Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/irjo.2021.110009Article Views : 21Article Downloads : 19
Electrophysiological study after ranibizumab in choroidal neovascularizat
Mona Abdelkader*, Ayman Fawzy and Wael El-doskey
Mansoura Ophthalmic Center, Faculty of Medicine, Mansoura University, Egypt
*Corresponding Author: Mona Abdelkader, Professor, Ophthalmology Center, Faculty of Medicine, Mansoura University, Mansoura, Egypt, 35516, Tel: 002-050-2202064; Mobile: 00201006278757; Fax 002-050-2256104; Email: monaabdelkader78@yahoo.com
Article Information
Aritcle Type: Research Article
Citation: Mona Abdelkader, Ayman Fawzy, Wael El-doskey. 2021. Electrophysiological study after ranibizumab in choroidal neovascularizat. Int Res J Optha. 3: 03-13.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2021; Mona Abdelkader
Publication history:
Received date: 08 April, 2021Accepted date: 21 April, 2021
Published date: 23 April, 2021
Abstract
Purpose: To study the effects of intra-vitreal injection of ranibizumab on pattern electroretinogram (PERG) and multifocal electroretinogram(MF-ERG) parameters in choroidal neovascularization and to test the retinal toxicity of ranibizumab.
Method: Fifty eyes of 50 patients with subfoveal choroidal neovascularization were included in the study. Thirty (30) eyes had neovascular age related macular degeneration (nAMD) and 20 eyes had myopic choroidal neovascularization. Ranibizumab was injected intravitreal monthly for3 months. Optical Coherence Tomography Angiography (OCT) was performed at the initial and final visits. Visual acuity, Pattern and multifocal electroretinography (PERG, MFERG) were performed before and at 1,2,3,6 months after intravitreal injection.
Results: There was no clinical significant reduction of parameters of either MFERG or PERG after intravitreal injection of ranibizumab. There was significant increase in visual acuity, decrease in central macular thickness ,decrease in total macular volume and improvement in the parameters of both PERG and MFERG after intravitreal injection.
Conclusion: Intravitreal injection of ranibizumab is effective in treatment CNV and safe. It has no retinal toxicity since no reduction in PERG& MFERG parameters.
Keywords: AMD; Ranibizumab; PERG; MFERG
Introduction
Age related macular degeneration(AMD) is one of the commonest causes of irreversible blindness [1]. Neovascular AMD is the most aggressive form of AMD . It is characterized by choroidal neovascularization(CNV), which is the development of abnormal blood vessels underneath the retina leading to damage and loss of photoreceptors by the processes of exudation, bleeding, and fibrovascular scarring [2]. Vascular endothelial growth factor is the main mediator of CNV .Intravitreal injections of vascular endothelial growth factor antagonists( anti-VEGF ) have been the best strategy for treating neovascular AMD [3]. Ranibizumab (Lucentis, Genentech, Inc., South San Francisco, CA) is a monoclonal antibody fragment (Fab). It is a 48-kilodalton (kDa) recombinant humanized immunoglobulin G1 kappa isotype that binds all isoforms of VEGF-A [4]. Anti-VEGF decreases the permeability and vascular growth [2]. Myopic macular neovascularization occurs in 5–10% of high myopic patients and may lead to visual loss due to chorioretinal atrophy and increased deterioration of the retinal pigment epithelium (RPE) near CNV [6]. Multifocal electroretinogram evaluates focal responses from central 30? of the retina while PERG quantifies ganglion cell function [7]. The aim of the study is to review the safety and effectivity of ranibizumab in management of choroidal neovascularization.
Subjects and Methods
All subjects were carried out in accordance with the tenets of the Declaration of Helsinki (1989) of the World Medical Association. The study was approved by Mansoura International Review Board Ethics Committee. Each patient signed a written consent. This retrospective study was carried out in Mansoura Ophthalmic Center during the period from May 2018 to December 2020. The study included 50 eyes of 50 patients with subfoveal active choroidal neovascularization (CNV). Thirty (30) eyes had neovascular age related macular degeneration (n AMD) and 20 eyes had myopic choroidal neovascularization.
