Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/jca.2022.110040Article Views : 0Article Downloads : 2
Study of leukopenia induced by a high doses of cyclophosphamide in mice
Idriss H. Mohamed*
Zoology Department, Faculty of Science, Omar AL-Mukhtar University, Albida, Lybia
*Corresponding Author: Idriss H. Mohamed, Idriss H. Mohamed, Zoology Department, Faculty of Science, Omar AL-Mukhtar University, Albida, Lybia; Email: idress.hamad@omu.edu.ly
Article Information
Aritcle Type: Research Article
Citation: Idriss H. Mohamed. 2022. Study of leukopenia induced by a high doses of cyclophosphamide in mice. J Chem Appl. 4: 21-26.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2022; Idriss H. Mohamed
Publication history:
Received date: 04 August, 2022Accepted date: 20 August, 2022
Published date: 25 August, 2022
Abstract
The study was conducted to evaluate the toxic effect of different concentrations of cyclophosphamide (CTX) by inducing leukopenia. Adult female Swiss albino mice weighting (20±4g) arranged in three groups of six animals each housed . a control mice saline PBS solution (group I), mice treated with 200 mg/Kg (4mg/mouse) cyclophosphamide, High dose (group II) and mice treated with treated 100 mg/kg (2mg/mouse) body weight Low dose (group III) for three weeks . Cyclophosphamide causes a significant decrease (P < 0.01) in total leukocyte count (TLC), total erythrocyte count (TEC), and red blood cell distribution (RDW) in all groups compared to the control group of mice. Observed leukopenia inform marked reduced in the absolute numbers of lymphocytes, monocytes, and neutrophils in comparison to the control group of mice. The results indicate that cyclophosphamide alters the blood profile only after high doses while low doses had the least impact on blood pictures. Cyclophosphamide causes a significant increased (P < 0.01) in activity of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum after CTX treatment with no effect on the serum albumin level compared to the control group. While lower doses had less effect on liver enzymes.
Keywords: Cyclophosphamide; Cytotoxic effect; Leukopenia; Total erythrocyte count (TEC); Alanine amino transferase (ALT); Total leukocyte count (TLC)
Introduction
Cyclophosphamide (CTX) is a class of antineoplastic drugs, widely used in the treatment of various types of cancer [1]. Due to this cytotoxic property it is used extensively in a variety of carcinomas singly or in combination with other drugs to treat a variety of leukemias, Hodgkin’s lymphoma, Multiple myeloma, Mycosis fungigoides, lymphoma, breast cancer, ovarian cancer and Retinoblastoma. Cyclophosphamide is also used at lower doses for some autoimmune diseases such as systemic lupus erythematosus, severe rheumatoid arthritis, Wegener’s granulomatosis, multiple sclerosis, some connective tissue disorders, minimal lesion glomerulonephritis, several forms of vasculitis, Nephrotic Syndrome and post organ transplantation for prevention of rejection of the organ [2]. Cyclophosphamide is a prodrug that gets activated to alkylating phosphoramide mustard in the liver and excreted primarily (70%) in urine in forms of metabolites [3]. Adverse effects of cyclophosphamide include alopecia, thrombocytopenia, mucosal ulcerations, leukopenia, hematuria, diarrhoea, hemorrhagic cystitis and petechial haemorrhage in lungs and small bowel [4,5]. Cyclophosphamide is also documented to cause hepatotoxicity in Fischer rats and cardiopulmonary toxicity in Mongrel dogs [6,7]. Recent studies suggest that CTX generates reactive oxygen species (ROS) like superoxide anion, hydroxyl radical, and hydrogen peroxide (H2O2) during its oxidative metabolism and depresses the antioxidant defense mechanisms in the liver [8-9]. Detailed analysis of this leucopenic effect in mice showed in our study, that establish the CTX decreasing effect on the absolute number of leukocytes in the peripheral blood (PBL) [10].CTX is transformed via hepatic enzymes to active alkylating metabolites. CTX cytotoxicity is due to the formation of phosphoramide mustard, which generates DNA crosslinks and leads to apoptosis [4]. Cells deficient in aldehyde dehydrogenase (ALDH), the enzyme involved in CTX detoxification, produce an active phosphoramide metabolite. Low levels of ALDH are expressed in lymphocytes and, therefore, these cells are sensitive to CTX. Conversely, hematopoietic stem cells (HSCs) express high levels of ALDH and resist CTX-mediated cytotoxicity [1].
