Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/irjo.2019.110004Article Views : 25Article Downloads : 10
GSTs (T1 and M1) population data in patients with glaucoma: genetic screening and ethical aspects
Ferreri F.M.B1* and Sapienza D2
1Institute of Ophtalmology, Department of Biomedical and Dental Sciences and Morpho-Functional Imaging University of Messina, Italy
2Institute of Legal Medicine, Department of Biomedical and Dental Sciences and Morpho-Functional Imaging University of Messina, Italy
*Corresponding Author: Ferreri F.M.B, Institute of Ophtalmology, Department of Biomedical and Dental Sciences and Morpho-Functional Imaging University of Messina, AOU Policlinico “G. Martino”, viale Gazzi 98125 Messina, Email: fferreri@unime.it
Article Information
Aritcle Type: Research Article
Citation: Ferreri F.M.B, Sapienza D. 2019. GSTs (T1 and M1) population data in patients with glaucoma: genetic screening and ethical aspects. Int Res J Optha. 1: 18-23.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Ferreri F.M.B
Publication history:
Received date: 04 December, 2019Accepted date: 16 December, 2019
Published date: 18 December, 2019
Summary
Background and Aim: Molecular epidemiology is an interdisciplinary field concerning the analysis of biological indicators and the investigation of individual DNA polymorphisms. The glutathione-S-transferases (GSTs) are one of the most studied metabolic gene families. They are involved in the genesis of oxidative stress and many authors hypothesize that some null polymorphic variants determine increasing toxic effects in tissues. Oxidative stress and antioxidant systems are very important in the onset and progress of glaucoma, one of the leading causes of blindness worldwide. We investigate the association of null variant of GST (M1 and T1) with the risk of primary open angle glaucoma (POAG) and we considered the ethical and legal implications of genetic procedures as a screening tool.
Materials and Methods: We conducted a case-control study including 103 unrelated carriers of glaucoma in a southern Italian population (living in Eastern Sicily) and 150 unrelated healthy individuals as controls, whose buccal swabs samples were genotyped for GST polymorphisms using a standardized multiplex PCR based method.
Results: In patients with glaucoma (primary open angle glaucoma, POAG) null genotype of the investigated genetic polymorphisms is very common compared to the healthy individuals. The obtained data suggest an influence of the (dual) null genotype on the normal metabolic pathway in the genesis of congenital glaucoma giving to these polymorphisms the role of so-called “indicators of susceptibility”.
Conclusions: We conclude that the increased frequency of null GSTs (M1, T1) in patients with glaucoma could be considered a risk factor for incidence of the disease. Screenings can be carried out only in compliance with legislative rules.
Keywords: Glaucoma; Glutathione S-transferase; GSTM1; GSTT1
Introduction
Glaucoma denotes a broad and heterogeneous range of ocular diseases, all of them sharing a damage to the optic-nerve which, on the long run, is able to cause irreversible visual field loss and vision impairment. According to an a popular and authoritative study conducted in 2006 by Quigley and Braman [1] glaucoma is the second leading cause of blindness worldwide: the aforementioned study reports that the estimated number of people affected by glaucoma is around 60 million in 2010 and such a number is expected to increase up to 79.6 million by 2020. The same study estimated that bilateral blindness due to glaucoma is likely to occur in around 4 million people, rising to more than 5 million people in 2020. The intraocular pressure (IOP) is universally considered as the most important risk factor for developing glaucoma: the well-known Ocular Hypertension Treatment Study (OHTS) proved that topical ocular hypotensive medication is effective in delaying or preventing the onset of Primary Open Angle Glaucoma (POAG) in those individuals affected by high IOP [2]. The IOP is the only treatable risk factor to prevent glaucoma progression: according to Gordon et al. [2], demographic and clinical factors that may influence POAG are older age, larger vertical or horizontal cup-disc ratio. The etiology of POAG is still unclear but very recent studies [3,4] point out that genetic variants, epigenetic modifications as well as environmental factors may contribute to glaucoma and they could explain the prevalence of one type of glaucoma in a specific area of the world. To this end, molecular epidemiology - an interdisciplinary field of research which focuses on the analysis of biological indicators and the technical analysis of individual DNA polymorphisms to define the role of some exogenous and endogenous factors as causes of metabolic disorders and other pathology as well as neoplastic diseases - provides powerful tools for better understanding of the genetic causes of POAG.
