81 |
36.22 |
37.28 |
|
Platelets (103/ul) |
392 |
194 |
151 |
144 |
131 |
|
Gluc (mg/dL) |
348 |
140 |
161 |
181 |
100 |
|
Urea (mg/dL) |
134.82 |
38.52 |
36.38 |
36.38 |
32.1 |
|
Creat (mg/dL) |
4.72 |
0.78 |
0.54 |
0.44 |
0.42 |
|
ECMO Flow (Liters/Minute) |
|
3 |
1.6-1.9 |
1.6-1.8 |
0.6-1.2 |
|
ECMO RPM (Revolutions per minute) |
|
3500 |
1800-2200 |
1800-2100 |
1200-1500 |
|
ECMO FiO2 (%) |
|
100 |
75-90 |
70-80 |
75-80 |
|
Respiratory Rate (breathes per minute) |
30 |
28 |
18 |
14 |
14 |
|
HR (beats per minute) |
160 |
133 |
78 |
79 |
97 |
|
MAP (mmHg) |
90/60 |
86/74 |
103/81 |
92/71 |
134/90 |
|
Drugs |
Adrenaline: 0.81 mcg/kg/min |
Noradrenaline: 0.49 mcg/kg/min |
Noradrenaline: 0.07 mcg/kg/min |
Noradrenaline: 0.04 mcg/kg/min |
Noradrenaline: 0.014 mcg/kg/min |
|
Noradrenaline: 0.81 mcg/kg/min |
Dobutamine: 8.1 mcg/kg/min |
||||
|
Methylene blue |
|
||||
|
Dobutamine: 12 mcg/kg/min |
|
||||
During the first 12 hours post-procedure was achieved decrease of serum lactate, also achieving weaning of dobutamine, adrenaline and methylene blue, and maintaining vasopressor support with norepinephrine at 0.005-0.003 mcg/kg/min. On the other hand, baseline parameters of ventilatory support (FiO2 35%, PEEP 5, FR 14/min) were established, while ECMO parameters were maintained at 1800-2300 rpm, with flows between 1.5-1.8 L/min. 48hr transthoracic echocardiogram showed improvement in ventricular function, and ECMO weaning protocol was initiated. Levosimendan was initiated as inotropic support at 0.2 mcg/kg/ min, a new echocardiogram was repeated 24hr later and the explant was successfully performed on the 4th postoperative day. Subsequent evolution was satisfactory, achieving extubation and weaning vasopressor and inotropic support 48 hours after explanting the device. Patient was discharged on the 15th post operatory day. In the 30-day follow-up his evolution remained without eventualities, continuing to oversee the departments of pediatrics and cardiovascular surgery.
Discussion
Phenylephrine is a sympathomimetic alpha-agonist with a powerful vasoconstrictor effect on veins and arteries. Hypertension caused by alpha adrenergic stimulation can increase peripheral vascular resistances, end diastolic volumes, filling pressure, and post charge over left ventricle. At the same time, cardiac output can be severely decreased, causing acute heart failure and acute pulmonary edema. Another side effect related to phenylephrine are arrhythmias, myocardial infarction and subarachnoid hemorrhage. Management of these complications is crucial, and it is necessary to remember that the key point is the capacity to increase heart rate and contractility as compensatory mechanisms to preserve cardiac output [1-4].
According to Schwalma et al, stimulation of alpha receptors by systemic absorption of topic vasopressors results in increased vascular resistances. In these cases, compensatory mechanisms required to prevent acute pulmonary edema and ventricular dysfunction include increased heart rate and myocardial contractility. However, interaction with different drugs alters this necessary response [5]. Moreover; in pediatric patients, topical phenylephrine has greater effects due to its increased systemic absorption and relative dose in relation to body weight, and to the pharmacological compartment where it has effect [6]. Many systemic effects are dose related, ophthalmic solution is available in concentrations of 2.5% and 10%and the nasal solution is inpresent0.025% and0.05%. However, even when using low concentrations of phenylephrine drops; care should be taken during application due to the risk of an unpredictable hypertensive response [1-2].
Usefulness of ECMO in overdose cases or drug poisoning has been previously reported, and the indication is pulmonary or cardiac failure that do not respond to conventional management. The use of ECMO should be considered if the risk of mortality exceeds 51%, and the therapy must be started immediately in those cases with a mortality risk of more than 80%, especially if we consider that this group of patients are young and previously healthy; and if we keep in mind that once the toxin or drug are removed from the body their condition will improve [3]. In these patients, ECMO provides adequate cardiac output and maintain tissue perfusion. In addition, it improves the redistribution of circulation and facilitates the metabolism and excretion of the drug. There is no guide to the appropriate time in which ECMO should be initiated in severely poisoned patients, and their timely installation depends on the clinical criteria [3]. Length of ECMO therapy will depend on several factors, including the severity of poisoning, cardiac dysfunction recovery, half-life of cardiac toxin and organic dysfunction at the time of initiation of treatment [3-7].
Current evidence of ECMO usefulness in patients with severe drug poisoning and refractory shock is limited to animal studies, case reports, and small cohort studies [3]. Toxicology consultation reports show that ECMO is still rarely used in intoxicated patients (10 /26,271 patients over a 3-year period) [8]. Human experience includes an observational cohort study that shows better recovery in six patients with severe poisoning and refractory shock who received VA ECMO therapy compared to using ECMO with other indications[7-8] On this point, Megarbane et al published a cohort study of 17 patients where ECMO was established in all patients with refractory cardiac arrest. Survival was better in shock patients due to poisoning than in the group of patients with cardiogenic shock [9]. Also, Masson et al conducted a retrospective analysis on 2 hospitals in France that involved patients with intoxication and shock who were crashed in to VA ECMO therapy; with better results observed in those patients treated with ECMO compared to conventional management with liquids, vasopressors and other support measures. However, due to the small number of patients who were treated with ECMO-VA in this cohort, these results should be carefully interpreted [10].
