Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/jca.2022.110039Article Views : 1Article Downloads : 0
Paracetamol as organic compound induced the decrease in the antioxidant enzymes and thiobarbituric acid-reactive substances in male rabbits
Hameda T Algalbati*1, Fathia M Atalhi2 and Fayrouz A Khaled1
1Chemistry Department, Faculty of Science, Omar El-Mokhtar University, El -Beyda-Libya
2Chemistry Department, Faculty of Education/ Janzour, Tripoli University, Libya
*Corresponding Author: Fayrouz A Khaled, Chemistry Department, Faculty of Science, Omar El- Mokhtar University, El -Beyda-Libya; Email: fayalzobair@yahoo.com
Article Information
Aritcle Type: Research Article
Citation: Hameda TA, Fathia MA, Fayrouz AK. 2022. Paracetamol as organic compound induced the decrease in the antioxidant enzymes and thiobarbituric acid-reactive substances in male rabbits. J Chem Appl. 4: 13-20.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2022; Hameda TA
Publication history:
Received date: 14 March, 2022Accepted date: 30 March, 2022
Published date: 06 April, 2022
Abstract
Paracetamol is accessible as a nonspecific medicine, with brand names counting Tylenol and Panadol among others. In 2019, it was the 145th most commonly prescribed medication in the world, with more than 4 million prescriptions. Therefore, the present study has been carried out to investigate the paracetamol toxicity on lipid peroxidation andd antioxidant enzymes in male New Zealand white rabbits. Animals were assigned to one of two treatment groups: 0 mg paracetamol kg BW [control]; 500 mg paracetamol/kg BW. Rabbits were orally managed the particular dosages each other day for 12 weeks. Results obtained showed that paracetamol significantly [P < 0.05] induced thiobarbituric acid-reactive substances [TBARS; the marker of lipid peroxidation] in plasma, while the activities of glutathione S-transferase [GST], superoxide dismutase [SOD] and catalase [CAT], and the level of sulfhydryl groups [SH-group] were decreased [P < 0.05] in blood plasma.
Keywords: Paracetamol; TBARS; Glutathione S-Transferase; Rabbits
Introduction
Paracetamol, moreover known as acetaminophen is figure 1 a medicine utilized to treat fever and gentle to direct torment [1]. At a standard measurement, paracetamol as it were somewhat diminishing body temperature, [2]. it is second rate to ibuprofen in that regard, [3] and the benefits of its utilize for fever are vague [4]. Paracetamol may calm torment in intense mellow headache but as it were somewhat in verbose pressure migraine [5]. Be that as it may, the aspirin/paracetamol/caffeine combination makes a difference with both conditions where the torment is mellow and is suggested as a first-line treatment for them [6]. Paracetamol is viable for post-surgical torment, but it is second rate to ibuprofen [7]. The paracetamol/ibuprofen combination gives encourage increment in power and is prevalent to either sedate alone [7]. The torment help paracetamol gives in osteoarthritis is little and clinically inconsequential [8]. The prove in its favor for the utilize in moo back torment, cancer torment and neuropathic torment is deficiently [9]. Within the brief term, common side impacts of paracetamol are sickness and stomach torment, and it appears to have tolerability comparable to ibuprofen [10]. Incessant utilization of paracetamol may result in a drop in hemoglobin level, demonstrating conceivable gastrointestinal dying [11], and anomalous liver function tests [12]. There's a steady affiliation of expanded mortality as well as cardiovascular [stroke, myocardial localized necrosis], gastrointestinal [ulcers, dying] and renal unfavorable impacts with taking higher measurements of paracetamol [13]. The sedate may too increment the hazard of creating hypertension [14]. Hoisted recurrence of asthma and developmental and regenerative disarranges is watched within the descendant of ladies with drawn out utilize of paracetamol amid pregnancy, in spite of the fact that whether paracetamol is the genuine cause of this increment is hazy [15]. A few considers propose that there's prove for the affiliation between paracetamol amid pregnancy and extreme introvertedness range clutter and consideration shortage hyperactivity clutter, whereas making clear advance research is required to set up any causal connect [16], which has provoked a few calls to restrain its utilize in pregnancy to the most reduced viable measurement for the most limited conceivable time [17].
Figure 1: Structure of paracetamol.