Exclusion Criteria
Previous therapy to CNV, media opacity (cataract, corneal opacity,..), any contra indication for ranibizumab (myocardial infraction ,pregnancy ,cerebral stroke in the last 6 months),glaucoma, any other retinal diseases (diabetic retinopathy ,retinal vein occlusion,…)
Ophthalmologic examination included best-corrected visual acuity (BCVA) measurement, slit lamp biomicroscopy of anterior and posterior segments, and Goldmann applanation tonometry. Fundus and structural Optical Coherence Tomography (OCT) images, fluorescein angiography, PERG and MF-ERG were performed for each patient at the initial visit. All tests were performed on the same day.
Best corrected visual acuity (BCVA) at the baseline and1week,1,3,6 months were converted to logMAR and recorded for each patient. Participants with CNV received intravitreal injections of 0.5 mg of ranibizumab monthly for 3 months. PERG, MFERG were performed at baseline, 1 week, 1 month, 3 months, and 6 months after intravitreal injection.
Optical Coherence Tomography Angiography (OCT)
Imaging was performed with SS-OCT at 100 000 A-scans per second (DRI triton OCT, Topcon, Tokyo, Japan). OCT was performed at the initial and final visits.
Electrophysiological tests
Were recorded using Roland Consult, Brandenburg Germany system .Were performed in accordance to International Society for Clinical Electrophysiology of Vision( ISCEV) guidelines [8]. Dawson Trick litzkow (DTL) electrode was applied to topically anaesthetized cornea with one ground electrode on the forehead and two temporal reference electrodes.
MF-ERG
A stimulus array of 61 hexagons covering a visual field of 30o was presented on a monitor 30 cm from the eye. Each hexagon alternated between black and white. Subjects fixated at the center of the stimulus. The recording period was comprised of eight segments of 30s. Ring form and trace array were obtained .MF ERG response characterized by an initial negative wave (N1), followed by a positive peak (P1). P wave latency and amplitude were measured.
PERG
The patient was seated in front of alternating checker-board pattern monitor and the mean width and height of the stimulus field was 15° monitor (check size=1 min, contrast was 97). The distance between the eye of the patient and the monitor was 1 meter while wearing his reading correcting glasses. They were watched for any gross eye movement or attention lapse during the procedure through the camera in the monitor. Two trials were given for each eye. The responses to pattern stimulus were recorded, including P50 wave (reflects macular photoreceptors and N95 wave (reflects post-photoreceptor, including the optic nerve). The P50 amplitude was measured from the trough of N35 to the peak of P50. The N95 amplitude was measured from the peak of P50 to the trough of N95.
Statistical Analyses
Statistical analysis were analyzed by SPSS program versions 25 using Microsoft Windows 7 (SPSS Inc., Chicago IL, USA), Parametric quantitative data were described using mean, standard deviation after testing normality using Kolmogrov Smirnov test.). The paired t test was used to analyze VA and electroretinographic variables Spearman correlation coefficient is used for correlation between variables. If P≤ 0.05 was considered statistically significant.
Results
Fifty eyes were included in the study. Thirty eyes had CNV secondary to AMD and twenty eyes had myopic CNV. Demographic features of patients and study design were included in Table 1A,B.
|
Table 1A: Demographic features of patients. |
|||
|
Features |
n AMD |
Myopic CNV |
P -value** |
|
Number |
30 |
20 |
|
|
Age, years |
62.6±12 |
49 ± 10 |
0.09 |
|
Sex, |
|
|
|
|
Female |
|
|
|
|
Number |
15 |
12 |
0.4 |
|
Percentage % |
50% |
60% |
|
|
Male |
|
|
|
|
Number |
15 |
8 |
0.1 |
|
Percentage % |
50% |
40% |
|
|
Eye |
|
|
|
|
Right |
16 |
11 |
0.8 |
|
Left |
14 |
9 |
0.7 |
This is table shows insignificant differences between groups.