In addition, there are several reports of refractory shock secondary to drug intoxication treated by ECMO, with reports of its usefulness in the management of flecainid poisoning, beta blockers, digoxin, calcium-antagonists, tricyclic antidepressants, bupropion, methamphetamines and mepivacaine, in adults and pediatrics. In another retrospective study of 17 patients diagnosed with severe intoxication with different cardiotoxic drugs, Daubin et al reported a successful ECMO explant rate of approximately 90%, and 76% of cases were discharged without any significant cardiac or neurological dysfunction; although complications such as bleeding, hypotension, thromboembolism, neurological events, limb ischemia and thrombosis formation in situ were reported [7-13].
In this case, absorption of topical phenylephrine caused the hemodynamic phenomena mentioned above, and drug interaction with atropine enhanced its toxicity and adverse effects. Patient was in cardiogenic shock and pulmonary edema refractory to pharmacological management and conventional measures, and we believe that he would not have been able to survive without ECMO support. Likewise, coordinating the multidisciplinary team needed for this type of treatment and determining the time to start therapy was crucial to obtain good results. During the days that extracorporeal circulatory support was maintained, there were no complications and no need for configuration changes or replacement of ECMO circuit components. Since the condition that led to cardiopulmonary failure to our patient was potentially reversible, indication of therapy was "bridge to recovery", providing the necessary hemodynamic and pulmonary support until metabolism of drugs was completely possible. Unfortunately, it was not possible to obtain serum concentrations of the circulating drugs, which would have supported the exact moment to explant the device, however, the echocardiographic controls performed, as well as the clinical and radiological improvement allowed us to determine the time of weaning.
Conclusions
The use of ECMO in patients with drug poisoning and refractory cardiopulmonary failure is becoming important as a therapeutic option; however, it is still underutilized. This condition has characteristics that make it unique, considering the number of potentially toxic substances, its pharmacokinetics and pharmacodynamics, so ECMO is an alternative in cases with poor response to conventional treatment, that can save patients' lives. At the best of our knowledge, this is the first successful case in a pediatric patient reported in our country. Current evidence is limited and more studies are needed to determine situations about the right time and circumstances to start therapy and achieve the best outcomes, although we consider it to be an area of opportunity that should be taken into account.
References
1. Abdelhalim AA, Mostafa M, Abdulmomen A, et al. 2012. Severe hypertension and pulmonary edema associated with systemic absorption of topical phenylephrine in a child during retinal surgery. Saudi Journal of Anaesthesia. 6: 285-288. Ref.: https://bit.ly/2pcM7Vk
2. Venkatakishnan J, Jagadeesh V, Kannan R. 2010. Pulmonary edema following instillation of topical phenylephrine eyedrops in a child under general anesthesia. Eur J Ophtalmol. 21: 115-117. Ref.: https://bit.ly/2B4jsVa
3. Gupta V, Wander GS. 2017. ECMO in Poisoning. J Card Crit Care TSS .1: 82-88. Ref.: https://bit.ly/2paFJ17
4. Lai YK. 1989. Adverse effect of intraoperative phenylephrine 10%: case report. British Journal of Ophtalmology. 73: 468-469. Ref.: https://bit.ly/2M41UPu
5. Schwalm JD, Hamstra J, Mulji A, et al. 2008. Cardiogenic shock following nasal septoplasty: a case report and review of the literatura. CAN J ANESTH. 55: 376-379. Ref.: https://bit.ly/2IGTAmw
6. Renu S. 2009. bTopical phenylephrine induced pulmonary odema: few suggestions. Pediatric Anesthesia. 19: 541-553. Ref.: https://bit.ly/2Muu84V
7. Subash SS, Nandanam NM, Alphonse PS, et al. 2017. Extracorporeal Membrane Oxygenation for Cardiotoxic Drug Overdose – A lifesaving Intervention. J Card Crit Care TSS. 1: 105-107. Ref.: https://bit.ly/2IFVRyq
8. Maskell KF, Ferguson NM, Bain J, et al.2017. Survival after cardiac arrest: ECMO rescue therapy after amlodipine and metoprolol overdose. Cardiovasc Toxicol. 17: 223-225. Ref.: https://bit.ly/2VwgD8Z
9. Mégarbane B, Leprince P, Deye N, et al. 2007. Emergency feasibility in medical intensive care unit of extracorporeal life support for refractory cardiac arrest. Intensive Care Med. 33: 758-764. Ref.: https://bit.ly/33nbAuk
10. Masson R, Colas V, Parienti JJ, et al. 2012. A comparison of survival with and whitout extracorporeal life support treatment for severe poisoning due to drug intoxication. Resuscitation. 83: 1413-1417. Ref.: https://bit.ly/313MDlV
11. Daubin C, Lehoux P, Ivascau C, et al. 2009. Extracorporeal life support in severe drug intoxication: a retrospective cohort study of seventeen cases. Crit Care. 13: R138. Ref.: https://bit.ly/35nJD7j
12. Weinberg RL, Bouchard NC, Abrams DC, et al. 2013. Venoarterial extracorporeal membrane oxygenation for the management of massive amlodipine overdose. Perfusion. 29: 53-56. Ref.: https://bit.ly/2VyPodU b
13. Heise CW, Skolnik AB, Raschke RA, et al. 2016. Two Cases of Refractory Cardiogenic Shock Secondary to Bupropion Successfully Treated with Veno-Arterial Extracorporeal Membrane Oxygenation. J. Med Toxicol. 12: 301-304. Ref.: https://bit.ly/2OCOUSx




