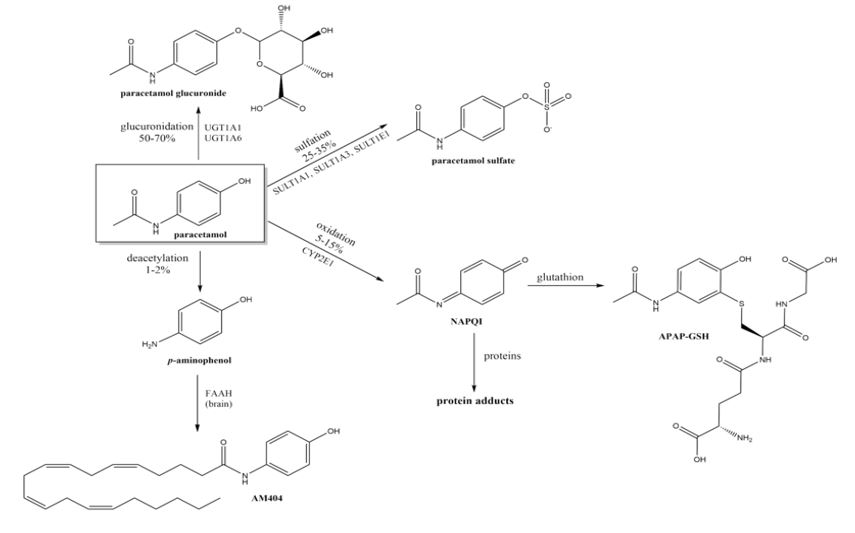
Figure 2: Important pathways of paracetamol metabolism.
Materials and Methods
Develop male Unused Zealand White rabbits [age of 7 months and beginning weight of (2.917 ?28.9 Kg) were utilized. Animals were independently housed in cages and weighed week after week all through 3-months exploratory period. Twenty develop male rabbits were arbitrarily separated into two break even with bunches [each five rabbits]. The primary bunch was utilized as control. The moment gather was utilized to think about the impact of paracetamol (500 mg/kg body weight) [18]. Blood tests were collected from the ear vein of all creatures each other week all through the 12-week exploratory period. Blood tests were set promptly on ice. Heparin was utilized as an anticoagulant. Plasma was gotten by centrifugation of tests at 860×g for 20 min, and was put away at -40?C. At the conclusion of exploratory period, put away plasma tests were specifically utilized for investigations. Plasma thiobarbituric acid-reactive substances [TBARS] were measured at 532 nm by utilizing 2-thiobarbituric corrosive (2,6-dihydroxypyrimidine-2-thiol; TBA An termination coefficient of 156,000 M−1 cm−1) was utilized for calculation [19]. Plasma glutathione S-transferas (GST; EC 2.5.1.18) activity was determined according [20], using para-nitrobenzylchloride as a substrate. Superoxide dismutase (SOD; EC 1.15.1.1) activity was measured in plasma according to [21]. Catalase [CAT; EC 1.11.1.6] activity was determined using the Luck method involving the decomposition of hydrogen peroxide [22]. Sulfhydryl groups were measured in plasma after reaction with 5,50-dithiobis-(2-nitrobenzoic acid) using the method of [23] Statistical analysis. Data were analyzed as a completely randomized design13 using the general linear model procedure of14. Means were statistically compared using least significant difference [LSD] test at 0.05 significant level13. The following model was used: Y ijk ¼ l ai bj abij eijk where Yijk = experimental observation; l=overall mean; ai=treatment effect; bj=week effect; abij=interaction effect of treatment and week; eijk=random error.
Results
Treatment with paracetamol caused significant (P < 0.05) increase in the overall means of plasma thiobarbituricacid-reactive substances (TBARS) concentrations and decrease in glutathione S-transferase (GST), superoxide dismutase (SOD) and catalase (CAT) activities, and the levels of SH-group compared to control animals (Table1 and figure 3 to 7).
|
Table 1: The activities of glutathione S-transferase [GST], superoxide dismutase [SOD] and catalase [CAT], and the levels of sulfhydral group [SH-group] and thiobarbituric acid-reactive substances [TBARS] in plasma of male rabbits treate with paracetamol. |
||
|
Parameters |
Groups |
|
|
Control |
Paracetamol |
|
|
(GST) |
0.87±0.01a |
0.78±0.04b |
|
(µmol/h) |
||
|
(SOD) |
1.27±0.01a |
0.90±0.05b |
|
(U/ml) |
||
|
CAT |
0.84± 0.02a |
0.69±0.02b |
|
(U/min/ml) |
||
|
SH-GROUP |
0.26±0.02a |
0.21±0.01b |
|
(mmol) |
||
|
TBARS |
2.96±0.12a |
4.00±0.16b |
|
(nmol/ml) |
||
|
abc Within row overall mean with different superscript letter differ significantly [P<0.05]. |
||

Figure 3: Change in GST during treatment of male rabbits with paracetamol.
