Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/jca.2019.110001Article Views : 2556Article Downloads : 43
Synthesis and Characteristics of Rice Husk Ash Based Magnetic Hydrogel (RHA-MH) for H2 Generation
Irvan Dahlan1,2* and Muhammad Syafieq Abd Halim1
1School of Chemical Engineering, Universiti Sains Malaysia, Engineering Campus, Seri Ampangan, 14300 Nibong Tebal, Pulau Pinang, Malaysia
2Solid Waste Management Cluster, Science and Engineering Research Centre, Universiti Sains Malaysia, Engineering Campus, Seri Ampangan, 14300 Nibong Tebal, Pulau Pinang, Malaysia
*Corresponding author: Irvan Dahlan, School of Chemical Engineering, Universiti Sains Malaysia, Engineering Campus, Seri Ampangan, 14300 Nibong Tebal, Pulau Pinang, Malaysia, Tel: +604-5996463; Fax: +604?5996908; Email: chirvan@usm.my
Article Information
Aritcle Type: Research Article
Citation: Irvan D, Muhammad SAH. 2019. Synthesis and Characteristics of Rice Husk Ash Based Magnetic Hydrogel (RHA-MH) for H2 Generation. J Chem Appl. 1: 01-09.
Copyright:This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Irvan D
Publication history:
Received date: 11 January, 2019Accepted date: 22 January, 2019
Published date: 23 January, 2019
Abstract: In this study, magnetic hydrogel was synthesized from rice husk ash (RHA-MH) using two methods, i.e. sol-gel and cross-linking methods. The prepared rice husk ash based magnetic hydrogel (RHA-MH) were used as a catalyst for H2 generation through hydrolysis of sodium borohydride (NaBH4). Factors effecting hydrolysis reaction such as effect of RHA calcination temperature and method used to synthesis the catalyst were investigated. The RHA-MH1200 synthesised via cross-linking and sol-gel methods (using RHA calcined at 1200°C) shows the shortest time taken (5.64 min and 11.18 min, respectively) to produce 40 mL of H2 gas as compared to RHA-MH600 (i.e. 10.47 min and 13.18 min respectively, for both methods using RHA calcined at 600°C). The prepared and spent RHA-MH were also characterized using Scanning Electron Microscopy (SEM)/Energy Dispersive X-ray (EDX) analysis. From SEM image, it could be concluded that silica (SiO2) could successfully be utilized as the supporting material for stabilizing magnetic iron oxide (Fe3O4) nanoparticles. The SEM image also showed that the overall structure was relatively porous. Moreover, the SEM images of magnetic hydrogel before and after the hydrolysis of NaBH4 exhibited similar physical structures.
Keywords: Hydrogen; NaBH4 hydrolysis; Rice husk ash based magnetic hydrogel (RHA-MH); Sol-gel; Cross-linking
Introduction
Recently, investigations about renewable and alternative energy resources worldwide have increased due to high demand, and the possibility of near future depletion of fossil fuel reserves [1]. The environmental concerns due to the destructive effects of fossil fuels which release harmful gases to the environment such as carbon dioxide, carbon monoxide, sulphur oxide, methane, nitrous oxide etc. which can result in global warming, climate change, natural disasters and deterioration of ecological systems [2]. This necessitates a novel approach of alternative, renewable and clean energy sources. Researchers seek to acquire clean and renewable energy by employing various technologies from wind power, solar, tidal, hydro power, biomass, geothermal energy, wave power and hydrogen energy technologies. Sahiner and Yasar [1] stated that hydrogen energy can be considered as potential alternative source to replace fossil fuel. Therefore, according to previous studies, researchers have focused on the production of hydrogen utilizing various methods such as hydrolysis, electrolysis, thrombolysis, and enzymatic.
