Indexing & Abstracting
Full Text
Review ArticleDOI Number : 10.36811/jcri.2021.110023Article Views : 107Article Downloads : 82
Demographic Variables, Co morbidities and Clinical Presentation of Spontaneous Intracerebral Hemorrhage (ICH) in a tertiary Care Hospital in Bangladesh: A Cross Sectional Study
Md. Shoriful Islam1, Richmond Ronald Gomes*2 and FM Monjur Hasan3
and FM Monjur Hasan3
1Junior Consultant, Medicine, Dhaka Medical College Hospital, Dhaka, Bangladesh
2Associate Professor, Medicine, Ad-din Women’s Medical College Hospital, Dhaka, Bangladesh
3Associate Professor, Medicine, Ad-din Sakina Women’s Medical College Hospital, Jashore, Bangladesh
*Corresponding Author: Richmond Ronald Gomes, Associate Professor, Medicine, Ad-din Women’s Medical College Hospital, Dhaka, Bangladesh; Email: rrichi.dmc.k56@gmail.com
Article Information
Aritcle Type: Review Article
Citation: Shoriful Islam, Richmond Ronald Gomes, FM Monjur Hasan. 2021. Demographic Variables, Co morbidities and Clinical Presentation of Spontaneous Intracerebral Hemorrhage (ICH) in a tertiary Care Hospital in Bangladesh: A Cross Sectional Study. J Case Rept Img. 3: 27-37.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2021; Shoriful Islam
Publication history:
Received date: 21 June, 2021Accepted date: 10 July, 2021
Published date: 13 July, 2021
Abstract
Spontaneous intracerebral hemorrhage (ICH) has remained the least treatable form of stroke despite recent improvements in medical treatment. Treatment usually supportive and medical such as ventilatory support, blood pressure reduction, osmotherapy, fever control, seizure control and nutritional support and treatment of co morbidconditions. This study was carried out to see demographic variability, clinical presentation, causes and outcome of spontaneous intracerebral hemorrhage.
Methods and Materials: This was a cross sectional observational prospective in study on 50 spontaneous ICH patients admitted in Medicine department of Khulna Medical College Hospital from November 2020 to April, 2021.
Result: The study showed that spontaneous ICH was most common in between 41-70 years. Their age frequency were 14 (28%) in 41-50 years, 15 (30%) in 51-60 years, 12 (24%) in 61-70 years, 5 (10%) in 71- 80 years and 4 (8%) in more than 81 years age group. Among the patients, 64% (32) were male and 36% (18) were female. Headache, vomiting and seizure was present in 28, 27 and 8 patients respectively. Diabetes mellitus was present in 22% (11) of patients and absent in 78% (39) patients. Range of blood pressure at presentation –<140/90 in 24%(12), Systolic BP:140-159/Diastolic BP:90-99 (mm Hg) in 10%(5), Systolic BP:160-179/Diastolic BP:100-109 (mm Hg) in 22%(11),Systolic BP:180 or more/Diastolic BP:110 or more (mm Hg) in 44%(22) patients. Dyslipidemia was present in 30% (15) & absent in 70% (35) patients. Glasgow Coma Scale Score was 8 or less in 42% (21) and 9 or more in 58% (29) patients.
Conclusion: Spontaneous ICH is common in Indian subcontinent. As our study showed that death occur due to ICH itself, associated co morbidities or complications, facilities for stroke care unit, high dependency unit and Intensive care unit is required in tertiary care health settings.