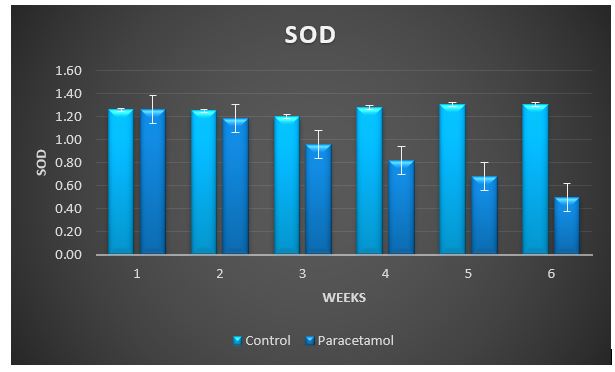
Figure 4: Change in SOD during treatment of male rabbits with paracetamol.
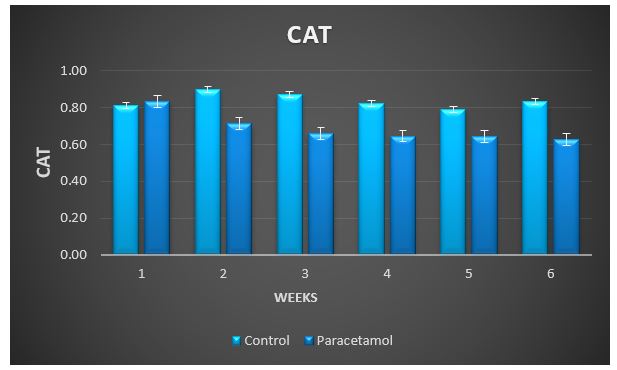
Figure 5: Change in CAT during treatment of male rabbits with paracetamol.
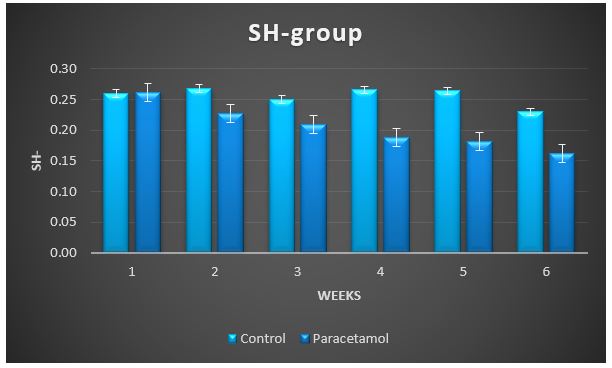
Figure 6: Change in SH-group during treatment of male rabbits with paracetamol.
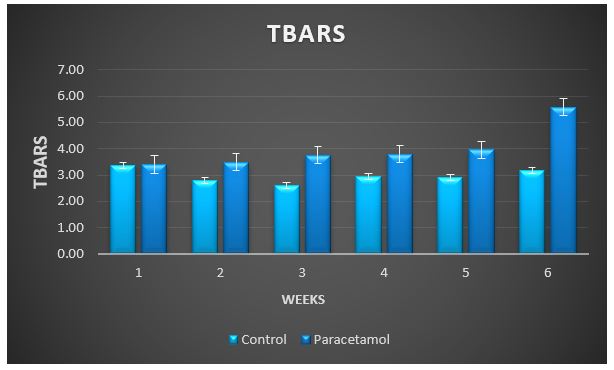
Figure 7: Change in TBARS during treatment of male rabbits with paracetamol.