Lately, numerous transition metal catalyst systems have been reported in hydrolysis reactions for hydrogen production. Ni-Ru nanoparticle composite catalyst, rhodium, AlCl3, alloys of Ni-Co-B, Ni (0) nanoclusters stabilize with hydrogen phosphate, Co-B/ Ni foam, carbon-supported Co-B and Co-P, Co-Ni-P, fluorinated cobalt, cobalt chloride, zirconia, and ruthenium are some examples [3,4]. However, it is convenient to use a hydrogel network structure for the preparation of metallic particles as hydrogels are three dimensional cross-linked hydrophilic polymer materials. Hydrogels can provide adaptable support networks with a built in three-dimensional polymeric structure advantageous for in situ metal nanoparticle synthesis. The structure containing the hydrophilic groups (-SO3H, -OH, -COOH, -NH2) allows water and other liquids to penetrate inside the hydrogel allowing them to swell and capture metal ions [5]. Because of these functional groups, hydrogels can respond to environmental effects such pH, ionic strength, temperature, electric and magnetic fields and can even recognize certain molecules. Additionally, hydrogels are biocompatible and have been used as a drug release system control, as sensors in biomedical applications, for separation and purification processes, in tissue engineering, as well as use as a support for catalyst materials [2].
Recently, Nurettin et al. [6], has discovered that metal particles and magnetic nanoparticles, such as Fe3O4 or γ-Fe2O3 (ferrites), can be prepared in situ within crosslinked hydrogel networks has become a promising method for environmental purification as it produces no contamination and it is easily separated from the medium under applied magnetic field. Hence, hydrogels with magnetic properties have become a new class of intelligent materials which attract interest as absorbents for the extraction of hydrogen from the solution.
In hydrolysis reaction, the presence of catalyst system could increase in the reaction rates with high yield. Thus, in order to increase the reaction rates the used of heterogeneous catalyst systems are preferred [1]. The advantages of heterogeneous catalysts can be separated from a reaction mixture in a straightforward manner, such as by filtration. In this way, expensive catalysts can be easily and effectively recovered, which is an important consideration for industrial manufacturing processes. However, one limitation of heterogeneous catalysis is that the availability of surface area of the catalyst. Once the surface area of the catalyst is completely saturated with reactant molecules, the reaction cannot proceed until products leave the surface, and some space opens up again for a new reactant molecule to adsorb or attach. Furthermore, in the utilization of metal nanoparticles as a catalyst, the most important problem is agglomeration. The aggregation of nanoparticles causes a reduction in the catalytic activity of metal particles as the surface area of the particles was decreased.
On the other hand, the cost associated with its magnetic hydrogel production is very high. Thus, needs a novel approach to develop a low-cost and environmentally friendly magnetic hydrogel. Using low-cost bio materials, such as agricultural wastes, clay materials, and biomass, may be a suitable approach because they are capable be used as a template in extracting hydrogen [7,8]. Therefore, in this study, an attempt was made to utilize industrial waste material as an alternative type of material to be incorporated to magnetic hydrogels, i.e. rice husk ash (RHA). RHA was obtained from the burning of rice husk. The type and content of silica (in RHA) were affect to the surface properties of hydrogel as the nanoparticles will only be adsorb in the crystalline region of the silica due to the higher purity and high crystallinity [9]. The main goal of this study is to synthesize and characterize rice husk ash based magnetic hydrogel (RHA-MH). RHA-MH were used as a catalyst for H2 generation through hydrolysis of sodium borohydride (NaBH4).
Materials and Methods
Chemical
The raw rice husk ash (RHA) was collected from Honseng Rice Mill Sdn Bhd, Nibong Tebal, Malaysia. Glutaric dialdehyde solution, iron (II,III) oxide nanoparticle (OD ¼ 20 nm, purity>99.9%), sodium borohydride and ethanol were obtained from Sigma-Aldrich (M) Sdn. Bhd. Sulphuric acid (H2SO4) were purchased from Fisher Chemicals. Iron (III) chloride hexahydrate (FeCl3.6H2O), 1-butyl-3-methylimidazolium chloride (bmimCl), and Span 80 were commercial products from Merck Sdn. Bhd. Vacuum pump oil is obtained from Robinair. Doubly distilled water and deionized water was used throughout this study. Other chemicals were of laboratory reagent grade and used without further purification.