|
Table 1B: Study design. |
||||||
|
6th month |
3rd month |
2nd month |
1st month |
1st week |
1st visit |
Parameters |
|
yes |
yes |
yes |
yes |
yes |
yes |
Visual acuity |
|
---- |
yes |
---- |
--- |
--- |
yes |
Optical coherence tomography angiography |
|
--- |
--- |
--- |
--- |
--- |
yes |
Fluorescein angiography |
|
yes |
yes |
yes |
yes |
yes |
yes
|
Intra ocular pressure measurement |
|
yes |
yes |
--- |
yes |
yes |
yes |
PERG |
|
yes |
yes |
|
yes |
yes |
yes |
MFERG |
|
---- |
----- |
yes |
yes |
----- |
yes |
Intravitreal injection |
Before injection; the mean best corrected vision in nAMD and myopic CNV were 0.87±0.24, 0.79 ±0.3 improved to 0.57 ±0.4, 0.44 ±0.2 after 3months respectively (Table 2).
|
Table 2: The changes of visual acuity among patients. |
||
|
BCVA |
nAMD |
Myopic CNV |
|
Before injection |
0.87±0.24 |
0.79 ±0.3 |
|
After injection |
|
|
|
1week |
0.7±0.27 |
0.58±0.42 |
|
1month |
0.63±0.18 |
0.51±0.33 |
|
3month |
0.57 ±0.4 |
0.44 ±0.2 |
|
6month |
0.51±0.35 |
0.41±0.12 |
|
P -value** |
0.04* |
0.03* |
*means statistical significant injection
This is table shows statistical significant improvement of visual acuity after.
The mean central macular thickness( CMT) at baseline in nAMD and myopic CNV were 380±20.78, 355±23.54 μm respectively that improved to 270±50.42, 250±45.65 μm. Statistically significant difference was found between the CMT at baseline and after treatment (p=0.04,0.03). Total macular volume (TMV) was 8.65±2.45 and 9.1±2.11 mm3 in nAMD and myopic CNV before treatment respectively and decreased to 7.52±1.78 and 7.98± 2.01 mm3 after treatment. There was significant improvement in TMV after injection (p=0.03, 0.01 ). The OCT changes during follow up period in (Table 3, Figure 1).
Figure1: Optical Coherence Tomography (OCT) before and after leucentis injection.
|
Table 3: OCT changes during follow up. |
||
|
OCT |
nAMD |
Myopic CNV |
|
CMT (µm) |
|
|
|
Before injection |
380 ± 20.78 |
355± 23.54 |
|
After injection |
270± 50.42 |
250±45.65 |
|
(6 month) |
||
|
P -value** |
0.04 |
0.03 |
|
TMV (mm3) |
|
|
|
Before injection |
8.65 ± 2.45 |
9.1±2.11 |
|
|
|
|
|
After injection |
7.52 ± 1.78 |
7.98±2.01 |
|
(6 month) |
||
|
P -value** |
0,03 |
0.01 |
This is table shows statistical significant reduction of macular thickness and total macular volume after injection. In PERG ;Baseline P50 and N95 amplitudes in nAMD and myopic CNV were1.01±0.99, 1.09±1.00; 1.2±1.08 μV, 1.4±1.1 respectively. While ,baseline P50 and N95 latencies in nAMD and myopic CNV were 60±9.8, 58±8.6; 105±13.5, 102±12.89 ms respectively. The changes in PERG parameters were recorded in (Table 4, Figure2). By 6 months, no decrease in P50 or N95 amplitudes from baseline was observed.
Fluorescein angiography shows area of hyperflourescence that increase throughout the angiogram corresponding to choroidal neovascularization (CNV); OCT shows fragmentation and thickness of RPE-choriocapillaries due to CNV with increase retinal thickness before injection. After injection; there is reduction in retinal thickness.