Discussion
In the present study, paracetamol produced a significant increase in MDA level with a small reduction in GSH. This pattern of change in MDA and GSH in plasma may indicate PCM induced lipid peroxidation and compromised antioxidant defense mechanism [24]. Damage triggered by oxidative stress can be controlled by exogenous antioxidants and endogenous antioxidants defense system of the host, the endogenous defense consists of enzymatic and non-enzymatic antioxidants, like as superoxide dismutase SOD, catalases CAT and glutathione GSH [25]. GSH is powerful nucleophilic, 3-peptide [L-γ-glutamyl cysteinyl glycine], antioxidant, important for cellular defense, that detoxifies, reactive oxygen species via binding with and eliminating of toxic molecules, thus handling the inflammatory cytokine chain reactions [26]. Reduced levels of GSH in various tissue makes the defense system weak, against reactive oxygen species, and may lead to per-oxidative damage. In present study, it is concluded that, paracetamol administration reduced the GSH levels in plasma by the process of lipid peroxidation, may have resulted free radical’s generation. High level of ROS destroys cellular lipids, proteins and nucleic acids. To avoid this situation antioxidant defenses have developed to eradicate most of these oxidant mediators. Imbalance between oxidative impairment and defensive mechanisms caused oxidative stress [27]. The elevated level of MDA is in accordance with previous study of [28,29], which showed the association between PCM toxicity and MDA. A significant elevation in MDA level weakling glutathione reductase [GR] activity, SOD and TAC was demonstrated in other studies.
Conclusion
Although paracetamol has a reasonable safety profile in therapeutic doses, its overdose remains the most important cause of decrease of antioxidant enzymes and increase of TBARS in plasma.
References
1. Saragiotto BT, Shaheed CA, Maher CG. 2019. Paracetamol for pain in adults. Bmj. 367. Ref.: https://pubmed.ncbi.nlm.nih.gov/31892511/ DOI: https://doi.org/10.1136/bmj.l6693
2. Chiumello D, Gotti M, Vergani G. 2017. Paracetamol in fever in critically ill patients-an update. Journal of critical care. 38: 245-252. Ref.: https://pubmed.ncbi.nlm.nih.gov/27992852/ DOI: https://doi.org/10.1016/j.jcrc.2016.10.021
3. Pierce CA, Voss B. 2010. Efficacy and safety of ibuprofen and acetaminophen in children and adults: a meta-analysis and qualitative review. Annals of Pharmacotherapy. 44: 489-506. Ref.: https://pubmed.ncbi.nlm.nih.gov/20150507/ DOI: https://doi.org/10.1345/aph.1m332
4. Ludwig J, McWhinnie H. 2019. Antipyretic drugs in patients with fever and infection: literature review. British journal of nursing. 28: 610-618. Ref.: https://pubmed.ncbi.nlm.nih.gov/31116598/ DOI: https://doi.org/10.12968/bjon.2019.28.10.610
5. Stephens G, Derry S, Moore RA. 2016. Paracetamol [acetaminophen] for acute treatment of episodic tension?type headache in adults. Cochrane Database of Systematic Reviews. 6.
6. Mayans L, Walling A. 2018. Acute migraine headache: treatment strategies. American family physician. 97: 243-251. Ref.: https://pubmed.ncbi.nlm.nih.gov/29671521/
7. Bailey E, Worthington HV, van Wijk A. 2013. Ibuprofen and/or paracetamol [acetaminophen] for pain relief after surgical removal of lower wisdom teeth. Cochrane Database of Systematic Reviews. 12.
8. Kolasinski SL, Neogi T, Hochberg MC. 2020. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis & Rheumatology. 72: 220-233. Ref.: https://pubmed.ncbi.nlm.nih.gov/31908163/ DOI: https://doi.org/10.1002/art.41142
9. Wiffen PJ, Derry S, Moore RA. 2017. Oral paracetamol [acetaminophen] for cancer pain. Cochrane Database of Systematic Reviews. 7.
