Indexing & Abstracting
Full Text
Review ArticleDOI Number : 10.36811/jca.2019.110003Article Views : 1671Article Downloads : 30
Nanocomposites and their employment as scavengers of water pollutants
Carolina Maria Bezerra de Araújo1, Ziani Santana Bandeira de Souza1, Maurício Alves da Motta Sobrinho1, Marcos Gomes Ghislandi2 and Tiago José Marques Fraga1*
1Department of Chemical Engineering, Federal University of Pernambuco (UFPE), 1235 Prof. Moraes Rego Av., Recife, PE, Brazil
2Engineering Campus-UACSA, Federal Rural University of Pernambuco (UFRPE), 300 Cento e sessenta e Três Av, Cabo de Santo Agostinho/PE, Brazil
*Corresponding author: Tiago José Marques Fraga, Department of Chemical Engineering, Federal University of Pernambuco (UFPE), 1235 Prof. Moraes Rego Av., Recife, PE, Brazil, zip code: 50670-901, Email: tiago_mfraga@hotmail.com
Article Information
Aritcle Type: Review Article
Citation: Carolina Maria Bezerra de A, Ziani Santana Bandeira de S, Maurício Alves da MS, et al. 2019. Nanocomposites and their employment as scavengers of water pollutants. J Chem Appl. 1: 18-31.
Copyright:This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Carolina Maria Bezerra de A
Publication history:
Received date: 01 March, 2019Accepted date: 14 March, 2019
Published date: 15 March, 2019
Abstract: The industrial effluents are of great environmental impact not only by volume and high toxicity. Many of these effluents present high organic load, which compromises photosynthesis of algae and, consequently, these water bodies life. In order to minimize the impact of these discharges, many researchers have been focusing on the development of smart and ecofriendly materials that have high capacity for removal of toxic agents. Among these new materials, nanocomposites with fascinating physicochemical properties which facilitate their interaction with the molecules of these aquatic pollutants deserve to be highlighted. The processes of enhancement of these materials through activation (chemical, thermal, electromagnetic) and functionalization, by which the removal capacity of these materials increases greatly, are also of great importance.
Keywords: Nanotechnology; Nanocomposites; Wastewater treatment; Activation; Functionalization
Introduction
The impact of organic and inorganic pollutants on the environment has been a matter of great concern to the governments of several countries and the scientific community, as these substances can cause the death of the microorganisms responsible for photosynthesis and the degradation of water bodies, if are discarded without proper treatment. Moreover, water pollutants are strictly regulated by legislation; in Brazil, there are national and regional laws which deal with the discharge of industrial effluents in water bodies [1]. As an example, Brazilian federal agency National Council of Environment (CONAMA) is responsible for establish the parameters of quality of industrial wastewater; in this sense, for the variables temperature, color, turbidity, biochemical oxygen demand (BOD), chemical oxygen demand (COD), among many others, are fixed a series of limits for wastewater disposal [1]. Nevertheless, substantial investigation must be provided in order to keep the legislation up to date and in total accordance with local reality. In this scenario, many unitary operations to treat effluents from the textile industry have been proposed over the past decades. The most significant primary treatments of crude wastewaters are flocculation [2], aeration [3], coagulation [4,5], ultrafiltration [6,7] and biodegradation [7]. Furthermore, there are processes employed after these so called primary treatments, which aim to eliminate the residual load of pollutants: decantation/sedimentation, advanced oxidative process (AOP)-among them photodegradation, photocatalysis, chemical oxidation and others; ultrasonic assisted processes; and, finally, adsorption, which applies a broad number of low cost materials [8-10].
Nanocomposites are placed as a cutting edge of the materials science and engineering, regarding the development of smart and highly effective nanoscavengers of chemical pollutants from water. Several inorganic matrixes are being currently researched for the development of capable nanomaterials: boron nitrides [11], iron oxides/magnetite nanoparticles [12,13], titanium dioxides [14,15] and oxygenated niobium composites [16]. Among the newly engineered materials used in the treatment of industrial and agricultural wastewater, the most prominent are carbon nanocomposites, which have been the object of several recent studies. Among these carbonaceous materials, graphene, for example, has attracted a huge interest in world research due to its unique structural characteristics and high adsorption performance [17,18]. Therefore, there has been a growing interest among the scientific community in investigating different aspects, particularly the surface modification, of graphene and other nanocomposites [19]. Among the disadvantages of the application of nanomaterials, it can be cited their high production cost, difficulty to scale-up processes and their final separation from the treated effluents, since several nanocomposites have hydrophilic properties [20]. Nevertheless, many researchers are focused on the solution of these issues. In this work it is highlighted the main spots over the application of several nanoparticles (and their modifications) employed in wastewater treatments, such as photodegradation, flocculation and adsorption. For that, recent works found in the literature were surveyed to evaluate their production process, pollutant removal efficiency and recyclability aspects. Moreover, perspectives, updates and strong/weak points of some processes are remarked to deliver the reader a qualified overview towards these outstanding materials.
Carbon based nanomaterials
Conventional processes for purification and treatment of water, while eliminating pathogens, are often not able to completely remove some contaminants such as dyes, drugs and other organic compounds [21,22]. Recently, a remarkable potential has been observed for the remediation of environmental problems as a result of nanotechnological developments. Compared to conventional materials, nanostructured adsorbents present greater efficiencies in water treatment, combined with the possibility of applying these materials to the photodegradation of organic compounds [23,24]. Graphene-based nanomaterials (GBN) have a high theoretical specific surface area (up to 2630 m2?g-1), great regeneration capacity, as well as exceptional electrical properties, being considered promising materials for the removal of contaminants. Graphene can be defined as 2D material formed by a hexagonal network of carbon atoms bonded together by sp2 bonds, presenting unique electronic, chemical and mechanical properties [25,26]. It is also considered as a precursor of the nanomaterials of the graphene family and other families of carbon nano-allotropes, including large fullerenes, carbon nanotubes (CNT) and graphite Figure 1 [27-29].