Keyword: Spontaneous; Intracerebral Hemorrhage; Osmotherapy; Seizure; Glasgow Coma Scale
Introduction
Cerebrovascular diseases are the third leading cause of death after heart disease and cancer in developed countries. They also come first in terms of causing death and disability in neurologic diseases in adults [1]. Nontraumatic intracerebral hemorrhage is bleeding into the parenchyma of the brain that may extendinto the ventricles and, in rare cases, the subarachnoid space. Spontaneous intracerebral hemorrhage is second most common causes of stroke following ischemic stroke. Depending on the underlying cause of bleeding; intracerebral hemorrhage is classified as either primary or secondary. Primary intracerebral hemorrhage accounting for 78%-88% of all cases, originates from the spontaneous rupture of small vessels damaged by chronic hypertension or amyloid angiopathy [2]. The world wide incidence of intracerebral hemorrhage from 10-20 cases per 100000 population [3,4], and increases with age [3,5]. Intracerebral hemorrhage is more common in men than women, particularly those older than 55 years of age [5,6], and in certain populations, including blacks and Japanese [3,7]. Hypertension is the most important risk factor for spontaneous intracerebral hemorrhage [8]. Intracerebral hemorrhage commonly affects cerebral lobes, basal ganglia, the thalamus, the brain stem (predominantly the pons), and the cerebellum [9] as a result of ruptured vessels. Extension into the ventricles occurs in association with deep, large hematomas.The classic presentation of intracerebral hemorrhage is sudden onset of a focal neurological deficit that progress over minutes to hours with accompanying headache, nausea, vomiting, decreased consciousness and elevated blood pressure. Computed tomography is the key part of the initial diagnostic evaluation. First, it clearly differentiates hemorrhage from ischemic strokes. In addition computed tomography demonstrates the side and location of the hemorrhage and may reveal structural abnormality as well as structural complications such as herniation, intraventricular hemorrhage or hydrocephalus [10]. Initial management should first be directed toward the basics of air way, breathing, and circulation, and detection of focal neurologicaldeficits [10].
Other supportive medical care includes reduction of the intracranial pressure by diuretics (Mannitol 20% and furosemide, use of anticonvulsants (Diazepam, Midazolam or Phenobarbital) and control of hyperthermia (In order to decrease the neural metabolism) achieved by: external refrigeration, cold saline, sedation, and mechanical ventilation [11]. The optimal level of patient’s blood pressure should be based on individual factors such as chronic hypertension, elevated intracranial pressure, age, presumed cause of hemorrhage, and interval since onset [12]. In general recommendation for treatment of elevated blood pressure in patients with ICH are more aggressive than those for patients with ischemic stroke [12]. Antihypertensive agents recommended for treatment of blood pressure in ICH, labetalol, enalapril, esmolol, hydralazine [10]. In one fourth of patients with intracerebral hemorrhage who are initially alert, a deterioration in the level of consciousness occurs within first 24 hours after onset of hemorrhage [13,14]. Expansion of the hematoma is the most common cause of underlying neurologic deterioration within the first three hours the onset of hemorrhage. Worsening cerebral edema is also implicated in neurologic deterioration that occurs within 24-48 hours after the onset of hemorrhage [14].
Materials and Method
This cross sectional, observational, prospective study was carried out in Medicine department of Khulna Medical College Hospital from November, 2020 to April 2021. Total 50 cases of spontaneous ICH were selected. Diagnosis was made by CT scan of brain. Data were processed and analyzed using SPSS (Statistical Package for Social Science) 17.0.
Inclusion criteria:
1. All patients with Spontaneous intracerebral hemorrhage (diagnosed by CT brain) admitted in Medicine wards of KMCH.
2. Voluntarily given consent.
Exclusion criteria:
1. Patients of traumatic intracerebral hemorrhage
2. History of prior stroke
3. Not willing to give informed consent
Results
Table 1 showing age distribution of patients with spontaneous ICH. It was most common in between 41-70 years. Their age frequency 14 (28%) were in 41-50 years, 15 (30%) were in 51- 60 years, 12 (24%) were in 61-70 years, 5 (10%) were in 71- 80 years, 4 (8%) were more than 81 years old.
|
Table 1: Frequency of age distribution of the participants. |
||
|
Age group (Yrs.) |
Frequency(No.) |
Percentage (%) |
|
41-50 |
14 |
28.0 |
|
51-60 |
15 |
30.0 |
|
61-70 |
12 |
24.0 |
|
71-80 |
5 |
10.0 |
|
More than 80 |
4 |
8.00 |
|
Total |
50 |
100.0 |
Figure 1 showing spontaneous ICH is more common in male than female. Among 50 cases, 64% (32) were male whereas 36% (18) were female.
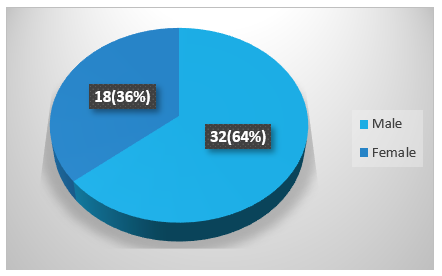
Figure 1: Sex distribution of the patients.