Material and Methods
Adult female Swiss albino mice weighting (20±4g) were procured and arranged in three groups of six animals each housed with ad libitum access to food and water. Group I Control animals were given orally an equal volume of diluents (0.9% sterile saline solution) for three weeks. Group II for induction of leukopenia mice (n=6/group) were treated for three weeks with intraperitoneal (i.p.) injection of 200 mg/Kg (4mg/mouse) cyclophosphamide (High dose). Group III mice were treated intraperitoneal (i.p.) with cyclophosphamide in the dose of 100 mg/kg (2mg/mouse) body weight (Low dose) a for three weeks.
Evaluation of Hematological Parameters
Blood samples with anticoagulant EDTA were analyzed for hematological parameters of (TEC), (TLC) and (RDW) counts, and an absolute number of neutrophils, monocyte, and lymphocytes according to Feldman [11].
Serum Biochemical Analysis
The serum liver enzymes aspartate transaminase (AST) and alanine transaminase (ALT) and the liver albumin were determined according to the manufacturer’s instructions (Biosystem, Egypt) [12-13].
Statistical analysis
Data are obtained from each experiment were analyzed by using Microsoft Excel (Seattle, WA). The differences between the experimental groups were assessed using the Student’s t-test. P > 0.05 was considered to indicate statistical significance by using Graph Pad Prism version 4.0 software (Graph Pad).
Results and discussion
We found that mice received treatment with CTX induced sharp decrease in both in the total number of leukocytes as well as in the number of neutrophils. This treatment, however, induced decreases in the number of lymphocytes and monocytes. These data are consistent with data reported by [14] on the effect of CTX in mice. Anaemia is a deficiency in the concentration of haemoglobin-containing red blood cells that is prevalent among cancer patients [15]. Leucopaenia is also a common adverse event in cancer therapy [16]. CTX treatment can reduce the number of TEC, TLC and RDW the peripheral blood analysis revealed the high-dose CTX treatments stably decreased the number of TLC and RBCs compared with that of the control group, which is consistent with the previous studies [17,18]. Meanwhile, our animal model showed a more sustained suppression on TLC and TEC. Unlike in previous reports, the number of TLC in the CTX treatment groups was increased, which needs further research. with the findings of several workers. The decrease in the TEC, TLC concentration and RDW leading to anaemia and leucopenia in patients treated with cyclophosphamide was reported by [19]. The leucocytic depression (leucopenia) was also reported by [20] It may be concluded that by inhibiting haematopoiesis, cyclophosphamide has dropped TLC leading to leucopenia after prolonged treatment.