Synthesis of RHA-MH via Cross-Linking Method
The RHA-MH via cross-linking method was prepared by modifying the literature procedure [10]. First, the raw RHA was further calcinated at different temperatures (i.e. 600°C, 800°C, 1000°C and 1200 °C) using furnace. In the preparation of RHA-MH, about 10g of calcinated RHA was dissolved in 200 mL of 0.1M iron chloride (FeCl3) aqueous solution. The solution was stirred at room temperature for 4 h at 1000 rpm. Subsequently, 0.5g of fine magnetic iron oxide (Fe3O4) nanoparticles was added into the mixture and dispersed sufficiently by stirring uniformly for 1 h. Then, 100 mL of ethanol was added and magnetic RHA-Fe(III) complex precipitation was allowed to occur overnight. Then, the magnetic RHA-Fe(III) complex precipitate was washed with ethanol to remove to remove an excess of FeCl3 and dried at 80°C. The dried sample was placed in 100 mL of ethanol in contact with 100 mL of 5wt% glutaraldehyde for 3 h. After chemical cross-linking, RHA-MH was collected by magnetic separation and the attracted sample was further washed with distilled water in order to remove the unreacted glutaraldehyde. Finally, the RHA-MH were dried at 80°C and store in air tight container.
Synthesis of RHA-MH via Sol-Gel Method
The RHA-MH via sol-gel method was prepared according to Liu, et. al. [8]. About 5g of calcinated RHA and 71.43 g of 1-butyl-3-methylimidazolium chloride ([bmim][Cl]) were dried at 80°C for 24 h in a vacuum oven before use. About 5g of RHA were dissolved in 71.43 g bmimCl at 100°C for 2 h using overhead stirrer. Then, 5 g of magnetic iron oxide (Fe3O4) nanoparticles was added to the solution and homogenized by continuous agitation for 30 min. After that, the mixture was dispersed in 100mL vacuum pump oil with 5wt% (5 mL) Span 80 under agitation of 1000 rpm for 4 h at 100 °C and cooled down to room temperature. During stirring, excess ethanol was added to the mixture to further regenerate the hydrogels microspheres from ionic liquid (ILs) of bmimCl. After reaching room temperature, the oil phase on top layer was poured out. The remaining mixture was further washed with five times ethanol followed by five time’s deionized water thoroughly in order to remove the residual ionic ILs. Finally, the RHA-MH were dried at 80 °C and stored in air tight container.
Hydrolysis of Sodium Borohydride (NaBH4)
Hydrolysis of sodium borohydride (NaBH4) was carried out with reference to the procedure outlined by Ozay et al. [2]. An experimental rig was used for hydrolysis of NaBH4. In the hydrolysis of NaBH4, several operating conditions were made constant throughout the entire study. However, the effect of RHA calcination temperature and method used to synthesise the catalyst on the hydrolysis of NaBH4 was studied. Overall, the experimental variables involved in the project were summarized as shown below:
(i) Constant variables: Concentration of NaBH4 solution at 100mM (0.2 g of sodium borohydride), reaction mixing rate at 150 rpm and the RHA-MH amount 0.2 g.
(ii) Manipulated variables: Type and content of RHA in RHA-MH (calcinated at 600, 800, 1000 and 1200 °C) and method used to synthesise the catalyst.
(iii) Responding variable: Time taken to produce 5 mL, 10 mL, 15 mL, 20 mL, 25 mL, 30 mL, 35 mL and 40 mL of hydrogen gas.
Characterization of RHA-MH
The surface morphologies of the selected RHA-MH were analysed using scanning electron microscopy (SEM FEI, Quanta™ 450 FEG). The sample was first mounted on the stub over double sided sticky carbon paper. Then, the stub with mounted sample was coated with gold for 2 min and 40 seconds at 30 miliamps. This gold-plated stub was then placed inside the SEM chamber under high vacuum. The chamber is equipped with probes for visualization of sample placed in the chamber. The SEM is equipped with an Energy Dispersive X-ray (EDX) that determine the composition of element on the surface targeted.