The glutathione-S-transferases (GSTs) are one of the most studied metabolic gene families in genesis of stress oxidative [9]. An important study in the role of GSTs in POAG is reported in Lu et al. [15], who performed a meta-analysis over 14 experimentations. Since many classical electrophilic carcinogens are substrates for the GST enzymes, it has generally been hypothesized that the common human polymorphic variants of these genes, which for GSTM1 and GSTT1 are homozygous deletions, should result in a decrease in conjugation and subsequent elimination of toxicogenic intermediates, resulting in the increasing of toxicity in tissue. A relevant percentage of the population (which varies on the basis of ethnicity) carries a null genotype of these genes [3,4]. Although mutations in several genes, including myocilin, optineurin, and CYP1B1 have been reported to cause POAG, as like some genetic polymorphism involved in detoxification of xenobiotic and oxidative stress such the super-family of GSTs, these genes account for less than 10% of cases worldwide [7, 8]. In the latest years, large scale genetic studies that have examined the blood samples and buccal swab of thousands of glaucoma patients. A risk factor is something that doesn’t always lead to a condition but increases the risk of having that condition. For glaucoma, these genetic factors have been implicated in individual susceptibility to different oxidative disease in pathology. How these genes cause or influence the likelihood of developing POAG is a question of major interest. Several studies have shown that the “null” GSTM1 and/or GSTT1 genotype (and the consequently decreased antioxidant activity) is correlated with a higher risk for oxidative stressrelated disease, such as neoplastic and inflammatory diseases [5]. An important contribution to clarify the role of GST polymorphisms as a risk factor for POAG (as well as on other types of glaucoma) is reported in [12]. Here, the authors carefully reviewed existing studies to evaluate the strength of the association between polymorphisms of GSTM1, GSTT1 and GSTP1 and glaucoma risk. The main finding of [12] is that either GSTM1 or GSTT1 null polymorphism was not associated with a POAG risk; such a negative association was also observed in Caucasian populations. The GSTP1 Ile 105 Val polymorphism was significantly correlated with increased POAG risk among Caucasian in a recessive model; a further interesting result was that an increased glaucoma risk was associated with the combined GSTM1 and GSTT1 null genotypes and with the combined GSTM1 null and GSTP1 Val genotypes. Amero et al. [13] reviewed recent genome-wide association studies which aim at identifying several single nucleotide polymorphisms at different loci (such as CAV1/CAV2, TMCO1, CDKN2B-AS1, CDC7-TGFBR3 to name a few) associated with POAG. The authors also discuss on the correlation of polymorphism with various clinical parameters influencing POAG, with a special focus on Middle East countries. In a subsequent study, Kumar et al. [14] highlights that at least 29 genetic loci have been linked to POAG and there exist at least 66 loci with 76 genes associated to POAG. To complete our discussion, it is important to cite the study by Malik et al. [16], who investigated the role of GSTM1/GSTT1 on Juvenile Open Angle Galucoma (JOAG) and they found that no significant association exists between GSTM1/ GSTT1 and JOAG. In the present study, we typed the polymorphism of GSTT1 and GSTM1 in 103 unrelated subjects with glaucoma and in 150 healty individuals as control [4] to define any relationship between incidence of glaucoma and GST polymorphyms. Finally, we considered the ethical and legal values of application of genetic screening as predictive factors in clinical and legal medicine.