Figure 2 demonstrates that spontaneous intracerebral hemorrhage was found in farmer/ day labor 26% (13), businessman 24% (12), housemaker 36% (18), and service holder14% (7).
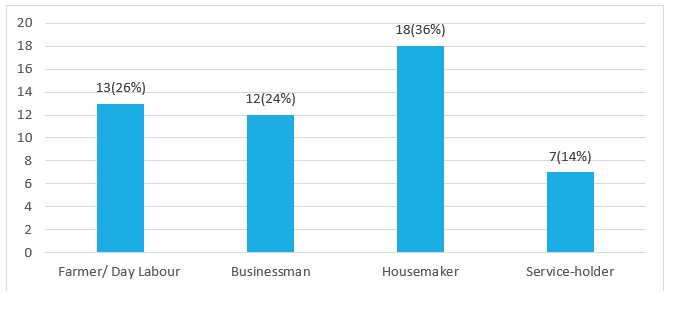
Figure 2: Occupation of the patients.
Table 2 demonstrates that spontaneous ICH is common in poor and middle class family. Among the patients, poor (monthly income less than 10000 taka) is 40% (20), middle class (monthly income 10000-50000 taka) is 58% (29), high class((monthly income more than 50000 taka)is 2% (1).
|
Table 2: Socio-economic condition of the participants |
||
|
Socio economic condition |
Frequency |
Percent |
|
Poor |
20 |
40 |
|
Middle class |
29 |
58 |
|
High class |
1 |
2 |
|
Total |
50 |
100.0 |
Figure 3 represents spontaneous ICH more common in smoker. Among 50 cases male smoker 25 (50%) and female 1 (2%) and nonsmoker male 7 (14%) and female 17 (34%).
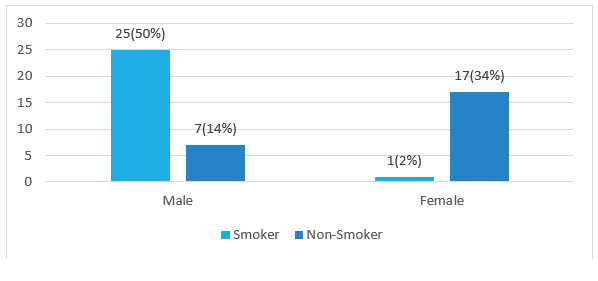
Figure 3: Showing distribution of smoker of the participants.
Figure 4 showing many patients with spontaneous ICH presented with headache. Headache was present in 56% (28) and was absent in 44% (22) patients.
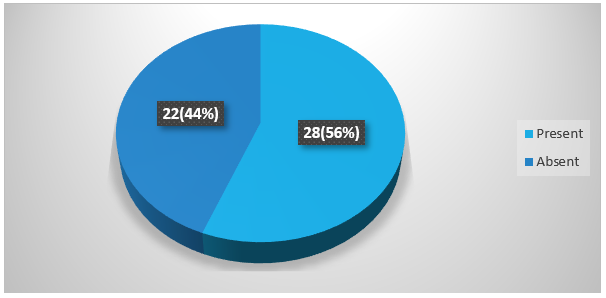
Figure 4: Percentage ofheadache among spontaneous ICH patients.
Figure 5 showing many patients with spontaneous ICH presented with vomiting. Vomiting was present in 54% (27) and was absent in 46% (23) patients.
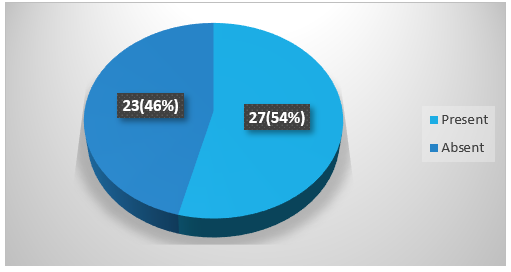
Figure 5: Percentage of vomiting among spontaneous ICH patients.
Figure 6 showing many patients with spontaneous ICH presented with seizure. Seizure was present in 8 (16%) and was absent in 42 (84%) patients.

Figure 6: Percentage of seizure among spontaneous ICH patients.