Figure 1: Illustration of graphene oxide (right) and graphene (left) nanosheets, as well as other carbon nano-allotropes (below graphene structure).
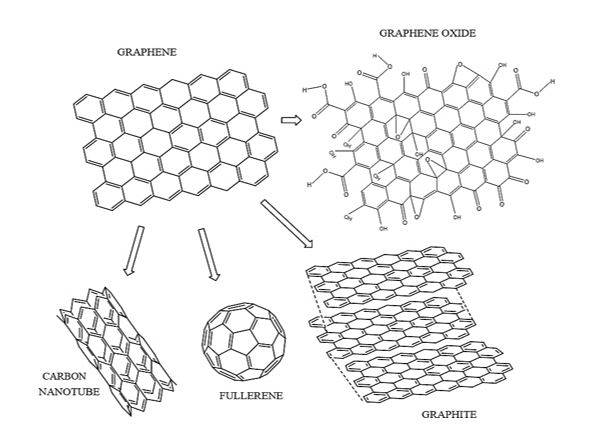
Figure 1 shows that structurally, graphene presents in an analogous way the structure of honeycombs, where each carbon atom forms three bonds with its first neighbors [22,30,31]. Graphene oxide (GO), which is the oxidized form of graphene, contains a variety of oxygen groups on its surface, a fact that contributes to the increase of adsorptive potential, besides facilitating its application directly or as a support in the photodegradation of organic pollutants [32].
According to recent studies, GO has shown to be a potential adsorbent, effective in the removal of dyes in water. For the removal of Methylene Blue (MB) using GO, adsorptive capacities above 500 mg.g-1 have been reported, with removal efficiencies above 99% in less than 10 min [17,26,33]. Peng et al. [34], on the other hand, using amorphous graphite in the GO production, observed an experimental adsorptive capacity above 2200 mg.g-1 for MB adsorption into GO, almost 5 times higher than the previous value. Yan et al. (2014) explained these disparity of results, as they depend on the GO synthesis method, as well as the steps employed during GO production. Thus, a sample that presents a higher degree of exfoliation and/or a greater amount of surface functional groups, might have a considerably increased capacity to remove the contaminant [35]. Some studies also highlight the use of GBN in the removal of color and turbidity from real textile wastewater samples, which reinforce the potential of a possible application of these materials as adsorbents in the treatment of industrial scale effluents. According to Araujo et al. [36], a sample of GO produced by modification of the method proposed by Hummers and Offeman [37], was able to remove in 30 min, approximately 85% of the turbidity and more than 60% of the apparent color from a textile wastewater sample, well above the value obtained by the conventional method, using coagulation-flocculation (35~50% apparent color removal). Carbon nanotubes are also nanomaterials that present great potential in applications of water remediation, having great potential as adsorbents for the removal of synthetic dyes. Gong et al. (2015) used porous cup-stacked carbon nanotube (P-CSCNT) for adsorption of Methylene Blue from wastewater. Although P-CSCNT showed good recyclability and reusability, it was found a maximum adsorption capacity of 319.1 mg.g-1, a much lower value compared to those found in works using GO to remove the same dye from aqueous medium [38].
The abundance of oxygen groups found on the surface of the GO was explored for studies related to the production of a wide range of materials, including aerogels, which could in the future make feasible the use of GBNs in the treatment of industrial scale waters and effluents [27]. A hydrothermal process was developed by Wang et al. (2017) to produce graphene aerogels using GO as a precursor [39]. In their study, the obtained aerogel was used as a model for the growth of carbon nanotubes (CNTs). The material was able to absorb a variety of organic liquids, including polydimethylsiloxane (PDMS), with a maximum adsorption capacity of 322.8 g.g-1. In addition, the aerogel exhibited excellent thermal stability and reuse for oil-water separation, which would make it possible to clean oil spills and water purification. Additionally, the exceptional electrical properties of graphene have also attracted applications in the areas of nanoelectronics, photocatalysis/photodegradation, and can also be incorporated into other materials on a macroscopic scale. In photocatalytic (as well as in adsorptive) processes, GBN has shown to be promising, mainly because it presents a high specific area, due to good optical transmittance and high intrinsic electronic mobility [30,40,41]. De Icaza-Herrera (2015) [24] reported the effectiveness of graphite and graphene oxides in the photocatalytic degradation of 4-chlorophenol in water. It was observed that 92 and 97% of 4-chlorophenol were degraded with graphite and graphene oxides, respectively; and that 97% of the total organic carbon was removed. In addition, the by-products normally observed in the reaction, which produce a yellow solution obtained by photolysis alone, were eliminated by the photocatalyst process using GBN.