Results and Discussion
SEM/EDX Characterization of RHA-MH by Cross-Linking Method
The SEM images of RHA-MH before and after the hydrolysis of NaBH4 are presented in Figure 1a,b, respectively.
Figure 1: SEM images of RHA-MH (a) before and (b) after hydrolysis of NaBH4.


It can be seen that the overall structure of RHA-MH was relatively porous. Moreover, the SEM images of RHA-MH before and after the hydrolysis of NaBH4 exhibited almost similar physical structures. The structure image of RHA-MH synthesized in this study was different if compared to structure image of magnetic chitosan-Fe(III) hydrogel prepared via cross-link method reported by Shen et al. [10]. Shen et al. [10] claimed that the surface of magnetic chitosan-Fe(III) hydrogel was irregular and nonporous structure. In this project, the RHA-MH was relatively porous. This would probably due to the use of different raw materials to synthesize magnetic hydrogel.
RHA is made up of many different chemical species especially silica (SiO2) and aluminium oxide (Al2O3) in majority. Therefore, an identification and evident of the silica (SiO2) structure was needed. From the EDX analysis it showed that the RHA surface compose of 15% of carbon (C), 40% of oxygen (O2), 10 % of silica (Si) and 35 % of iron (Fe) by weight. The amount of silica is not very high in this case because the prepared RHA-MH which consist of SiO2 as the raw material of RHA has been covered or agglomerate by Fe3O4 nanoparticle. Besides that, it is possible that the SiO2in prepared RHA-MH has been modified during the chemical reaction. Since the catalyst do not change in structure after the reaction the composition of element on the surface of spent catalyst is almost the same which is 10% of carbon (C), 40% of oxygen (O2), 15 % of silica (Si) and 35 % of iron (Fe) by weight.
SEM/EDX Characterization of RHA-MH by Sol-Gel Method
The prepared and spent RHA-MH1200°C were characterized by using SEM/EDX technique. Unfortunately, due to wet looking and oily surface of RHA-MH prepared via sol-gel method, SEM machine cannot scan the sample and the image produce are all black and not clear. The only image that can be captured is the SEM images of spent RHA-MH after the hydrolysis of NaBH4 are presented in Figure 2. The black colour background is not the surface of catalyst, but it is double-sided tape used to attach the sample. From the EDX analysis it showed that there was Si and iron oxide (Fe3O4) nanoparticles on the surface.
Figure 2: SEM images of RHA-MH sample after hydrolysis of NaBH4 at magnification of (a) 200X and (b) 1000X.

Effect of RHA Calcination Temperature
The effect of calcinated RHA ( at different temperatures) during the preparation RHA-MH via cross-link and sol-gel methods for hydrolysis of NaBH4 was investigated and the results are shown in Figure 3,4, respectively. It can be seen that increasing RHA calcination temperature have resulted in shorter time to produce 40 mL of H2 gas. For RHA-MH synthesized via cross-link method (Figure 3), it took only 5.64 min to produce 40mL of H2 using RHA-MH1200°C, however, 10.47 min was needed to produce 40mL of H2 gas using RHA-MH600°C which was twice the time taken. Similar observation can also be seen in Figure 4 for RHA-MH1200°C synthesized via sol-gel method, where 11.18 min and 13.18 min were needed for RHA-MH1200°C and RHA-MH600°C to produce 40mL of H2 gas.
Figure 3: Volume of hydrogen plot versus time for catalyzed reaction by RHA-MH synthesized via cross-link method (100mM NaBH4, 150 rpm mixing rate, 0.2 g RHA-MH).

Figure 4: Volume of hydrogen plot versus time for catalyzed reaction by RHA-MH synthesized via sol-gel method (100mM NaBH4, 150 rpm mixing rate, 0.2 g RHA-MH).