Table 3 shows GCS Score distribution. GCS Score was 8 or less in 42% (21), 9 or more in 58% (29) cases.
|
Table 3: GCS score of the participants. *GCS-Glasgow coma scale. |
||
|
GCS Score |
Frequency |
Percent |
|
8 or less |
21 |
42 |
|
9 or more |
29 |
58 |
|
Total |
50 |
100 |
Table 4 presents that number of disorientated patients with spontaneous intracerebral hemorrhage. Among the cases orientation absent in 76% (38) and orientation present in 24% (12).
|
Table 4: Orientation of the patients |
||
|
Orientation |
Frequency |
Percentage (%) |
|
Absent |
38 |
76.0(%) |
|
Present |
12 |
24.0(%) |
Table 5 showing that sensory deficit was present in 46% (23) and absent in 54% (27) patients.
|
Table 5: Sensory deficit distribution of the participants |
||
|
Sensory deficit |
Frequency |
Percent |
|
Present |
23 |
46 |
|
Absent |
27 |
54 |
|
Total |
50 |
100 |
Table 6 presents diabetes mellitus among 50 cases. Diabetes mellitus was present in 22% (11), absent in 78% (39) cases.
|
Table 6: Frequency of diabetes among the respondents. |
||
|
Diabetics status |
Frequency |
Percent |
|
Diabetics |
11 |
22.0 |
|
Non-diabetics |
39 |
78.0 |
|
Total |
50 |
100.0 |
Table 7 presents blood pressure(on presentation) distribution: SBP: Less than 140/DBP: Less than 90 (mm Hg) in 24%(12),SBP:140-159/DBP:90-99 (mm Hg) in 10%(5),SBP:160-179/DBP:100-109 (mm Hg) in 22%(11),SBP:180 or more/DBP:110 or more (mm Hg) in44% (22).
|
Table 7: Blood pressure (on presentation) distribution of the patients. |
||
|
Blood pressure |
Frequency |
Percent |
|
SBP*: Less than 140/ DBP: Less than 90 |
12 |
24 |
|
SBP:140-159/ DBP:90-99 |
5 |
10 |
|
SBP:160-179/ DBP:100-109 |
11 |
22 |
|
SBP:180 or more/ DBP:110 or more |
22 |
44 |
|
Total |
50 |
100.0 |
|
*SBP- Systolic Blood Pressure; ^DBP- Diastolic Blood Pressure. |
||
Table 8 showing dyslipidemia was present in 30% (15) & absent in 70% (35) patients.
|
Table 8: Dyslipidemia distribution among the participants |
||
|
Dyslipidemia |
Frequency |
Percent |
|
Present |
15 |
30 |
|
Absent |
35 |
70 |
|
Total |
50 |
100 |
Discussion
Spontaneous ICH is a third leading cause of death worldwide. It also common in developing country like Bangladesh.In our study, age of the patients with spontaneous ICH was above 40 years. It most commonly occurred in 40-70 years age group with 82% (41) and number of patients above 70 years of age with spontaneous ICH was 18% (9). Doctor M et al, [15] in their study found the maximum number of cases i.e. 38 (76%) were between the age groups 45 to 74 years and age ranged from 35 to 74 years. Thacker, et al reported, out of 50 cases of ICH, 39 (78%) were in the age group of 41-70 years and age ranged from 16-85 years. Kafle R, [15], Age distribution of patient’s presentation with spontaneous intracerebral bleeding was as follows. Less than 20 years of age: 1 patient. 20-29: 1 patient .30-39: 2 patients. 40-49: 11 patients. 50-59:19 patients. 60-69: 29 patients. 70-79: 20 patients. Above 80 years: 17 patients. In study by Bhatia, R et al, [17]. The mean age was 57.32±12.84 years and 140 (65.4%) were males. Hsiang, et al, [18] in a study of 60 consecutive Chinese patients showed that unlike the western studies, the majority of their patients were about a decade younger. Ong and Raymond, [19] in a study found the median age was 65 years. Juvela et al, [20] conducted a prospective study of 156 consecutive patient with an age range of 16 to 60 years. Study by Adnan et al, [21] showed that compared with woman, men had a younger age of onset. All studies have shown a steep rise in incidence with increasing age. In this study, spontaneous ICH is more common in male 64% (32) than female 36% (18). In study by Ong and Raymond, [19] showed that male to female ratio was 1:0.77. Adnan, et al, [21] in a study showed that compared with woman, men had a younger age of onset (54 versus 60 years; p<0.001). Juvela, et al, [20] in a study of consecutive patients, 96 were men and 60 were women where male female ratio was 1:0.63. Zaharia B, et al, [11] studied, from 93 studied cases 51 were men and 42, women. 52.6% were in the 5th and 6th decade. Results of all the above studies regarding age distribution correspond with our study. In the study regarding the occupation of the patients having Spontaneous ICH, farmer/day labor was 26% (13), businessman was 24% (12), and house- wife was 36% (18), and service holder was14% (7). Giulia et al, [23] found men with low SEP(socioeconomic position) with an ischemic event were more likely to be hospitalized for a new stroke than men with high SEP. Women with low SEP with hemorrhagic stroke were more likely to be hospitalized for cardiovascular disease compared with women with high SEP.