Other nanocomposites
With the growing need to develop more efficient methods for the treatment of complex wastewater, such as effluents containing dyes and heavy metals, the use of non-carbonaceous nanocomposites has become promising. The main characteristics of these nanomaterials are high surface area, high chemical activity, and exclusive antimicrobial, optical and electronic properties. These properties make these materials excellent candidates for different wastewater treatment processes such as disinfection, biological treatment, adsorption and photocatalytic oxidation/degradation [43-45]. The most common forms of iron oxide in nature are magnetite (Fe3O4), hematite (α-Fe2O3) and maghemite (γ-Fe2O3). Iron oxide nanomaterials have been extensively researched due to their characteristics such as nano-range size, high surface area and superparagliatism [46]. Recent studies report the potential of using this material in nanocomposites for different effluent treatment processes due to its versatility as a Fenton reaction catalyst, as well as its magnetic properties that allow an easy recovery after the process [47,48].In order to remove the Cr (VI) and Pb (II) ions from the aqueous solution, Koushkbaghi and collaborators [49] developed dual layer membranes, obtained by the incorporation of aminated-Fe3O4 nanoparticles into the chitosan/polyvinyl alcohol nanofibers that were coated on the porous polyethersulfone membrane. In a batch adsorption system, the maximum adsorption capacity was 509.7 and 525.8 mg·g-1 for the Cr (VI) and Pb (II) ions, respectively. In the ultrafiltration membrane system, maximum recoveries of about 85% and 95% (wt%) of the Pb (II) and Cr (VI) ions respectively obtained with the use of 2% by weight of aminated-Fe3O4. A good reusability of the nanofibrous membrane was also observed.For the treatment of Methylene Blue (MB) dye solution, Chen et al. (2018) [47] used Fe3O4 nanoparticles coated with SiO2 and C to form a nanospheric photocatalyst, and then applied it in Fenton-like reaction to degrade MB. The material presented high catalytic activity in pH range of 4-9 and better results were obtained for higher temperature conditions, nanosphere content and H2O2 concentration. At pH 6.0, the material had a discoloration efficiency of 90%, a much higher result compared to Fe3O4-H2O2 and H2O2 systems, which presented 14% and 5% discoloration, respectively. According to the authors, the decolorization efficiency even at pH close to neutrality may be associated with the presence of SiO2 that modified the optimal pH of the Fenton reaction, in addition to the accessible surface and the C layers capable of concentrating the dye and accelerating the reaction. The presence of Fe3O4 also allowed for easy magnetic separation.Nanostructured materials based on TiO2 present great potential in the photocatalytic removal and adsorption process due to their semiconductor character and their porous structure, mainly in dye removal [50]. In addition, the design of nanocomposites can increase the absorption potential of TiO2, enhancing the removal capacity under visible light irradiation [45].The presence of bismuth improved photodegradation of TiO2 in visible light, as observed by Ali et al. (2019) [52]. For the treatment of industrial textile effluents, they synthesized the bismuth-TiO2 nanotube using two different methods. In the two-step method, the TiO2 nanotube was synthesized by electrochemical anodization and then the bismuth was fixed by electrochemical deposition. In the one-step method, the electrochemical anodization of TiO2 nanotubes and the bismuth attachment occurred in parallel. Compared to the TiO2 nanotubes, bismuth-TiO2 nanocomposites obtained by one- and two-steps showed respectively, 2.5 and 2.0 higher photocatalytic activity, and the TOC reduction in industrial effluents was 44% and 56%, compared to a reduction of 22% with TiO2. According to the researchers, the improvement of photodegradation efficiency may be associated with different factors: high surface area of the nanocomposite; deposition of bismuth that causes the energy band-gap decrease and consequently, the increase of the TiO2 absorption in the visible region; bismuth ability to capture photoexcited electrons, reducing the recombination of electron-hole pairs (e-/h+).
The TiO2/CoSe nanocomposite synthesized by Warkhade et al. (2018) [53] was also able to increase photocatalytic efficiency under visible light in the treatment of dye solutions. It was observed that the treatment of the solution of Rhodamine B using the nanocomposite resulted in the degradation of 99.86% of the dye, while the degradation efficiency of TiO2 and CoSe was only 15% and 18%, respectively. The photocatalytic degradation of the solution of Methylene Blue with TiO2/CoSe showed 97.85% efficiency. The improvement is attributed to the retarded rate of recombination of photoexcitons, increasing the visible light harvesting ability. Another promising nanomaterial for use in effluent treatment processes is boron nitride because of its semiconductor nature, high thermal stability, strength and conductivity. In addition, its chemical inertia can facilitate the recyclability of this material [54].Shahabuddin et al. (2018) [55] synthesized polyaniline conductive nanocomposites doped with three different concentrations of 2D hexagonal boron nitride by the in situ oxidative polymerization technique. These materials were used for the treatment of an aqueous solution of dyes, through the photocatalytic process under UV irradiations. The researchers observed that the nanocomposites obtained showed higher photocatalytic performance compared to the hexagonal boron nitride and the polyaniline nanotube isolated. Better conditions were obtained for the nanocomposite containing 2% by weight of hexagonal boron nitride, with degradation efficiency 93% and 95% for methylene blue and methyl orange in 90 minutes of testing.
Lin et al. (2019) [56] investigated the adsorption, photolysis and photocatalytic oxidation under UV radiation, using TiO2/boron nitride nanocomposites as a photocatalyst for the treatment of a model solution containing ibuprofen. The adsorption capacity of the nanocomposite was about 14 mg·g-1. It was observed that the presence of boron nitride improved the light absorption efficiency and the high surface area of TiO2 facilitated the separation of the (e-/h+) pairs. The photocatalysis was very efficient, with a percentage of degradation of about 100% after 2 h of experiment, in comparison to the photolysis process with 27% of degradation. Nanocomposites also appear as an alternative to the addition of disinfectants such as chlorine, which at high dosages could form carcinogenic by-products [57]. Silver (Ag) nanoparticles, porous CuO microspheres and bimetallic nanoparticles of porous CuO decorated with Ag nanoparticles were evaluated for the antimicrobial power against the Escherichia coli, Salmonella and Listeria bacteria, and the microorganisms present in the local river water. Chen and coworkers [58] observed that the bimetallic CuO/Ag nanoparticle showed the highest disinfection efficiency, being able to completely inhibit the growth of the evaluated bacteria with a minimum dosage of 50 μg/mL. According to the authors, the antimicrobial efficacy of the nanomaterials is due to the high ratio surface/volume that allows a greater interaction with the microorganisms.