Figure 2: Pattern electroretinogram (PERG) before and after injection, there is increase in amplitude P50; N95 after injection.
|
Table 4: The changes in PERG parameters. |
||||
|
PERG |
P50 |
P50 |
N95 amplitude |
N95 latency |
|
Amplitude |
Latency |
µv |
ms |
|
|
µv |
ms |
|
|
|
|
Before injection |
|
|
|
|
|
|
|
|
|
|
|
nAMD |
1.01±0.99 |
60±9.8 |
1.2±1.08 |
105±13.5 |
|
myopic CNV |
1.09±1.00 |
|
|
|
|
|
|
58± 8.6 |
1.4±1.1 |
102±12.89 |
|
After injection |
|
|
|
|
|
( 1st week) |
|
|
|
|
|
nAMD |
1.01±0.99 |
62±9.8 |
1.1±1.08 |
104±13.5 |
|
myopic CNV |
|
|
|
|
|
|
1.09±1.00 |
59± 8.6 |
1.42±1.1 |
100±12.89 |
|
1st month |
|
|
|
|
|
nAMD |
1.11±0.6 |
60±7.8 |
1.31±0.9 |
|
|
myopic CNV |
|
|
|
102±11.5 |
|
|
1.21±0.9 |
58± 8.6 |
1.61±0.99 |
|
|
|
|
|
|
100±10.09 |
|
3th month |
|
|
|
|
|
nAMD |
1.34±0.99 |
58.9±4.8 |
1.41±0.99 |
|
|
myopic CNV |
|
|
1.61±0.94 |
101±10.5 |
|
|
1.41±0.99 |
57.7± 7.6 |
|
|
|
|
|
|
|
99±9.89 |
|
6th month |
|
|
|
|
|
nAMD |
1.61±0.39 |
58.1±9.8 |
1.54±0.99 |
|
|
myopic CNV |
|
|
1.66±0.56 |
100±9.9 |
|
|
1.81±0.92 |
57.5± 8.6 |
|
|
|
|
|
|
|
98.5±11.8 |
|
P -value** |
≤0.04 |
≤0.03 |
≤0.01 |
≤0.01 |
This table shows improvement of PERG parameters(reduction in latency and increase in amplitude); with no worsing of the parameters throughout fellow up period. As regards ,MFERG, there were statistical significant improvement of MFERG parameters (P amplitude and latency) after injection compared with baseline (Table 5, Figure 3,4).
Figure 3: MFERG (Trace array form)before and after leucentis injection.
A: Before injection ;no apparent wave ;just irregular line.
B: After injection; there is increase in amplitude and appearance of wave with its peak and trough.
|
Table 5: MFERG parameters changes. |
||
|
MFERG parameters |
nAMD |
Myopic CNV |
|
R1 R2, R3 R4,5 |
R1 R2, R3 R4,5 |
|
|
Amplitude |
34.9±10.1 30±6.4 19±7.8 9±4.9 |
37±8.3 35±4.8 25±7.9 11.5±6.3 |
|
Before injection |
||
|
After injection |
|
|
|
1week |
49±11.5 41.2±7.8 26±8.4 15±6.9 |
50±5.8 46±6.7 30±9.9 19±5.9 |
|
1month |
|
|
|
3month |
52±8.3 45±5.8 30±8.7 16±6.7 |
54±8.9 46±7.8 32±9.7 19±8.9 |
|
|
53±6.3 46±4.3 32±8.3 18±4.9 |
54±7.4 47±8.3 33±7.5 19.3±9.2 |
|
6month |
55±6.6 46±4.7 33±6.9 18±6.1 |
|
|
|
|
55±8.1 47.4±6.1 34.1±6 19±8.3 |
|
P -value** |
≤ 0.04 0.03 0.01 0.044 |
≤ 0.03 0.001 0.08 0.07 |
|
Latency |
|
|
|
|
|
|
|
Before injection |
69.8±5.1 67±3.4 66±2.7 66.4±3.6 |
65.9±3.4 64±3.2 64.1±4.3 62.±.91 |
|
After injection |
|
|
|
1week |
61.3±4.3 62.2±2.8 61.2±2.9 61±3.1 |
60.5±2.4 60.9±2.3 60.99±3.1 60.1±2.4 |
|
1month |
|
|
|
|
60±3.1 60±2.9 60.1±2.2 60.5±2.5 |
58±4 59±2.7 59.5±1.9 59±1.5 |
|
3month |
60±2.4 59±2.7 59.5±1.9 59±1.5 |
|
|
|
59±1.9 59±2.7 59±1.8 59.5±1.9 |
59±1.6 59±1.7 59±1.9 59.5±1.2 |
|
6month |
|
58±1.9 57±2.7 57±1.8 59.5±1.9 |
|
|
|
|
|
P -value** |
≤0.001 |
≤0.005 |
This table shows improvement of MF-ERG parameters ;with no worsing of the parameters. No patients had increased intraocular pressure or cataract progression through fellow up period 6 months after intravitreal injections of ranibizumab, and no severe ocular adverse events developed.