Materials and Methods
The study was performed on 103 subjects with primary open angle glaucoma (POAG). Written informed consent was obtained from all subjects enrolled in the study. Selected patients were mainly from the province of Messina (Italy) with an age from 43 to 60 (mean 52, sd 4.5) and none of them underwent ocular surgery; patients were not affected by diabetes and systemic hypertension. A complete ophtalmologic examination was performed; glaucoma diagnosis has been formulated according to the following parameters: IOP (intraocular pressure) above 21 mmHg, generalized/focal enlargement of the cup in the optic disk and a slight alteration of visual field (measured through the Humphrey Visual Field Analyzer). All selected patients were treated with only one type of drugs (eye drop). As for the GSTM1 and GSTT1 genotyping analysis, we used buccal swabs to obtain a sample of cells for DNA analysis. Swabs were put in separate vials and kept at −20?C until experiments were performed. In our tests, we used specific standard markers (DNA pGEM marker purchased from Promega) and for DNA analysis, we used the standard - Chelex® 100 method for DNA extraction [5]. The genes of interest were amplified by PCR, using a - PCR sprint (Hybaid) thermal-cycler. A multiplex system was created for the simultaneous amplification of the genetic loci of GSTM1 and GSTT1; to verify the correct completion of the PCR, the gene of beta-globin, that is unrelated to GST and with a molecular weight clearly distinct from that of both GSTM1 and GSTT1 genes, was amplified in the same system as a control. The oligonucleotide sequences of primers were those previously reported in literature [6], namely: for GSTM1, forward primer: 5′ -GAA CTC CCT GAA AAG CTA AAG C-3′ and reverse primer: 5′ -GTT GGG CTC AAA TAT ACG GTG G-3′; for GSTT1, forward primer: 5′ -TTC CTT ACT GGT CCT CAC ATC TC-3′; reverse primer: 5′ -TCA CCG GAT CAT GGC CAG CA-3′; and for BETA- GLOBIN: forward primer: 5′ -CAA CTT CAT CCA CGT TCA CC-3′; reverse primer: 5′ -GAA GAG CCA AGC ACA GGT AC-3′. Each PCR was performed with 2.5 ml extract (5-250 ng DNA), 0.5 mM of each primer, 2.5 ml Taq buffer (10× PCR Buffer II, Applied Biosystems, USA), 2 ml MgCl2 25 mM (Applied Biosystems, USA), 0.5 ml dNTPs mix (10 mM PCR Nucleotide Mix, Promega, Germany), 1 U Taq polymerase (DyNAzyme II DNA Polymerase, Finnzymes, Finland) in a total volume of 25 μL. Thirty amplification cycles (denaturation at 95 ?C for 1 min, annealing at 60 ?C for 1 min and extension at 72 ? C for 1 min) were performed. Finally, the products of the reaction were vertically electrophoresed at 2000 V, max mA, max W for 150 min on 0.4 mm layer polyacrylamide denaturing gels (6% - urea 7 M) in TBE buffer 1×, and then revealed by silver staining [17,18]. The migration bands of interest (480 bp for GSTT1 and 215 bp for GSTM1) were identified by comparison with the molecular weights of a specific standard marker (DNA pGEM® marker, Promega, Germany). The electropherogram of GSTT1(480 bp) and GSTM1(215 bp) is shown in figure 1. The beta-globin gene has been employed as a confirmation that PCR actually happened.
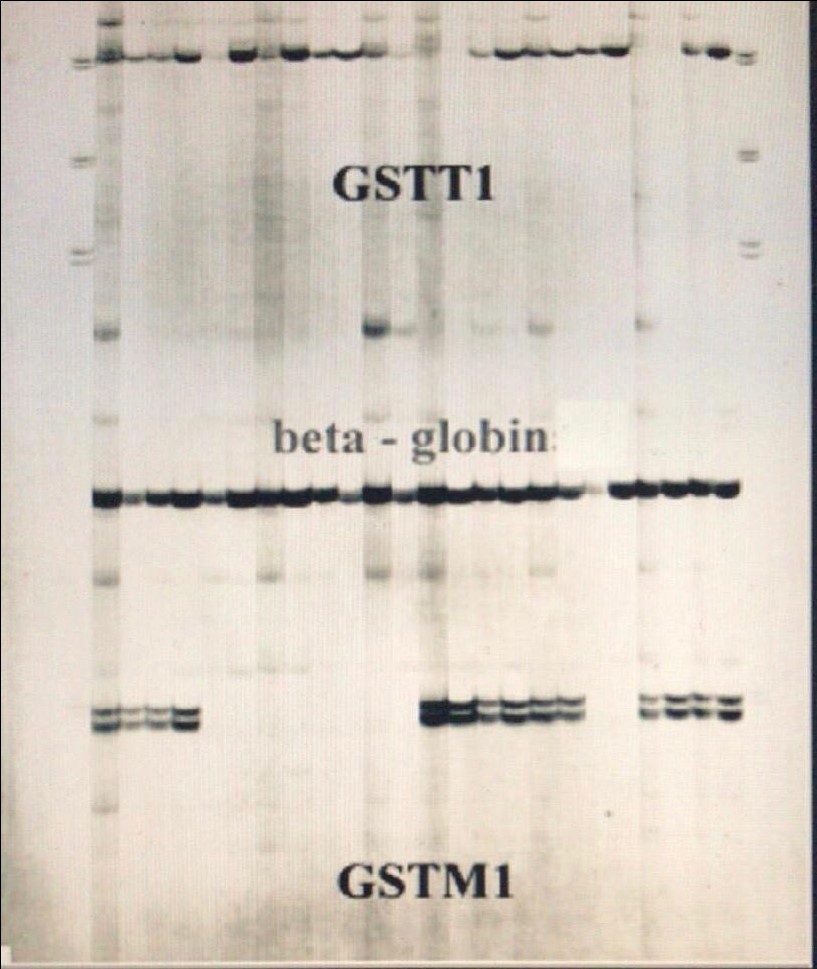
Figure 1: PCR product from different genotypes.