Despite having a high capacity to treat wastewater, the high cost of nanocomposites to be taken into consideration. In addition, the composition and irregularity of shape and size can cause secondary contamination to the environment and may be harmful to human health. Thus, the toxicity of these materials should also be thoroughly evaluated [59,60]. The efficiency, cost and safety of materials must be synergistically related.
Activation and functionalization
Technically, activation process is a route employed to promote modifications on a determined material surface in order to confer it chemical selectivity towards other types of pollutants. Many carbons, crude biomass and clay materials have been activated with the purpose to increase their adsorption capacity [61-63]. For that, it may be carried on by acid activation, with the addition of strong acids or their solution; and basic activation, generally with NaOH or KOH Figure 2. Chemical activation may take place with an experimental apparatus which allow the reflux of the acid/base or a bath in which the particles stay in contact with the activation agent under steady steering [63] and controlled temperature. Another enhancement process that is widely applied to macro and microporous materials is the thermic activation, which is reported to increase the availability of active sites on catalysts and adsorbents. However, chemical activation with a strong base is responsible for the formation of hydroxyl groups, distributed over the entire grain surface. These groups confer high selectivity to material when it is applied to remove positively charged molecules. Moreover, some nanoparticles can be activated by electromagnetic methods [64] that grant the flux of electron over the material surface and consequently turning it negatively or positively charged. On adsorptive processes, the activation of adsorbents is a key point to enhance sorbent removal efficiency. In this sense, some researchers have activated charcoals with NaOH to increase their affinity towards metallic cations, or cationic dyes [62]. Since chemical activation usually changes material composition in an irreversible way, it must only be performed if a substantial removal capacity increase can be reached. Therefore, researchers must be careful about operational costs increases (given in USD/kg of removed pollutants) when activate nanomaterials; for that a cost/benefit analysis must be thoroughly carried on prior to choose the activation path.
Preferentially researchers should option for easily reversible or non-destructible processes in order to make it possible the application of the same material as scavenger of a wide variety of pollutants or its easy recovery after wastewater treatment. In that sense, chemical activation processes are not fully recommended.
Figure 2: Scheme of basic activation of raw diatomite grain.

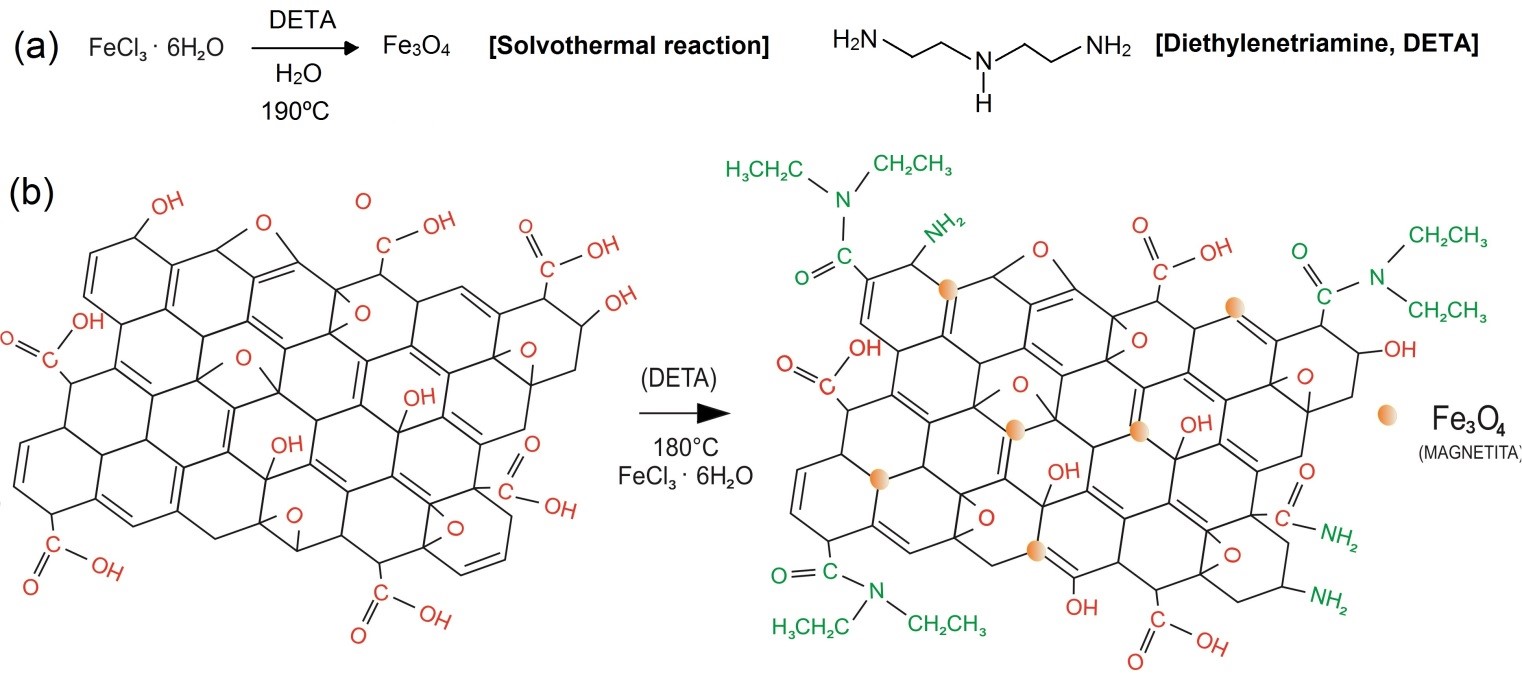
Functionalization, on the other hand, consists in a chemical reaction to insert specific functional groups over a nanomaterial surface and edges. It is reported in the literature two types of functionalization: covalent and non-covalent [18]. When functional moieties are stacked over the basal plane with weak interaction forces, it can be said that the functionalization is non-covalent. The nature of these transactions is characterized by Van der Waals forces, electrostatic, hydrophobic and stacking interactions. Furthermore, these interactions are characterized as being of lower energies (<100 kJ?mol-1) [18,65]. When the nature of the interaction between functional group and the nanomaterial basal plane possesses the strength nearly to a chemical bond (>100 kJ?mol-1), it can be said that this functionalization is covalent. As an example, it is reported in the literature the stacking of magnetic nanoparticles (Fe3O4, -Fe3O4, Fe2O3, etc.) over graphene, graphene oxide (Figure 3b) and titanium oxides are classified as of non-covalent nature [13,18]. Moreover, magnetic nanoparticles can be easily synthetized via solvothermal method, as depicted in Equation 1; it also can be synthetized and anchored over GO surface in the same time, as reported by several works in the literature [26,66,67] (Figure 3a). According to Othman et al. (2018), the main advantage of magnetic-functionalized nanocomposites is that it can be easily separated from liquid phase by the incidence of a magnetic field [68]; thus, the material can be fully characterized to be sure of its