Figure 4: MFERG (Ring form)before and after injection.
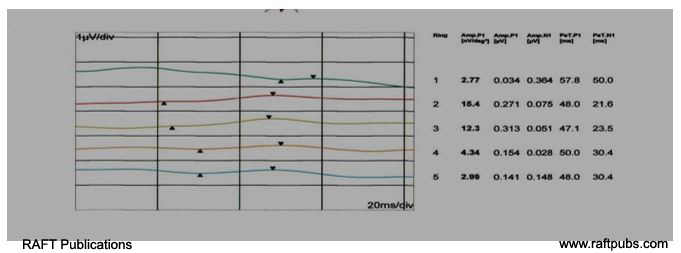
A: MFERG (Ring form) before injection;there is reduction in amplitude and delay in latency.
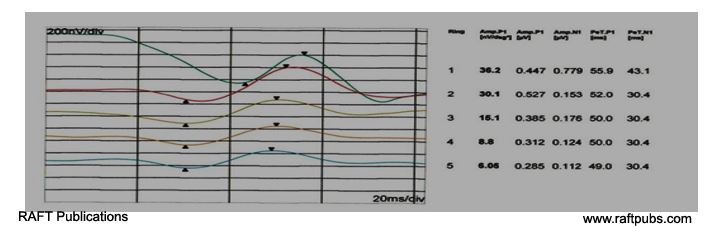
B:MFERG (Ring form) after injection; there is increase in amplitude and improvement of latency.
Discussion
There are three types of choroidal neovascularization ;type1 are located under the retinal pigment epithelium, type 2 are located in subretinal space. Both types are originate from choroidal vasculature while type 3 to originate from the deep retinal capillary plexus, grow downward into the neurosensory retina.9 Most of CNV in AMD are a mixture of subtypes [10]. Anti VEGF is the mainstay of treatment of CNV ;There are many articles about the structural and anatomical changes after treatment with ranibizumab [11-15]. However, there is few studies about the changes of retinal function after anti VEGF [16-18]. In this study; MFERG,PERG were performed to study the effect of ranibizumab injection on retinal function and its safety. In this study; there were increase in visual acuity after ranibizumab throughout fellow up period Similarly, Nguyen et al, [5], Cheng et al, [6], Arrigo et al, [11], Kim et al, [12], reported improvement of vision after anti-VEGF. Visual acuity improvement after ranibizumab, indicates improvement of less than one degree of central retina while PERG tests the central 15° and function of Ganglion cell in this area. Anti-VEGF is neuroprotection mediator by inhibiting apoptosis of retinal Muller cells and photoreceptors and promoting cell survival. Saint-Geniez observed decline of retinal function after neutralization of VEGF [19].