In our study, we found that spontaneous ICH was common in poor and middle class family. Among the patients, poor was 40% (20), solvent 58% (29), very good 2% (1). In a study by Giulia et al, [22] showed that stroke incidence strongly differs between socioeconomic groups reflecting a heterogeneous distribution of lifestyle and clinical risk factors. Strategies for primary prevention should target less affluent people. In this study, we found that spontaneous ICH was more common in smoker. Among 50 cases, male smoker were 25 (50%) and female 1(2%) and nonsmoker male was 7(14%) and female was 17 (34%).Kafle RD, [16], showed that 21 percent of patients were smoker. In study by Zaharia, et al, [11] found that, cigarette smoker (13.1%). Doctor, et al, [15], showed in their study, history of smoking was present in 24 cases (48%), all were male and 17 patients (34%) were currently smoking. Craig S. Anderson reported history of smoking in 29% of patients and ex-smoking in 19% of patients out of 60% cases of spontaneous intracerebral hemorrhage. In our study, among 50 cases 22% (11) were diabetics, and 78% (39) were non -diabetics. Kafle, RD [16], in his study found that nine percent of patients had a history of diabetes mellitus. Zaharia, et al, [11] studied, diabetes mellitus was present in (18%) cases. Doctor, et al. [15], in their study they found 10 (20%) diabetic. In our study we found that spontaneous intracerebral hemorrhage is commonly associated with high blood pressure. Among 50 patients, Systolic blood pressure(SBP): Less than 140/diastolic blood pressure(DBP): Less than 90 (mmHg) found in 24% (12),SBP:140-159/DBP:90-99(mmHg) in 10% (5),SBP:160-179/DBP:100-109 (mmHg) in 22%(11),SBP:180 or more/DBP:110 or more (mmHg) in 44%(22). Kafle RD, [16] found blood pressure at the time of admission: 32 percent of patients had blood pressure more than 180/110 mmHg, 28 percent had blood pressure more than 160/110 mmHg, 6 percent had blood pressure of more than 140/90. Rest of the patients (34 percent) had normal blood pressure at the time of admission. Hsiang et al, [18] conducted a prospective hospital-based study of 60 consecutive Chinese patients where they found 50% of the patients had complains of hypertension, but only 20% of these patients were treated with antihypertensive medication. Ong and Raymond, [19] studied to identify the prevalence of major risk factor for stroke and to determine predictors of one-month mortality. They found hypertension was the commonest risk factor (71.5%) followed by diabetes mellitus (40.2%) and dyslipidemia (37%). Study by Adnan et al, [21] showed most common risk factors in intracerebral hemorrhage were preexisting hypertension (77.0%). In their study among the 91 non hypertensive patients, 21 (23.0%) were diagnosed with hypertension after onset.
In our study we found that dyslipidemia was present in 30% (15) & absent 70% (35) patients. In the study of Ong and Raymond, [19] showed that dyslipidemia (37%) 74.8% of the cases were ischemic in origin and 25.2% of hemorrhagic. Following spontaneous ICH, patients present with various clinical symptoms of which headache is more common. In my study headache was present in 56% (28) and absent in 44% (22). Doctor M, et al, [15] also showed that headache was present in spontaneous intracerebral hemorrhage 22 (44%). Vomiting was present in 54% (27) and was absent in 46% (23). Seizure was present in 16% (8) patients and was absent in 84% (42) patients. Kafle R, [16], in a study found that vomiting was present in 45% of patients with spontaneous intracerebral hemorrhage. The other complains were headache (43%), loss of consciousness (17%), Seizure (13%). Doctor, et al, [15] in their study they showed that vomiting was present in 23 (46%) cases.