ferromagnetic and crystalline properties. In stance, magnetization analysis (emu?mg-1) must be carried on to evaluate if the functionalized nanocomposite has diamagnetic, paramagnetic or ferromagnetic behavior. Moreover, X-Rray Diffraction (XRD) together with Raman and Infrared spectroscopies (FTIR) must be performed to be certain of the presence of these functional groups in the sample, as well as the disorder degree over the crystalline structure, which is given by ID/IG ratio on Raman spectra [69]. Advanced microscopy techniques, such as Atomic Force Microscopy (AFM) and Transmission Electron Microscopy (TEM) can be employed to unravel nanocomposites morphology, which is important to explain their easy selectivity towards some pollutants.
It is important to keep in mind that the development of nanocomposites is always associated to a high diligence regarding the purity of reactants, strict control of the variables and materials characterization, which may also increase their costs. E.g. the modification of some clays may increase their production cost 40 times in comparison to crude ones, even though their removal capacity may double after surface enhancements [70]. Controlling the surface chemistry of adsorbents, catalysts and photocatalysts is crucial to unravel how they might behave towards specific species of contaminants. Moreover it is related to carry out several characterization techniques, such as FTIR infrared spectroscopy, X-ray photoelectron spectroscopy XPS, zeta potential, among others. Some of these techniques are very costly, when considering equipment maintenance, consumables purchasing and operator training and recycling. Yet, this associated cost can be lowered when the material is highly recyclable compared to conventional ones [26].
Figure 3: Amino-functionalization and Fe3O4 nanoparticles anchoring over GO carbonaceous lattice by solvothermal process.
In Table 1 some of the main findings from previous works are listed, when nanomaterials and their nanocomposites were applied as scavengers of water pollutants (organic and inorganic). All the values included in Table 1 are given at room temperature (298±5 K).
| Table 1: Comparison of removal efficiencies of different contaminants in aqueous medium applying various nanomaterials and different processes and conditions (average temperature within 298±5K). | |||||||
|---|---|---|---|---|---|---|---|
| Nanomaterial | Pollutant | Removal process | Removal efficiency | Initial concentration | Initial pH | Contact time | Reference |
| GO | Methylene Blue | Adsorption | >97% | 100 mg?L-1 | 5.5 | 10 min | [16,32] |
| Cup-stacked CNT | Methylene Blue | Adsorption | ~88% | 25 mg?L-1 | 6.0 | 180 min | [37] |
| P-CSCNT | Methylene Blue | Adsorption | ~90% | 100 mg?L-1 | 6.0 | 180 min | [37] |
| Amino-Fe3O4-functionalized GO | Methylene Blue | Adsorption | >99% | 100 mg?L-1 | 12.0 | 20 min | [25] |
| Agar-GO 3D aerogel | Methylene Blue | Adsorption | 90% | 200 mg?L-1 | 6.0 | 180 min | [26] |
| TiO2-kaolinite | Acid Dark Blue 5R | Adsorption | 97% | 100 mg?L-1 | 8.0 | 20 min | [63] |
| ZnO microspheres-reduced GO | Methylene Blue | Photocatalysis | >90% | 10 mg?L-1 | N/A | 35 min | [22] |
| Bi-TiO2 | Methylene Blue | Photocatalysis | 33% | 5 mg?L-1 | 7.0 | 150 min | [52] |
| GO | 4-chlorophenol | Photocatalysis | 97% | 30 mg?L-1 | 7.0 | 100 min | [41] |
| Graphite oxide | 4-chlorophenol | Photocatalysis | 92% | 30 mg?L-1 | 7.0 | 100 min | [41] |
| TiO2-BN | Ibuprofen | Photocatalysis | 27% | 5 mg?L-1 | 7.0 | 120 min | [56] |
Conclusion and perspectives
According to the relevant works mentioned in this literature review, it was verified that in fact nanomaterials can be potential compounds, effective and efficient in removing contaminants from water and wastewater. Moreover, in the presented studies, the potentiality of these materials was observed in the removal of various organic and recalcitrant compounds, which again reinforces the possibility of more practical applications. One the other hand, between the most significant challenges regarding the use of nanocomposites in wastewater treatment is their production scale-up, which can lead to substantial increases in costs. However, the technological mastery and the development of natural and low cost reactants have great potential to suppress this obstacle. Moreover, the toxicity of nanocomposites towards aquatic fauna and flora is not fully investigated, which delivers a wide field of research in this matter.