Also, Luke and colleagues found reduction in the amplitude of electroretinogram in 4% of patients with statistically insignificant differences after injection ranibizumab [20]. While, Sheybani et al, [21] Oner, et al, [22], found no reduction in retinal function after anti VEGF. In this study, there were increase in the amplitude of PERG with reduction in latency after ranibizumab injection during fellow up (without any decrease in ganglion cell function) in both myopic and age related neovascularization. As regard to MFERG, MFERG reflects the function of photoreceptors and bipolar than inner retinal cell function. So, MFERG response is useful in assessment of cone and bipolar cells [23,24]. Any damage to these cells ,will lead to decline MFERG response [25]. In this study; there were no reduction of MFERG parameters; there were increase in amplitudes and reduction in latencies of MFERG waves in the central and peripheral rings. While Feigl et al, [26] reported reduction in MFERG response after 3 injection of ranibizumab for recurrent CNV. But; he did not conclude whether the reduction due to progression the disease or toxic effect of ranibizumab. Xie et al, [27], said that thicker sclera and a thinner choroid are risk factors for choroidal neovascularization. Myopic CNVs regress rapidly with anti-VEG [28] while nAMD respond more variably to anti-VEGF [29,30]. As regards OCT changes, there were reduction in retinal thickness, intra-retinal and sub-retinal fluid after ranibizumab injection during fellow up in all cases in this study. Similarily, Change et al, [31] reported decrease in CNV area ,flow area after anti-VEGF injection. In the opposite, Giorno et al, [28] revealed disappearance of the feeder vessel without significant reduction in CNV area. The limitations of this study were the small sample size and short fellow up (only 6 months). A longer follow-up is needed to know if repeated injection cause retinal toxicity or increase the complication.
References
1. Klein R, Peto T, Bird A, et al. 2004. The epidemiology of age-related macu- lar degeneration. Am J Ophthalmol. 137: 486-495. Ref.: https://pubmed.ncbi.nlm.nih.gov/15013873/ Doi; https://doi.org/10.1016/j.ajo.2003.11.069
2. Lim LS, Mitchell P, Seddon JM, et al. 2012. Age-related macular degeneration. Lancet 379: 1728-1738. Ref.: https://pubmed.ncbi.nlm.nih.gov/22559899/ DOI: https://doi.org/10.1016/s0140-6736(12)60282-7
3. Arrigo A, Romano F, Aragona E, et al. 2020. Optical coherence tomography angiography can categorize different subgroups of choroidal neovascularization secondary to age-related macular degeneration. Retina. 40: 2263-2269. Ref.: https://pubmed.ncbi.nlm.nih.gov/32032255/ Doi: https://doi.org/10.1097/iae.0000000000002775
4. Ferrara N, Damico L, Shams N. 2006. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 26: 859-870. Ref.: https://pubmed.ncbi.nlm.nih.gov/17031284/ Doi: https://doi.org/10.1097/01.iae.0000242842.14624.e7
5. Nguyen CL, Oh LJ, Wong E, et al. 2018. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration: a meta-analysis of randomized controlled trials. BMC Ophthalmol. 18: 130. Ref.: https://pubmed.ncbi.nlm.nih.gov/29843663/ Doi: https://doi.org/10.1186/s12886-018-0785-3
6. Cheng LN, Lin YX, Liu, et al. 2020. Assessment of conbercept therapy for high myopia macular neovascularization by optical coherence tomography angiography. 10: 16959. Ref.: https://pubmed.ncbi.nlm.nih.gov/33046787/ Doi: https://doi.org/10.1038/s41598-020-74073-1
7. Odom JV, Bach M, Brigell M. 2016. ISCEV standard for clinical visual evoked potentials (2016 update). Doc Ophthalmol. 133: 1-9. Ref.: https://pubmed.ncbi.nlm.nih.gov/27443562/ Doi: https://doi.org/10.1007/s10633-016-9553-y