Results
In patients with POAG, null genotype of the investigated genetic polymorphisms is increased compared to healty subjects.
|
Table 1: Main features of Sicilian and Turkey cohorts [3] under investigation. |
||||
|
|
Eastern |
Turke |
||
|
Genotyp |
N |
Frequenc |
n |
Frequenc |
|
Tota |
150 |
|
13 |
|
|
GSTM1 |
8 |
5.7 |
6 |
5.9 |
|
GSTT1 |
3 |
2.7 |
2 |
1.3 |
|
GSTM1/GSTT1 |
1 |
1% |
1 |
9 |
We first compared the results we achieved on a population from Eastern Sicily with results reported in [3], which consider a population from Turkey (see Table 1). Both cohorts were constituted by healthy individuals and we found that the fraction of individuals with GSTM1 null in Eastern Sicily (54.7%) was roughly equal to that observed in Turkey sample. Percentage of individuals belonging to GSTT1 null and GSTM1/GSTM1 null in Eastern Sicily cohort were larger than that observed in Turkey population (24.7% and 12% in Eastern Sicily sample against 17.3% and 9% in Turkey sample). Then, we turned our attention on Eastern Sicily population and were interested in assessing whether meaningful differences emerge between healthy individuals and individuals with glaucoma (see Table 2).
|
Table 2: Main features of healthy individuals and individuals with POAG recruited in our study. |
||||
|
|
Healthy individuals |
Individuals affected by POAG |
||
|
Genotyp |
N |
Frequenc |
n |
Frequenc |
|
Tota |
150 |
|
103 |
|
|
GSTM1 null |
82 |
54.7% |
68 |
66% |
|
GSTT1 null |
37 |
24.7% |
23 |
22.3% |
|
GSTM1 and GSTT1 null |
18 |
12% |
8 |
7.7% |
|
GSTM1 and GSTT1 no null |
13 |
8.6% |
4 |
3.8% |
The data indicates an influence of the null genotype of GSTM1 gene on the normal metabolic pathway in the genesis of POAG. Specifically, the percentage of individuals belonging to the GSTM1 null class goes up to 66% (with an increase of about 11.3% against the cohort of healthy individuals). As a consequence, the proportion of individuals with glaucoma belonging to the GSTM1 and GSTT1 no null class precipitates to 3.8% (and, thus, it is 2.26 times smaller than the proportion of healthy individuals in that class). These results give to these polymorphisms the role of indicators of susceptibility.
Statistical Analysis. Difference between group proportions was tested through the χ2 square test and they indicate that obtained results were statistically significant (p-value<0.05).
Conclusions
We conclude that the increased frequencies of GSTM1 null in patient with POAG could be a risk factor for incidence of the disease. So, it is necessary a replacement of the primary and secondary prevention strategy in people with positive markers (alimentary, no smoking and/or alcohol status) but it is necessary to avoid any discrimination in these subjects especially in relation to the stipulation of policies covering illness and work opportunity that involve the use of VDT, for example. We plan to refine our analysis by adding further, genetic-based features to predict the onset of glaucoma and a potential candidate is certainly represented by the Val genotype, in compliance with previous studies [12]. In the tertiary prevention, the protection of the subject with individual susceptibility to these diseases, must to consider the impact of these genetic biomarkers in the quality and life expectancy to the well-being of the individual and the community.