Even though numerous papers have presented batch studies using various types of nanomaterials for adsorption and/or photodegradation of contaminants in aqueous medium, the difficulty of applying these materials to continuous system still represents a bottleneck. That is because as they are extremely small, nanomaterials are often easy to be carried and difficult to be separated during the recycle, which is an obstacle to industrial applications. Thus, there is a need for more research and developments in this area.
References
- Brasil. 2011. res 35705 Classificação de corpos d’água padrão de lançamento de efluentes.[Ref.]
- Bratby J. 2016. Coagulation and flocculation in water and wastewater treatment, Third Edit, IWA Publishing, London. [Ref.]
- Freeman AI, Surridge BWJ, Matthews M, et al. 2018. New approaches to enhance pollutant removal in artificially aerated wastewater treatment systems, Sci. Total Environ. 627: 1182-1194. [Ref.]
- Man LW, Kumar P, Teng TT, et al. 2012. Design of experiments for Malachite Green dye removal from wastewater using thermolysis-coagulation-flocculation, Desalin. Water Treat. 40: 260-271. [Ref.]
- GilPavas E, Dobrosz-Gómez I, Gómez-García MÁ. 2018. Optimization of sequential chemical coagulation - electro-oxidation process for the treatment of an industrial textile wastewater, J. Water Process Eng. 22: 73-79. [Ref.]
- Hidalgo AM, Gómez M, Murcia MD. et al. 2018. Behaviour of polysulfone ultrafiltration membrane for dyes removal, Water Sci. Technol. 77: 2093-2100. [Ref.]
- Goswami L, Kumar RV, Pakshirajan K, et al. 2019. A novel integrated biodegradation-microfiltration system for sustainable wastewater treatment and energy recovery, J. Hazard. Mater. 365: 707-715. [Ref.]
- Lin Y, Ma J, Liu W, et al. 2019. Efficient removal of dyes from dyeing wastewater by powder activated charcoal/titanate nanotube nanocomposites: adsorption and photoregeneration, Environ. Sci. Pollut. [Ref.]
- Fraga TJM, Carvalho MN, Fraga DMDSM, et al. 2018. Treated residue from aluminium lamination as adsorbent of toxic reactive dyes-a kinetic, equilibrium and thermodynamic study, Environ. Technol. [Ref.]
- Baptisttella AMS, Araújo AAD, Barreto MC, et al. 2018. The use of metal hydroxide sludge (in natura and calcined) for the adsorption of brilliant blue dye in aqueous solution, Environ. Technol. [Ref.]
- S Yu, Wang X, Pang H, et al. 2018. Boron nitride-based materials for the removal of pollutants from aqueous solutions: A review, Chem. 333: 343-360. [Ref.]
- Liu F, Zhou K, Chen Q, et al. 2019. Application of magnetic ferrite nanoparticles for removal of Cu(II) from copper-ammonia wastewater, J. Alloys Compd. 773: 140-149. [Ref.]
- Liu M, Wen T, Wu X, et al. 2013. Synthesis of porous Fe3O4 hollow microspheres/graphene oxide composite for Cr(vi) removal, Dalt. Trans. [Ref.]
- Yang X, Chen Y, Liu X, et al. 2018. Influence of titanium dioxide nanoparticles on functionalities of constructed wetlands for wastewater treatment, Chem. Eng. J. 352: 655-663. [Ref.]
- Mousavi SV, Bozorgian A, Mokhtari N, et al. 2019. A novel cyanopropylsilane-functionalized titanium oxide magnetic nanoparticle for the adsorption of nickel and lead ions from industrial wastewater: Equilibrium, kinetic and thermodynamic studies, Microchem. J. 145: 914-920. [Ref.]
- Heitmann AP, Patrício PSO, Coura IR, et al. 2016. Nanostructured niobium oxyhydroxide dispersed Poly (3-hydroxybutyrate) (PHB) films: Highly efficient photocatalysts for degradation methylene blue dye, Appl. Catal. B Environ. 141-150. [Ref.]
- De Araujo CMB, De Assis Filho RB, Baptisttella AMS, 2018. Systematic study of graphene oxide production using factorial design techniques and its application to the adsorptive removal of methylene blue dye in aqueous medium, Mater. Res. Express. [Ref.]
- Georgakilas V, Tiwari JN, Kemp KC, et al. 2016. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications, Chem. Rev. 116: 5464-5519. [Ref.]
- Kyzas GZ, Deliyanni EA, Bikiaris DN, 2018. Graphene composites as dye adsorbents: Review, Chem. Eng. Res. Des. 129: 75-88. [Ref.]
- Park S, Ruoff RS. 2009. Chemical methods for the production of graphenes, Nat. Nanotechnol. 4: 217-224. [Ref.]
- Upadhyay RK, Soin N, Roy SS, 2014. Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: A review, RSC Adv. 4: 3823-3851. [Ref.]