|
Table 1: Effect of different treatments on the number of TEC, TLC, and RDW. |
|||
|
Parameters |
PBS |
200mg/Kg (High dose) |
100mg/kg (Low dose) |
|
TLC (×103) |
3.8±0.48 |
1.80±0.48** |
2.00 ±0.41 |
|
TEC (×106) |
5.98±0.16 |
1.71±0.30** |
3.60±0.32 |
|
RDW (×104) |
20.21±0.59 |
15.08±0.57* |
17.65±3.17 |
|
CTX, cyclophosphamide; PBS, Phosphate buffer saline; TLC, total leukocyte count total erythrocyte count; TEC, total erythrocyte count; and RDW, Red blood cell distribution width; ns, non-significant; *and**significant at P≤0.05 and 0.01, respectively. |
|||
|
Table 2: Effect of different treatments on the number of monocyte, neutrophils, and lymphocytes. |
|||
|
Parameters |
PBS |
200mg/Kg (High dose) |
100mg/kg (Low dose) |
|
Monocyte\ cmm |
763.70±2.70 |
584.08±11.10 |
633.25±14.41 |
|
Neutrophil \ cmm |
643.33±9.04 |
589.67±11.72 |
785.33±17.67 |
|
Lymphocyte\ cmm |
2606.23±39.89 |
1043.00±5.50 |
2384.67±38.16 |
|
CTX, cyclophosphamide; PBS, Phosphate buffer saline; monocyte; neutrophil; and lymphocyte; ns, non-significant respectively. |
|||
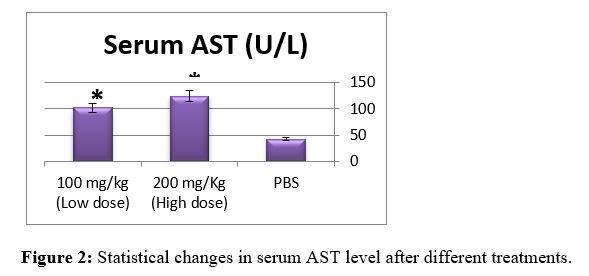
As shown in Table 3, CTX treatment significantly increased the activities of AST and ALT in sera with no effect on the serum albumin level as compared to control group(p<0.01). Any damage to the liver cells will result in an increase in liver enzymes (ALT & AST) [21]. The elevation of AST and ALT in our present findings is consistent with [22]. The liver is the richest source of both AST and ALT enzymes and, thus, the levels of both these enzymes are expected to increase as a result of damage to the liver cells [23]. That related to chemical-induced tissue injury along with hepatocellular necrosis [24]. The elevation of AST was observed in our study is consistent with the data reported by [25]. ALT is now frequently detected to estimate the degree of liver dysfunction due to CTX or advanced liver cirrhosis as well as the expectation of heart failure development [26]. In support of these reports, our data shown in Table 3 indicate that CTX treatment resulted in a significant increase in the activities of AST and ALT in sera with no effect on the serum albumin level as compared to the control group . CPA belongs to this cytotoxic alkylating group of drugs. It is bio-activated by hepatic cytochrome P450 enzymes ensuing in the productionof its two metabolites: phosphoramidemustard and acrolein. Acrolein, a byproductmetabolite of CTX, obstructs the tissue antioxidant defense system, producesreactive oxygen species (ROS), and interacts with protein amino acids causing structural and functional changes [27].
|
\ Table 3: Effect of different treatments on serum liver function parameters. |
|||
|
Parameters |
PBS |
200mg/Kg (High dose) |
100mg/kg (Low dose) |
|
Serum ALT (U/L) |
31.39 ± 1.48 |
96.18 ± 2.07** |
67.61 ± 1.19 |
|
Serum AST (U/L) |
42.37 ± 1.46 |
123.78 ± 4.24** |
101.67 ± 3.38* |
|
Albumin(mg/dl) |
45.01±0.91 |
45.76±1.10 |
46.35±3.27 |
|
CTX, Cyclophosphamide; PBS, Phosphate buffer saline; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase and Albumin; ns, non-significant; * and ** significant at P≤0.05 and 0.01, respectively. |
|||


References