Silica, as formed by the chemical reaction of silicon (Si) and oxygen (O2) can be either crystalline or amorphous. Depending upon the temperature, pressure and the rate of cooling, solid silica can take up different forms. According to Nuruddin et al. [11], RHA calcined at 600 °C (1atm) are in amorphous state on the other hand RHA calcined at 1200°C (1atm) are crystalline. Thus, in this study, silica inside RHA-MH1200°C are in crystalline form whereas inside RHA-MH600°C are amorphous for both methods used to synthesize the catalyst. Crystalline and amorphous silica are identical chemically (SiO4). But they attain different physical forms as their internal structures are very different due to different arrangements of Si and O layers (like ABA--- and ABC-- packing of layers) but each Si is always surrounded by four O atoms (SiO4). Since crystalline silica contain only Si and O2 it has very large surface area and porous structure compare to amorphous silica [9]. Thus, due to large effective surface area available, large volume of iron oxide (Fe3O4) can be absorbed and dispersed into the pores and surface of crystalline silica inside RHA-MH1200°C and eventually there would be more active sites or effective surface area to catalyze the reaction. RHA-MH600°C can only absorb small amount of iron oxide (Fe3O4) nanoparticle due to limited surface area and non-porous. Iron oxide (Fe3O4) nanoparticle acts as a catalyst provides an alternative route for the hydrolysis reaction with a lower activation energy that will further discuss in following section and silica acts as a template that provide surface area for iron oxide (Fe3O4) deposition. The more iron oxide (Fe3O4) that were attached to the surface of RHA the higher the number of active sites available for catalysis to happen, hence the higher the rate of reaction. Thus, this proved that RHA-MH1200°C help to produce 40 mL H2 gases faster compare to RHA-MH600°C for both methods.
Effect of Method Used to Synthesis Catalyst in H2 Generation
The effect of method used to synthesis the catalyst for hydrolysis of sodium borohydride (NaBH4) was investigated. The best catalyst which produce higher yield of H2 gas in short period of time are chosen from two different methods which are sol-gel method and cross-link method to give a reasonable comparison. Figure 5 shows this comparison.
Figure 5: Volume of hydrogen plot vs. time for uncatalyzed reaction (100mM NaBH4, 150 rpm mixing rate) & catalysed reaction (100mM NaBH4, 150 rpm mixing rate, 0.2 g RHA-MH).
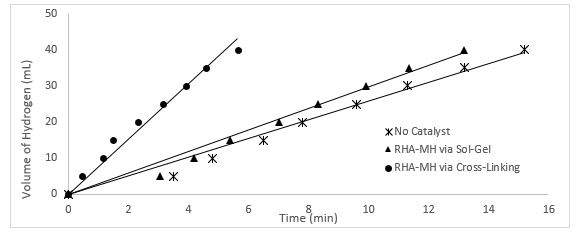
As could be clearly illustrated in Fig. 5, there is an obvious different in the rate of production of hydrogen gas using catalyst from different method. For example, it took 5.64 min to produce 40mL of H2 when 0.2 g of RHA-MH by cross-linking method was used as catalyst. However, 11.18 min was required to produce 40mL of H2 gas when 0.2g of RHA-MH by sol-gel method was used. Catalyst produce by cross-linking method catalysed the hydrogen production twice as fast as catalyst produce by sol-gel method. This observation could be explained in term of the raw material and the way the catalyst is synthesised. Apart from that, the finding from this study was consistent with the outcomes of study done by Ferreira [12] which show that the uncatalyzed aqueous hydrolysis reaction of NaBH4 proceeds at a very slow rate. In more concentrated solutions, the rate was diminished probably due to solubility and mobility inhibition by the NaBO2 product that is produce from the reaction. It was found that the addition of alkali stabilizers such as NaBO2, KOH and NaOH depressed the hydrogen evolution, a property utilized in many practical chemical hydride systems to promote stable long term storage. Besides that, Ferreira [12] also stated that the present of catalyst will shorter time taken to produce given volume of hydrogen, where in his study, the hydrolysis of NaBH4 using Ru-Ni based metal catalyst could produce 78% yield of H2 in just 19000 sec compare to hydrolysis of NaBH4alone to produce same yield which took 26000 sec.