8. McCulloch DL, Marmor MF, Brigell MG, et al. 2015. Erratum to: ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol. 131: 81-83. Ref.: https://pubmed.ncbi.nlm.nih.gov/26059396/ Doi: https://doi.org/10.1007/s10633-015-9504-z
9. Nagiel A, Sarraf D, Sadda SR, et al. 2015. Type 3 neovascularization: evolution, association with pigment epithelial detachment, and treatment response as revealed by spectral domain optical coherence tomography. Retina. 35: 638-647. Ref.: https://pubmed.ncbi.nlm.nih.gov/25650713/ Doi: https://doi.org/10.1097/iae.0000000000000488
10. Spaide RF, Jaffe GJ, Sarraf D, et al. 2019. Consensus nomenclature for reporting neovascular age-related macular degeneration data: consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology. 127: 616-636. Ref.: https://pubmed.ncbi.nlm.nih.gov/31864668/ Doi: https://doi.org/10.1016/j.ophtha.2019.11.004
11. Arrigo A, Aragona E, Di Nunzio C, et al. 2020. Quantitative Optical Coherence Tomography Angiography Parameters in Type 1 Macular Neovascularization Secondary to Age-Related Macular Degeneration. Transl Vis Sci Technol. 9: 48. Ref.: https://pubmed.ncbi.nlm.nih.gov/32934898/ Doi: https://doi.org/10.1167/tvst.9.9.48
12. Kim JM, Cho HJ, Kim Y, et al. 2019. Responses of Types 1 and 2 Neovascularization in Age-Related Macular Degeneration to Anti-Vascular Endothelial Growth Factor Treatment: Optical Coherence Tomography Angiography Analysis. Semin Ophthalmol. 34: 168-176. Ref.: https://pubmed.ncbi.nlm.nih.gov/31132283/ Doi: https://doi.org/10.1080/08820538.2019.1620791
13. Arrigo A, Aragona E, Capone L, et al. 2018. Advanced Optical Coherence Tomography Angiography Analysis of Age-related Macular Degeneration Complicated by Onset of Unilateral Choroidal Neovascularization. Am J Ophthalmol. 195: 233-224. Ref.: https://pubmed.ncbi.nlm.nih.gov/30098346/ Doi: https://doi.org/10.1016/j.ajo.2018.08.001
14. Levine ES, Custo Greig E, Mendonça LSM. 2020. The long-term effects of anti-vascular endothelial growth factor therapy on the optical coherence tomography angiographic appearance of neovascularization in age-related macular degeneration. Int J Retin Vitr. 6: 39. Ref.: https://pubmed.ncbi.nlm.nih.gov/32844038/ Doi: https://doi.org/10.1186/s40942-020-00242-z
15. Dias JO, Andrande GC, Kniggendorf VF. 2017. Evaluation after vitreal ZIV-Aflibercept for exudative age-related macular degeneration. RETINA. 37: 1499-1507. Ref.: https://pubmed.ncbi.nlm.nih.gov/27798520/ Doi: https://doi.org/10.1097/iae.0000000000001385
16. Berrow EJ, Bartlett HE, Eperjesi F, et al. 2010. The electroretinogram: a useful tool for evaluating age-related macular disease? Doc Ophthalmol. 121: 51-62. Ref.: https://pubmed.ncbi.nlm.nih.gov/20232109/ Doi: https://doi.org/10.1007/s10633-010-9226-1
17. Moschos MM, Brouzas D, Apostolopoulos M, et al. 2007. Intravitreal use of bevacizumab (avastin) for choroidal neovascularization due to ARMD: a preliminary multifocal-ERG and OCT study. Doc Ophthalmol. 114: 37-44. Ref.: https://pubmed.ncbi.nlm.nih.gov/17216267 Doi: https://doi.org/10.1007/s10633-006-9036-7
18. Skaat A, Solomon A, Moroz I, et al. 2011. Increased electroretinogram a-wave amplitude after intravitreal bevacizumab injection for neovascular age-related macular degeneration. Acta Ophthalmol. 89: 269-273. Ref.: https://pubmed.ncbi.nlm.nih.gov/20946333/ Doi: https://doi.org/10.1111/j.1755-3768.2010.02005.x
19. Saint-Geniez M, Maharaj ASR, Walshe TE. 2008. Endogenous VEGF is required for visual function: evidence for a survival role on Mu¨ller cells and photoreceptors. PLoS One. 3: 3554. Ref.: https://pubmed.ncbi.nlm.nih.gov/18978936/ Doi: https://doi.org/10.1371/journal.pone.0003554
20. Lu¨ke M, Januschowski K, Lu¨ke J, et al. 2009. The effects of ranibizumab (Lucentis) on retinal function in isolated perfused vertebrate retina. Br J Ophthalmol. 93: 1396-1400.