10. Conaghan PG, Arden N, Avouac B. 2019. Safety of paracetamol in osteoarthritis: what does the literature say?. Drugs & aging. 36: 7-14. Ref.: https://pubmed.ncbi.nlm.nih.gov/31073920/ DOI: https://doi.org/10.1007/s40266-019-00658-9
11. Roberts E, Nunes VD, Buckner S. 2016. Paracetamol: not as safe as we thought? A systematic literature review of observational studies. Annals of the rheumatic diseases. 75: 552-559. Ref.: https://pubmed.ncbi.nlm.nih.gov/25732175/ DOI: https://doi.org/10.1136/annrheumdis-2014-206914
12. Leopoldino AO, Machado GC, Ferreira PH. 2019. "Paracetamol versus placebo for knee and hip osteoarthritis". Cochrane Database Syst Rev. 2: 13273. Ref.: https://pubmed.ncbi.nlm.nih.gov/30801133/ DOI: https://doi.org/10.1002/14651858.cd013273
13. Choueiri TK, Je Y, Cho E. 2014. Analgesic use and the risk of kidney cancer: A meta?analysis of epidemiologic studies. International journal of cancer. 134: 384-396. Ref.: https://pubmed.ncbi.nlm.nih.gov/23400756/ DOI: https://doi.org/10.1002/ijc.28093
14. MacIntyre IM, Turtle EJ, Farrah TE. 2022. Regular Acetaminophen Use and Blood Pressure in People With Hypertension: The PATH-BP Trial. Circulation. 145: 416-423. Ref.: https://pubmed.ncbi.nlm.nih.gov/35130054/ DOI: https://doi.org/10.1161/circulationaha.121.056015
15. McCrae JC, Morrison EE, MacIntyre IM. 2018. Long?term adverse effects of paracetamol–a review. British journal of clinical pharmacology. 84: 2218-2230. Ref.: https://pubmed.ncbi.nlm.nih.gov/29863746/ DOI: https://doi.org/10.1111/bcp.13656
16. Gou X, Wang Y, Tang Y. 2019. Association of maternal prenatal acetaminophen use with the risk of attention deficit/hyperactivity disorder in offspring: A meta-analysis. Australian & New Zealand Journal of Psychiatry. 53: 195-206. Ref.: https://pubmed.ncbi.nlm.nih.gov/30654621/ DOI: https://doi.org/10.1177/0004867418823276
17. Black E, Khor KE, Kennedy D. 2019. Medication use and pain management in pregnancy: a critical review. Pain Practice. 19: 875-899. Ref.: https://pubmed.ncbi.nlm.nih.gov/31242344/ DOI: https://doi.org/10.1111/papr.12814
18. Sayed MM, El-Kordy EA. 2014. The protective effect of curcumin on paracetamol-induced liver damage in adult male rabbits: biochemical and histological studies. Egyptian Journal of Histology. 37: 629-639.
19. Tappel AL, Zalkin H. 1959. Lipide peroxidation in isolated mitochondria. Archives of Biochemistry and Biophysics. 80: 326-332.
20. Habig WH, Pabst MJ, Jakoby WB. 1974. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. Journal of biological Chemistry. 249: 7130-7139. Ref.: https://pubmed.ncbi.nlm.nih.gov/4436300/
21. Misra HP, Fridovich I. 1972. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. Journal of Biological chemistry. 247: 3170-3175. Ref.: https://pubmed.ncbi.nlm.nih.gov/4623845/
22. Misra HP, Fridovich I. 1972. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. Journal of Biological chemistry. 247: 3170-3175. Ref.: https://pubmed.ncbi.nlm.nih.gov/4623845/
23. Nam GW, Lee DW, Lee HS. 2002. Native-feather degradation by Fervidobacterium islandicum AW-1, a newly isolated keratinase-producing thermophilic anaerobe. Archives of Microbiology. 178: 538-547. Ref.: https://pubmed.ncbi.nlm.nih.gov/12420177/ DOI: https://doi.org/10.1007/s00203-002-0489-0
24. Kaplowitz N. 2000. Mechanisms of liver cell injury. Journal of Hepatology. 32: 39-47. Ref.: https://pubmed.ncbi.nlm.nih.gov/10728793/ DOI: https://doi.org/10.1016/s0168-8278(00)80414-6
25. He L, He T, Farrar S. 2017. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cellular Physiology and Biochemistry. 44: 532-553. Ref.: https://pubmed.ncbi.nlm.nih.gov/29145191/ DOI: https://doi.org/10.1159/000485089
26. Pes L. 2013. Study of the non-proteinogenic delta-amino acid ACCA, its biological investigation and application.
27. Chan KWK, Ho WS. 2015. Anti-oxidative and hepatoprotective effects of lithospermic acid against carbon tetrachloride-induced liver oxidative damage in vitro and in vivo. Oncology reports. 34: 673-680. Ref.: https://pubmed.ncbi.nlm.nih.gov/26081670/ DOI: https://doi.org/10.3892/or.2015.4068
28. Farghaly HS, Hussein MA. 2010. Protective effect of curcumin against paracetamol-induced liver damage. Australian Journal of Basic and Applied Sciences. 4: 4266-4274.
29. Ajiboye TO. 2016. Lophirones B and C Attenuate Acetaminophen?Induced Liver Damage in Mice: Studies on Hepatic, Oxidative Stress and Inflammatory Biomarkers. Journal of Biochemical and Molecular Toxicology. 30: 497-505. Ref.: https://pubmed.ncbi.nlm.nih.gov/27161652/ DOI: https://doi.org/10.1002/jbt.21814




