1. Pass GJ, Carrie D, Boylan N. 2005. Role of hepatic cytochrome P450s in the pharmacokinetics and toxicity of cyclophosphamide: studies with the hepatic cytochrome P450 reductase null mouse. Cancer Research. 65: 4211-4217. Ref.: https://pubmed.ncbi.nlm.nih.gov/15899812/ DOI: https://doi.org/10.1158/0008-5472.can04-4103
2. Ataya KM, Valeriote FA, RamahiAtaya AJ. 1989. Effect of Cyclophosphamide on the Immature Rat Ovary. Cancer Res. 49:1660-1664. Ref.: https://pubmed.ncbi.nlm.nih.gov/2924314/
3. Foley GE, Friedman OM, Drolet BP. 1961. Studies on the Mechanism of Action of Cytoxan Evidence of Activation in Vivo and in Vitro. Cancer Res. 21: 57-63. Ref.: https://pubmed.ncbi.nlm.nih.gov/13700518/
4. Poblador MS, Rojas C, Raya A, et al. 1989. The effects of cyclophosphamide on the prolactin cells of the normal rat. Histol Histopath. 4: 27-30. Ref https://pubmed.ncbi.nlm.nih.gov/2485186/
5. Sharbaugh RJ, Grogan JB. 1969. Suppression of Reticuloendothelial Function in the Rat with Cyclophosphamide. J Bacteriol. 100: 117-122. Ref.: https://pubmed.ncbi.nlm.nih.gov/4981054/ DOI: https://doi.org/10.1128/jb.100.1.117- 122.1969
6. Lavin P, Koss LG. 1971. Effects of a Single Dose of Cyclophosphamide on various Organs in the Rat. American Journal of Pathology. 62: 159-165. Ref.: https://pubmed.ncbi.nlm.nih.gov/5540655/
7. O'Connell TX, Berenbaum MC. 1974. Cardiac and Pulmonary Effects of High Doses of Cyclophosphamide and Isophosphamide. Cancer Research July. 34: 1586-1591. Ref.: https://pubmed.ncbi.nlm.nih.gov/4833912/
8. Bhattacharya A, Lawrence RL, Krishnan A,et al. 2003. Effect of dietary n-3 and n-6 oils with and without food restriction on activity of antioxidant enzymes and lipid peroxidation in livers of cyclophosphamide treated autoimmune-prone NZB/W female mice,” Journal of the American College of Nutrition. 22: 388-399. Ref.: https://pubmed.ncbi.nlm.nih.gov/14559931/ DOI: https://doi.org/10.1080/07315724.2003.10719 322
9. Stankiewicz A, Skrzydlewska E, Makie?a M. 2002. Effects of amifostine on liver oxidative stress caused by cyclophosphamide administration to rats,” Drug Metabolism and Drug Interactions. 19: 67-82. Ref.: https://pubmed.ncbi.nlm.nih.gov/12751907/ DOI: https://doi.org/10.1515/dmdi.2002.19.2.67
10. Brode S, Cooke A. 2008. Immunepotentiating effects of the chemotherapeutic drug cyclophosphamide. Crit Rev Immunol. 28: 109-126. Ref.: https://pubmed.ncbi.nlm.nih.gov/18540827/ DOI: https://doi.org/10.1615/critrevimmunol.v28.i2. 20
11. Feldman BF, Zinkl JG, Jain NC, 2000. Schalm’s Veterinary Hematology. 1st Edn., Wiley, Ames, ISBN-10: 0683306928. 1344.
12. Burits CA, Ashwood ER. 1999.Tietz text book of clinical chemistry.Philadelphia, WB Saunders. 1840-1845.
13. Kind PR, King EJ. 1954. Estimation of plasma phosphates by determination of hydrolyzed phenol with antipyrine. Jof ClinPath. 7: 322-326. Ref.: https://pubmed.ncbi.nlm.nih.gov/13286357/ DOI: https://doi.org/10.1136/jcp.7.4.322
14. Huyan XH, Lin YP, Gao T, et al. 2011. Immunosuppressive effect of cyclophosphamide on white blood cells and lymphocyte subpopulations from peripheral blood of Balb/c mice: Int Immunopharmacol. 11: 1293-1297. Ref.: https://pubmed.ncbi.nlm.nih.gov/21530682/ DOI: https://doi.org/10.1016/j.intimp.2011.04.011
15. Grant MD, Piper M, Bohlius J, et al. 2013. Epoetin and Darbepoetin for Managing Anemia in Patients Undergoing Cancer Treatment: Comparative Effectiveness Update [Internet]. Agency for Healthcare Research and Quality (US), Rockville.