- Jauris IM, Schopf PF, dos Santos CL, et al. 2015. Adsorção do fármaco nimesulida em nanoestruturas de carbono, Discip. Sci. 16: 245-256. [Ref.]
- Qin J, Zhang X, Yang C, et al, 2017. ZnO microspheres-reduced graphene oxide nanocomposite for photocatalytic degradation of methylene blue dye, Appl. Surf. Sci. 392: 196-203. [Ref.]
- Sadegh H, Ali GAM, Gupta VK, et al. 2017. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment, J. Nanostructure Chem. 7: 1-14. [Ref.]
- Yang X, Chen C, Li J, et al. 2012. Graphene oxide-iron oxide and reduced graphene oxide-iron oxide hybrid materials for the removal of organic and inorganic pollutants, RSC Adv. 2: 8821-8826. [Ref.]
- Fraga TJM, de Lima LEM, de Souza ZSB, et al. 2018. Amino-Fe3O4-functionalized graphene oxide as a novel adsorbent of Methylene Blue: kinetics, equilibrium, and recyclability aspects, Environ. Sci. Pollut. Res. [Ref.]
- Chen L, Li Y, Du Q, et al. 2017. High performance agar/graphene oxide composite aerogel for methylene blue removal, Carbohydr. Polym. 155: 345-353. [Ref.]
- Chowdhury S, Balasubramanian R. 2014. Recent advances in the use of graphene-family nanoadsorbents for removal of toxic pollutants from wastewater, Adv. Colloid Interface Sci. 204: 35-56. [Ref.]
- Suárez-Iglesias O, Collado S, Oulego P, et al. 2017. Graphene-family nanomaterials in wastewater treatment plants, Chem. Eng. J. 313: 121-135. [Ref.]
- Junges TA, Jauris IM, Rossato J. 2015. Adsorção de diazepam com óxido de grafeno: uma abordagem de primeiros princípios, Discip. Sci. 16: 151-160. [Ref.]
- Shi H, Li W, Zhong L, et al. 2014. Methylene Blue Adsorption from Aqueous Solution by Magnetic Cellulose/Graphene Oxide Composite: Equilibrium, Kinetics, and Thermodynamics, Ind. Eng. Chem. Res. 53: 1108-1118. [Ref.]
- Banerjee P, Sau S, Das P, et al. 2015. Optimization and modelling of synthetic azo dye wastewater treatment using Graphene oxide nanoplatelets: Characterization toxicity evaluation and optimization usingArtificial Neural Network, Ecotoxicol. Environ. Saf. 119: 47-57. [Ref.]
- Assis Filho RB, Araujo CMB, Baptisttella AMS, et al. 2019. Environmentally friendly route for graphene oxide production via electrochemical synthesis focused on the adsorptive removal of dyes from water, Environ. Technol. just accep. [Ref.]
- Peng W, Li H, Liu Y, et al. 2016. Adsorption of methylene blue on graphene oxide prepared from amorphous graphite: Effects of pH and foreign ions, J Mol Liq. 221: 82-87. [Ref.]
- Yan H, Tao X, Yang Z, et al. 2014. Effects of the oxidation degree of graphene oxide on the adsorption of methylene blue, J. Hazard. Mater. 268. [Ref.]
- Bezerra de Araujo CM, Filipe Oliveira do Nascimento G, Rodrigues Bezerra da Costa G, et al. 2018. Alves da Motta Sobrinho, Adsorptive removal of dye from real textile wastewater using graphene oxide produced via modifications of hummers method, Chem. Eng. Commun. 1-13. [Ref.]
- Hummers WS, Offeman RE. et al. 1958. Preparation of Graphitic Oxide, J. Am. Chem. Soc. 1339. [Ref.]
- Gong J, Liu J, Jiang Z, et al. 2015. A facile approach to prepare porous cup-stacked carbon nanotube with high performance in adsorption of methylene blue, J. Colloid Interface Sci. 445: 195-204. [Ref.]
- Wang C, Yang S, Ma Q, et al. 2017. Preparation of carbon nanotubes/graphene hybrid aerogel and its application for the adsorption of organic compounds, Carbon N. Y. 118: 765-771. [Ref.]
- Gorgolis G, Galiotis C. 2017. Graphene aerogels: a review, 2D Mater. [Ref.]
- Yang Y, Xu L, Wang H, et al. TiO2/graphene porous composite and its photocatalytic degradation of methylene blue, Mater. Des. 108: 632-639. [Ref.]
- De Icaza-Herrera M, Martínez-Hernández AL, Velasco-Santos C, et al. 2015. 4-Chlorophenol Removal From Water Using Graphite and Graphene Oxides As Photocatalysts, J. Environ. Heal. Sci. Eng. 13: 0-10. [Ref.]
- Ghodke SA, Sonawane SH, Bhanvase BA, et al. 2018. Advanced Engineered Nanomaterials for the Treatment of Wastewater, Elsevier Inc.[Ref.]
- Yang L, Ding L, Deng F, et al. 2019. Principles for the Application of Nanomaterials in Environmental Pollution Control and Resource Reutilization, Elsevier Inc.[Ref.]
- Wang D, Zhang M, Zhuang H, et al. 2017. The photocatalytic properties of hollow (GaN) 1-x (ZnO) x composite nanofibers synthesized by electrospinning, Appl. Surf. Sci. 396: 888-896. [Ref.]
- Xu P, Ming G, Lian D, et al. 2012. Science of the Total Environment Use of iron oxide nanomaterials in wastewater treatment?: A review, Sci. Total Environ. 424: 1-10. [Ref.]