In this study, we found that patients with spontaneous intracerebral hemorrhage GCS Score were 8 or less in 42% (21), 9 or more in 58% (29). Kafle RD, [16]. In his study showed GCS at presentation .The majority of patients (66%) admitted to the hospital had a Glasgow coma scale score of 15. In our study, we found disorientation is present in 76% (38) of the patients with spontaneous ICH. Sensory deficits were present in 46% (23) and absent in 54% (27) patients. In the study of Doctor, et al. [15] showed that altered sensory perception in 30 (60%) patients.
Conclusion
Spontaneous ICH is a major cause of morbidity and mortality among stroke patients. Hypertension is the most common cause of spontaneous ICH. Other risk factors are smoking, dyslipidemia, diabetes mellitus and family history of stroke. Mainstay of treatment is supportive, including airway maintenance, diabetes control, blood pressure control, treatment and prophylaxis of convulsion, temperature control, nutritional support, careful fluid therapy and rehabilitation is also needed for improved mortality and morbidity. As our study showed that death occur due to ICH itself, associated co morbidities or complications, facilities for stroke care unit, high dependency unit and Intensive care unit is required in tertiary care health settings.
Limitation of Study
The present study did not represent the actual scenario of spontaneous ICH in Bangladesh because the study was conducted in one tertiary level hospital (Khulna Medical College and Hospital (KMCH). Sample size and duration of the study was short. Actual measurement of intracranial pressure was not possible. Advanced investigation facilities (Cerebral angiogram, MRI of brain) were limited. There was no advanced life support available.
References
1. Sacco RL. 1997. Risk factors, outcomes, and stroke subtypes for ischemic stroke. Neurology. 49: 39-44. Ref.: https://pubmed.ncbi.nlm.nih.gov/9371148/ DOI: https://doi.org/10.1212/wnl.49.5_suppl_4.s39
2. Foulkes MA, Wolf PA, Price TR, et al. 1988. The Stroke Data Bank: design, methods, and baseline characteristics. Stroke.19: 547-554. Ref.: https://pubmed.ncbi.nlm.nih.gov/3363586/ DOI: https://doi.org/10.1161/01.str.19.5.547
3. Broderick JP, Brott T, Tomsick T, et al. 1992. The risk of subarachnoid and intracerebral hemorrhages in blacks as compared with whites. N Engl J Med. 326: 733-736. Ref.: https://pubmed.ncbi.nlm.nih.gov/1738378/ DOI: https://doi.org/10.1056/nejm199203123261103
4. Furlan AJ, Whisnant JP, Elveback LR. 1979. The decreasing incidence of primary intracerebral hemorrhage: a population study. Ann Neurol. 5: 367-373. Ref.: https://pubmed.ncbi.nlm.nih.gov/375807/ DOI: https://doi.org/10.1002/ana.410050410
5. Giroud M, Gras P, Chadan N, et al. 1991. Cerebral hemorrhage in a French prospective population study. J Neurol Neurosurg Psychiatry. 54: 595-598. Ref.: https://pubmed.ncbi.nlm.nih.gov/1895123/ DOI: https://doi.org/10.1136/jnnp.54.7.595
6. Sacco RL, Mayer SA. 1994. Epidemiology of intracerebral hemorrhage. In: Feldmann E, ed. Intracerebral hemorrhage. Armonk NY. Futura Publishing. 3-23.