References
1. Quigley HA, Broman AT. 2006. The number of people with glaucoma worldwide in 2010 and 2020. British journal of ophthalmology. 90: 262-267. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/16488940
2. Gordon MO, Beiser JA, Brandt JD, et al. 2002. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Archives of ophthalmology. 120: 714-720. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/12049575
3. Yilmaz SG, Palamar M, Onay H, et al. 2016. LOXL1 gene analysis in Turkish patients with exfoliation glaucoma. International ophthalmology. 36: 629-635. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/26758070
4. Pasquale LR, Kang JH, Wiggs JL. 2014. Prospects for gene-environment interactions inexfoliation syndrome. J Glaucoma. 23: 64-67. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/25275911
5. Walsh PS, Metzger DA, Higuchi R. 1991. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques. 10: 506-513. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/1867860
6. Pavanello S, Simioli P, Lupi S, et al. 2002. Exposure levels and cytocrhome P450 1A2 activity, but not N -Acetyltransferase, Glutathione S-transferase (GST) M1 and T1, influence urinary mutagen excretion in smokers. Cancer epidemiolgy, biomarker & prevention. 11: 998-1003.
7. Huang W, Wang W, Zhou M, et al. 2013. Association of Glutathione S-transferasepolymorphism (GSTM1 and GSTT1) with primary open-angle glaucoma: an evidence-based metaanalysis. Gene. 526: 80-86. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/23747403
8. Aslan M, Cort A, Yucel I. 2008. Oxidative and nitrative stress markers in glaucoma. Free Radic Biol Med. 45: 367-376. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/18489911
9. Seidegard J, Vorachek WR, Pero RW, et al. 1988. Hereditary differences in theexpression of the human glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proc Natl Acad Sci USA. 85: 7293-7297. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/3174634
10. Asmundo A, Sapienza D. 2006. Analisi dei polimorfismi genetici GSTT1 e GSTM1 nella provincia di Messina (Sicilia orientale). Atti XX Congresso Nazionale GEnetisti Forensi Italiani, Bologna 9-11 Settembre 2004: “Il DNA nella Società attuale: test genetici, disastri di massa, identificazione criminale” pubblicati in Medicina Legale criminologia e deontologia medica ISBN 11. 88-14-12471-X Giuffrè editore, S. p. A. Milano - 2006 pag 203.
12. Spatari G, Saitta S, Cimino F, et al. 2012. Increased serum levels of advanced oxidation protein products and glycation end products in subjects exposed to low-dose benzene. Int J Hyg Environ Health. 215: 389-392. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/22153878
13. Yu Y, Weng Y, Guo J, et al. 2013. Association of Glutathione-S-transferases Polymorphisms with Glaucoma: A Meta-Analysis. PLoS ONE. 8: 54037. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/23342067
14. Abu-Amero, Khaled, Altaf A. et al. 2015. An updated review on thegenetics of primary open angle glaucoma. International journal of molecular sciences. 12: 28886-28911. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/26690118
15. Kumar, Sunil, et al. 2016. Candidate genes involved in the susceptibility of primary open angleglaucoma. Gene. 2: 119-131. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/26621382
16. Lu, Yan, et al. 2013. Are glutathione S-transferase polymorphisms (GSTM1, GSTT1) associatedwith primary open angle glaucoma? A meta-analysis. Gene. 1: 311-315. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/23827458
17. Malik, Manzoor Ahmad, et al. 2017. Glutathione S-transferase (GSTM1, GSTT1) polymorphisms and JOAG susceptibility: a case control study and meta-analysis in glaucoma. Gene. 628: 246-252. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/28710033
18. Budowle B, Chakraborty R, Giusti AM, et al.1991. Analysis of the variable number of tandem repeat locus D1S80 by the polymerase chain reaction followed by high resolution polyacrylamide gel electrophoresis. Am J Hum Genet. 48: 137-144.
19. Bassam BJ, Caetano Anollés G, Gresshoff PM. 1991. Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem.196: 80-83. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/1716076




