21. Sheybani A, Brantley M, Apte RS. 2011. Pattern Electroretinography in Age-Related Macular Degeneration. Arch Ophthalmol. 129: 580-584. Ref.: https://pubmed.ncbi.nlm.nih.gov/21555610/ Doi: https://doi.org/10.1001/archophthalmol.2011.83
22. Oner A, Gumus K, Arda H, et al. 2009. Pattern electroretinographic results after photodynamic therapy alone and photodynamic therapy ini combination with intravitreal bevacizumab for choroidal neovascularization in agerelated macular degeneration. Doc Ophthalmol. 119: 37-42. Ref.: https://pubmed.ncbi.nlm.nih.gov/19225818/ Doi: https://doi.org/10.1007/s10633-009-9167-8
23. Maturi RK, Yu M. 2003. Multifocal electroretinography and its clinical application. In: Ciulla TA, Regillo CD, Harris A, editors. Retina and optic nerve imaging. Philadelphia: Lippincott Williams & Wilkins. 213-301.
24. Hood DC, Frishman LJ, Saszik S, Viswanathan S. Retinal origins response. Invest Ophthalmol Vis Sci. 43: 1673-1685 Ref.: https://pubmed.ncbi.nlm.nih.gov/11980890/
25. Hood DC. 2000. Assessing retinal function with the multifocal. 19: 607-646. Ref.: https://pubmed.ncbi.nlm.nih.gov/10925245/ Doi: https://doi.org/10.1016/s1350-9462(00)00013-6
26. Feigl B, Greaves A, Brown B. 2007. Functional outcomes after multiple treatments with anibizumab in neovascular age-related macular degeneration beyond visual acuity. Clin Ophthalmol. 1: 167-175. Ref.: https://pubmed.ncbi.nlm.nih.gov/19668506/
27. Xie, Chen Yu, Zhou, et al. 2020. Fan and XuMorphologic Features of Myopic Choroidal Neovascularization in Pathologic Myopia on Swept-Source Optical Coherence Tomography. Front Med (Lausanne). 7: 615902. Ref.: https://pubmed.ncbi.nlm.nih.gov/33425961/ Doi: https://doi.org/10.3389/fmed.2020.615902
28. Giorno P, Iacono P, Scarinci F, et al. 2019. Microvasculature changes of myopic choroidal neovascularization and the predictive value of feeder vessel disappearance after ranibizumab treatment revealed using optical coherence tomography angiography. Ophthalmologica. 243: 263-270. Ref.: https://pubmed.ncbi.nlm.nih.gov/31838464/ Doi: https://doi.org/10.1159/000504755
29. Faes L, Ali Z, Wagner S, et al. 2019. Effect of total anti-VEGF treatment exposure on patterns of choroidal neovascularisation assessed by optical coherence tomography angiography in age-related macular degeneration: a retrospective case series. BMJ Open Ophthalmol. 4: 000244. Ref.: https://pubmed.ncbi.nlm.nih.gov/31179393/
30. Miere A, Butori P, Cohen SY, et al. 2019. Vascular remodeling of choroidal neovascularization after anti-vascular endothelial growth factor therapy visualized on optical coherence tomography angiography. Retina. Ref.: https://pubmed.ncbi.nlm.nih.gov/29210939/ Doi: https://doi.org/10.1097/iae.0000000000001964
31. Cheng Y, Li Y, Huang X, et al. 2019. Application of optical coherence tomography angiography to assess anti-vascular endothelial growth factor therapy in myopic choroidal neovascularization. 39: 712-718. Ref.: https://pubmed.ncbi.nlm.nih.gov/29256987/ Doi: https://doi.org/10.1097/iae.0000000000002005




