7. Suzuki K, Kutsuzawa T, Takita K, 1987. et al. Clinico-epidemiologic study of stroke in Akita, Japan. Stroke. 18: 402-406. Ref.: https://pubmed.ncbi.nlm.nih.gov/3564096/ DOI: https://doi.org/10.1161/01.str.18.2.402
8. Brott T, Thalinger K, Hertzberg V. 1986. Hypertension as a risk factor for spontaneous intracerebral hemorrhage. Stroke. 17: 1078-1083. Ref.: https://pubmed.ncbi.nlm.nih.gov/3810704/ DOI: https://doi.org/10.1161/01.str.17.6.1078
9. Mutlu N, Berry RG, Alpers BJ. 1963. Massive cerebral hemorrhage: clinical and pathological correlations. Arch Neurol. 8: 644-661. Ref.: https://pubmed.ncbi.nlm.nih.gov/14065777/ DOI: https://doi.org/10.1001/archneur.1963.00460060074008
10. Broderick JP, Adams HP, Barsan W, et al. 1999. Guidelines for the management of spontaneous intracerebral hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 30: 905-915. Ref.: https://pubmed.ncbi.nlm.nih.gov/10187901/ DOI: https://doi.org/10.1161/01.str.30.4.905
11. Zaharia.B, Plesea.IE, Georgescu.CC, et al. 2005. Morphoclinical study of intracerabral hemorrhage with intraventricularextention. Romanian Journal of Morphology and Embryology. 46: 199-206. Ref.: https://pubmed.ncbi.nlm.nih.gov/16444306/
12. Adams HP Jr, Brott TG, Furlan AJ, et al. 1996. Guidelines for thrombolytic therapy for acute stroke: a supplement to the guidelines for the management of patients with acute ischemic stroke: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Circulation. 94: 1167-1174. Ref.: https://pubmed.ncbi.nlm.nih.gov/8790069/ DOI: https://doi.org/10.1161/01.cir.94.5.1167
13. Qureshi AI, Safdar K, Weil J, et al. 1995. Predictors of early deterioration and mortality in black Americans with spontaneous intracerebral hemorrhage. Stroke. 26: 1764-1767. Ref.: https://pubmed.ncbi.nlm.nih.gov/7570722/ DOI: https://doi.org/10.1161/01.str.26.10.1764
14. Mayer SA, Sacco RL, Shi T. 1994. Neurologic deterioration in noncomatose patients with supratentorialintracerebral hemorrhage. Neurology. 44: 1379-1384. Ref.: https://pubmed.ncbi.nlm.nih.gov/8058133/ DOI: https://doi.org/10.1212/wnl.44.8.1379
15. Doctor NM, Pandya RB, Vaghani CV. 2013. A study on clinical profile, risk factors and mortality in hypertensive intracerebral hemorrhage in a tertiary care hospital in surat city. National journal of medical research. 4: 381-384.
16. Kafle DR. 2013. Outcome of patients with spontaneous intracerebral hemorrhage at a tertiary care hospital in Nepal. Journal of Nobel medical college. 4: 35-43.
17. Bhatia R, Singh H, Singh S, et al. 2013. A prospective study of in-hospital mortality and discharge outcome in spontaneous intracerebral hemorrhage. Neurol India. 61: 244-248. Ref.: https://pubmed.ncbi.nlm.nih.gov/23860142/ DOI: https://doi.org/10.4103/0028-3886.115062
18. Hsiang J, Zhu X, Wong L. 2009. Putaminal and thalamic hemorrhage in ethnic chiness living in Hong Kong .Surgical Neurology. 46: 441-445. Ref.: https://pubmed.ncbi.nlm.nih.gov/8874542/ DOI: https://doi.org/10.1016/s0090-3019(96)00157-7
19. Ong TZ, Raymond AA. 2002. Risk Factors for stroke and predictors of one-month morality. Singapore Med J. 43: 517-521. Ref.: https://pubmed.ncbi.nlm.nih.gov/12587706/
20. Juvela S. 1995. Risk Factors for impaired outcome After Spontaneous Intracerebral Hemorrhage Arch Neutol. 52: 1193-1200. Ref.: https://pubmed.ncbi.nlm.nih.gov/7492294/ DOI: https://doi.org/10.1001/archneur.1995.00540360071018
21. Adnan IQ Suri AKM, Safdar K, Jeffery RO. 1997. Intracerebral Hemorrhage in Blacks: Risk Factors, Subtypes, and outcome. Stroke. 28: 961-964. Ref.: https://pubmed.ncbi.nlm.nih.gov/9158633/ DOI: https://doi.org/10.1161/01.str.28.5.961
22. Giulia C, Nera A, Ffrancesco F. 2009. Socioeconomic Differences in stroke incidence and prognosis under a Universal Healthcare system. Stroke. 40: 2812-2819. Ref.: https://pubmed.ncbi.nlm.nih.gov/19478229/ DOI: https://doi.org/10.1161/strokeaha.108.542944




