Conclusions
RHA-MH were successfully synthesized via two different methods, which were cross-linking method and sol-gel method. Besides that, the implementation of new emerging technology such as Fe3O4 nanoparticle provides an easy and efficient way to separate the substance from solution using external magnetic field. SEM/EDX images of RHA-MH prepared by two different methods showed some similarities. The factors affecting hydrolysis of NaBH4, such as effect of RHA calcination temperature and effect of method used to synthesis of catalyst were investigated. RHA-MH1200°C gave high rate of production of hydrogen in short period of time in both synthesis method. This is due to purity and crystallinity of silica. The time taken to produce a maximum 40mL of hydrogen gas is shorter compare to uncatalyzed reactions.
Acknowledgements
The authors wish to acknowledge the financial support from Universiti Sains Malaysia and Iconic Grant Scheme (A/C. 1001/CKT/870023) for research associated with the Solid Waste Management Cluster.
References
- Sahiner N, Yasar AO. 2014. H2 Generation from NaBH4 and NH3BH3 using metal catalysts prepared within p(VI) capsule particles. Fuel Process. Technol. 125: 148-154.[Ref.]
- Ozay O, Aktas N, Inger E, et al. 2011. Hydrogel assisted nickel nanoparticle synthesis and their use in hydrogen production from sodium boron hydride. Int J Hydrogen Energy. 36: 1998-2006.[Ref.]
- Sahiner N, Ozay O, Inger E, et al. 2011. Superabsorbent hydrogels for cobalt nanoparticle synthesis and hydrogen production from hydrolysis of sodium boron hydride. Appl Catal B. 102: 201-206.[Ref.]
- Sahiner N, Ozaya O, Inger E, et al. 2011. Controllable hydrogen generation by use smart hydrogel reactor containing Ru nano catalyst and magnetic iron nanoparticles. J Power Sources. 196: 10111.[Ref.]
- Sahiner N, Ozay H, Ozay O, et al. 2010. New catalytic route: Hydrogels as templates and reactors for in situ ni nanoparticle synthesis and usage in the reduction of 2- and 4-nitrophenols. Appl Catal. 385: 201-207.[Ref.]
- Nurettin, Sahiner, Ozgur, et al. 2011. The on demand generation of hydrogen from co-ni bimetallic nano catalyst prepared by dual use of hydrogel: As template and as reactor. Int. J. Hydrogen Energy. 36: 15250-15258.[Ref.]
- Liu Z, Wang H, Li B, et al. 2012. Biocompatible magnetic cellulose−chitosan hybrid gel microspheres reconstituted from ionic liquids for enzyme immobilization. J Mater Chem. 22: 15085-15091.[Ref.]
- Liu Z, Wang H, Liu C, et al. 2012. Magnetic cellulose-chitosan hydrogels prepared from ionic liquids as reusable adsorbent for removal of heavy metal ions. Chem Commun, 48: 7350.[Ref.]
- Rodrigues FHA, Fajardo AR, Pereira AGB, et al. 2012. Chitosan-graft-poly(acrylic acid)/rice husk ash based superabsorbent hydrogel composite: Preparation and characterization. J Polym Res. 19: 1-10.[Ref.]
- Shen C, Shen Y, Wen Y, et al. 2011. Fast and Highly Efficient Removal of Dyes under Alkaline Conditions Using Magnetic Chitosan-Fe(III) Hydrogel. Water Res. 45: 5200-5210.[Ref.]
- Nuruddin MF, Shafiq N, Kamal NLM. 2015. The effects of types of rice husk ash on the porosity of concrete. In the proceeding of the 7th Asia Pacific Structural Engineering and Construction Conference & 2nd European Asian Civil Engineering Forum (APSEC/EACEF 2009). 602.[Ref.]
- Ferreira MJF. 2009. Catalytic generation and storage of hydrogen from hydrolysis of sodium borohydride under pressure - Application in a hydrogen/oxygen fuel cell. MSc Thesis, University of Porto, Portugal. [Ref.]




