- Chen L, Feng S, Yang H, et al. Decoloration of methylene blue by heterogeneous Fenton-like oxidation on Fe 3 O 4 /SiO 2 /C nanospheres in neutral environment, Mater. Chem. Phys. 213: 231-238. [Ref.]
- Song HJ, You S, X Jia H, et al. 2015. MoS2 nanosheets decorated with magnetic Fe3O4 nanoparticles and their ultrafast adsorption for wastewater treatment, Ceram. Int. 41: 13896-13902. [Ref.]
- Aliabadi M, Ramandi HF, Irani M, et al. 2017. Aminated-Fe3O4 nanoparticles filled chitosan/PVA/PES dual layers nanofibrous membrane for the removal of Cr (VI) and Pb(II) ions from aqueous solutions in adsorption and membrane processes, Chem. Eng. J. 337: 169-182. [Ref.]
- Wei Y, Zhu J, Gan Y, et al. 2018. Titanium glycolate-derived TiO2nanomaterials: Synthesis and applications, Adv. Powder Technol. 29: 2289-2311. [Ref.]
- Wang D, Xu Y, Sun F, et al. 2016. Enhanced photocatalytic activity of TiO 2 under sunlight by MoS 2 nanodots modification, Appl. Surf. Sci. 377: 221-227. [Ref.]
- Ali I, Han GB, Kim JO. 2019. Reusability and photocatalytic activity of bismuth-TiO2 nanocomposites for industrial wastewater treatment, Environ. Res. 170: 222-229. [Ref.]
- Warkhade SK, Zodape SP, Pratap UR, et al. 2018. Rutile TiO2/CoSe nanocomposite: An efficient photocatalyst for photodegradation of model organic dyes under visible light irradiation, J. Mol. Liq. 279: 434-443. [Ref.]
- Singla P, Goel N, kumar V, et al. 2015. Boron nitride nanomaterials with different morphologies: Synthesis, characterization and efficient application in dye adsorption, Ceram. Int. 41. [Ref.]
- Shahabuddin S, Khanam R, Khalid M, et al. 2018. Synthesis of 2D boron nitride doped polyaniline hybrid nanocomposites for photocatalytic degradation of carcinogenic dyes from aqueous solution, Arab. J. Chem. 11: 1000-1016. [Ref.]
- Lin L, Jiang W, Bechelany M, et al. 2019. Adsorption and photocatalytic oxidation of ibuprofen using nanocomposites of TiO2 nanofibers combined with BN nanosheets: Degradation products and mechanisms, Chemosphere. 220: 921-929. [Ref.]
- Nnaji CO, Jeevanandam J, Chan YS, et al. 2017. Engineered nanomaterials for wastewater treatment: current and future trends, Elsevier.[Ref.]
- Chen X, Ku S, Weibel JA, et al. 2017. Enhanced Antimicrobial Efficacy of Bimetallic Porous CuO Microspheres Decorated with Ag Nanoparticles, ACS Appl. Mater. Interfaces. 9: 39165-39173. [Ref.]
- Lu F, Astruc D. 2018. Nanomaterials for removal of toxic elements from water Precipitaion Adsorption Heavy metals Removal Ion Exchange Filtration Coagulation Bio-sorprtion, Coord. Chem. Rev. 356: 147-164. [Ref.]
- Kabir E, Kim K, Yip ACK, et al. 2018. Environmental impacts of nanomaterials, J. Environ. Manage. 225: 261-271. [Ref.]
- El-Sayed GO, Yehia MM, Asaad AA. 2014. Assessment of activated carbon prepared from corncob by chemical activation with phosphoric acid, Water Resour. Ind. 66-75. [Ref.]
- Juan Y, Ke-qiang Q. 2009. Preparation of Activated Carbon by Chemical Activation under Vacuum, Environ. Sci. Technol. 43. [Ref.]
- Hai Y, Li X, Wu H, et al. 2015. Modification of acid-activated kaolinite with TiO2 and its use for the removal of azo dyes, Appl. Clay Sci. 114: 558-567. [Ref.]
- Schmidt AM. 2006. Electromagnetic Activation of Shape Memory Polymer Networks Containing Magnetic Nanoparticles, Macromol. Rapid Commun. 27: 1168-1172. [Ref.]
- Spyrou K, Rudolf P. 2014. An Introduction to Graphene, in: V. Georgakilas (Ed.), Funct. Graphene, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany.[Ref.]
- Ai L, Zhang C, Chen Z. 2011. Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite, J. Hazard. Mater. 192: 1515-1524. [Ref.]
- Zhao D, Gao X, Wu C, et al. 2016. Facile preparation of amino functionalized graphene oxide decorated with Fe 3 O 4 nanoparticles for the adsorption of Cr (VI), Appl. Surf. Sci. 384: 1-9. [Ref.]
- Othman NH, Alias NH, Shahruddin MZ, et al. 2018. Adsorption kinetics of methylene blue dyes onto magnetic graphene oxide, J. Environ. Chem. Eng. 6: 2803-2811. [Ref.]
- Vecera P, Chacón-Torres JC, Pichler T, et al. 2017. Precise determination of graphene functionalization by in situ Raman spectroscopy, Nat. Commun. 8: 15192. [Ref.]
- Fraga TJM, Fraga DMDSM, da Silva TC, et al. 2018. Adsorption of reactive dyes onto thermally treated waste from aluminum lamination, Water Pract. Technol. 13: 629-641. [Ref.]




