16. Liu K, Wang GB, Cheng B, et al.2007. Clinical comparison of GC regimen (gemcitabine and cisplatin) versus FEC regimen (fluorouracil, epirubicin, and cyclophosphamide) as neoadjuvant chemotherapy for breast cancer. Ai Zheng. 26: 427-430. Ref.: https://pubmed.ncbi.nlm.nih.gov/17430667/
17. Botnick LE, Hannon EC, Vigneulle R, et al. 1981. Differential effects of cytotoxic agents on hematopoietic progenitors. Cancer Res. 41: 2338-2342. Ref.: https://pubmed.ncbi.nlm.nih.gov/7237432/
18. Petrucci MT, Avvisati G, La Verde G, et al. 2003. Intermediate-dose cyclophosphamide and granulocyte colonystimulating factor is a valid alternative to highdose cyclophosphamide for mobilizing peripheral blood CD34+ cells in patients with multiple myeloma. Acta Haematol. 109: 184- 188. Ref.: https://pubmed.ncbi.nlm.nih.gov/12853690/ DOI: https://doi.org/10.1159/000070967
19. Drabeck J, Hofirek B. 1977. Cytostatic effect of cyclophosphamide on blood picture in sheep. Acta. Veterinaria. Brno. 46: 293-300.
20. Thatcher N, Wagstaff J, Willinson P, et al. 1982. Intermittent high dose cyclophosphamide with and without prednisolone study of the relationships between toxicity response and survival in metastatic lung cancer. 50: 1051-1056. Ref.: https://pubmed.ncbi.nlm.nih.gov/7049346/ DOI: https://doi.org/10.1002/1097- 0142(19820915)50:6%3C1051::aidcncr2820500605%3E3.0.co;2-x
21. Cole GW, Bradley W. 1973, Hospital admission laboratory profile interpretation. The SGOT and SLDH-SGOT ratio used in the diagnosis of hepatic disease: Hum Pathol. 4: 85- 88. Ref.: https://pubmed.ncbi.nlm.nih.gov/4713686/ DOI: https://doi.org/10.1016/s0046- 8177(73)80049-8
22. Senthilkumar S, Ebenezar KK, Sathish V, et al. 2006, Modulation of the tissue defense system by squalene in cyclophosphamide induced toxicity in rats.: Arch Med Sci. 2: 94- 100.
23. Davila JC, Lenherr A, Acosta D. 1989. Protective effect of flavonoid on drug-induced hepatotoxicity in vitro. Toxicology. 57: 267- 286. Ref.: https://pubmed.ncbi.nlm.nih.gov/2756528/ DOI: https://doi.org/10.1016/0300- 483x(89)90116-9
24. Cole GW, Bradley. 1973. Hospital admission laboratory profile interpretation. The SGOT and SLDH-SGOT ratio used for the diagnosis of hepatic disease. Hum Pathol. 4: 85- 88. Ref.: https://pubmed.ncbi.nlm.nih.gov/4713686/ DOI: https://doi.org/10.1016/s0046- 8177(73)80049-8
25. Senthilkumar S, Ebenezar KK Sathish V, et al. 2006. Modulation of the tissue defense system by squalene in cyclophosphamide induced toxicity in rats. Arch Med Sci. 2: 94- 100.
26. Soni S, Shrivastava V. 2013. Carbendazim induced histopathological changes and some enzyme activities (GOT, GPT,ACP and ALP) in liver and kidneys of male Rattus rattus. Int J Pharm Sci Health Care. 5: 19-35.
27. Khorwal G, Chauhan R, Nagar M. 2017. Effect of cyclophosphamide on liver in albino rats: acomparative dose dependent histomorphological study. International Journal of Biomedical andAdvance Research. 8: 102- 107.




















