Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ijrmsh.2019.110001Article Views : 3225Article Downloads : 29
Robson’s class and caesarean scar defects
Carlo Alovisi1, Roberta Amadori2*, Carlotta Alovisi1 and Daniela Surico2
1Department of Obstetrics and Gynaecology, Maria Vittoria Hospital, Torino, Italy
2Department of Obstetrics and Gynaecology, University of Eastern Piedmont, 28100 Novara, Italy
*Corresponding author: Roberta Amadori, Department of Obstetrics and Gynaecology, University of Eastern Piedmont, 28100 Novara, Italy, Email: ierama@tin.it
Article Information
Aritcle Type: Research Article
Citation: Carlo Alovisi, Roberta Amadori, Carlotta Alovisi, et al. 2019. Robson’s class and caesarean scar defects. Int J Reprod Med Sex Health. 1: 01-07.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Carlo Alovisi
Publication history:
Received date: 16 February, 2019Accepted date: 07 March, 2019
Published date: 08 March, 2019
Abstract: Caesarean scar defect (CSD) may lead to the occurrence of gynecologic symptoms such as abnormal uterine bleeding secondary to intermittent passage of retained menstrual blood within the CSD pelvic pain, and infertility. This prospective cohort study was conducted at the Department of Obstetrics at Maria Vittoria Hospital in Turin (Italy), from January 2013 to December 2013 to analyze the effects of two different suturing techniques (single layer and double layer closure of the hysterotomy) and Robson's class impact on the incidence of CSD. All procedures were performed using a modified Stark technique by the same single senior surgeon. The way of closure of the uterine incision was alternated every three months, in order to have two groups of partecipants: one with a single layer and the other with a double layer closure technique. Single layer was carried out as one continuous locking stitch; double layer was performed with a first closure identical to the single layer and an additional suture with a continuous unlocked stitch used to imbricate the first layer. Both ways of closure of the uterine incision were performed using monofilament synthetic absorbable polydioxanone suture. Twelve months after their caesarean section, the women had an ultrasound examination of the uterine scar performed by a single experienced operator blinded to suture technique and the Robson class. The trial recruited 85 cases. 21 patients (24.8%) belonged to Robson's class 1, 5(6%) to class 2, 1(1.3%) to class 4, 35(41%) to class 5, 13(15.4%) to class 6, 6(7%) to class 7, 4(4.5%) to class 8. During the ultrasound follow up we found 10 CSD (11,8%): 8/10 CSD (80%) were found in Robson's class 5, 1 in class 1 and 1 in class 6 (p 0.008), with no correlation with single- or double-layer suture (p 0.141). To our knowledge, no previous studies evaluated the correlation with Robson classification and CSD.
Keywords: Uterine Scar Defects; Robson's Classification; Caesarean Scar; Hystmocele; Uterine Niche
Introduction
Caesarean section (CS) is one of the most common major surgery performed worldwide. The surgical technique of CS is not standardized, and many steps of the procedure as it is commonly performed today are not based on evidences from randomized trials [1]. Although several obstetric complications such as placenta accreta, scar dehiscence, and ectopic scar pregnancy (due to inappropriately healed uterine lower segment incision) have been reported, gynecologic sequela are increasingly described in the last decade [2]. The standard low-segment transverse incision accounts for 90% of all and the risk of long term postoperative morbidity is linked to the uterine scar, that sometimes has an incomplete healing, appearing like a pouch [3]. A histopathological study of hysterotomy specimens from caesarean section scar or defect (CSD) suggested that some possible mechanisms underlying the pathogenesis of these conditions: the presence of congested endometrial fold (found in 61% cases) and small polyps in the scar recess (16%) are causes of abnormal bleeding; secondly, lymphocytic infiltration (65%) and distortion of the lower uterine segment (75%) could contribute to pelvic pain, and, finally, iatrogenic adenomyosis confined to the scar (28%) could cause dysmenorrhea [4]. Alternative terms for a niche are, deficient caesarean scar [5], diverticulum [6], pouch [7] and isthmocele [8], determining diffilculties in literature review, but the most accepted definition is CSD [9]. Since the suturing technique and mechanical factors affecting the surgical area are major determinants of the surgical outcome in terms of incision integrity in almost any surgical wound, there is a controversy about the association between the risk of uterine rupture and the different way of hysterotomy closure [10]. As uterine rupture is quite uncommon, many studies have considered scar defects or the thickness of residual myometrium as a marker of incomplete healing of the uterine incision [11-12]. Transvaginal ultrasound evaluation is highly accurate in detecting caesarean scar assessment and its worldwide use has increased the identification of CSD [13]. As not all women with a history of CS develop a niche, it is of interest to identify the risk factors that may predict their development [14]. The aim of this study was to analyze the effects of two different suturing techniques (single layer and double layer closure of the hysterotomy) and Robson’s class impact on the incidence of post-operative defective healing of the uterine incision in non-pregnant women.
This prospective cohort study was conducted at the Department of Obstetrics and Gynaecology at Maria Vittoria Hospital in Turin (Italy), including patients undergoing CS from January 2013 to December 2013. They were undergoing delivery by lower segment CS through a transverse abdominal incision, and had no more than one previous CS. Then the women were recalled for ultrasound evaluation 12 months after CS. All patients gave their informed consent prior to their inclusion in the study. This study was approved by the ethics committee. All caesarean deliveries were performed using a modified Stark technique by the same single senior surgeon (C.A.). The way of closure of the uterine incision was alternated every three months, in order to have two groups of partecipants: one with a single layer and the other with a double layer closure technique: patients who delivered in January, February, March, July, August and September underwent to single layer closure, patients who delivered in April, May, June, October, November and December to double layer. We classified the CS’s urgency according to RCOG guidelines [15]. Single layer was carried out as one continuous locking stitch; double layer was performed with a first closure identical to the single layer and an additional suture with a continuous unlocked stitch used to imbricate the first layer. Both ways of closure of the uterine incision were performed using monofilament synthetic absorbable polydioxanone suture (PDS loop 1; Ethicon, Inc., Somerville, NJ, USA). Intravenous prophylactic antibiotics (2 g. ampicillin +1 g. sulbactam) and an oxytocin bolus 5 IU was routinely administered, followed by an infusion with 10 IU in 2 h. (Syntocinon®) in both groups. Twelve months after their CS, the women were invited by phone to have an ultrasound examination of the uterine scar. Sonographic assessment was performed by a single experienced operator blinded to suture technique and the Robson class (R.A.) using a Voluson 730 Logic 9 (GE Healthcare, Milwaukee, WI, USA) ultrasound machine, equipped with a 7-9-MHz transvaginal probe. CSD was diagnosed in the presence of a hypoechogenic area (a filling defect) within the myometrium of the lower uterine segment, at the site of a previous caesarean incision, according to previous reports [16]. During the assessment, a detailed medical history was taken by a specifically trained nurse. The uterine position was also recorded. Uterine anteflexion was defined as the anterior deviation of the long axis of the uterine endometrial cavity toward the axis of the cervix, and uterine retroflexion as posterior deviation of this axis [17]. The variables considered in the study were: age, number of deliveries, gestational age, timing of surgery, Robson classification, intra- and/or post- operative complications, pain, menstrual disorders and the presence/absence of the CSD.
Statistical analysis: All outcomes were analysed in the groups into which the women were allocated irrespective of the technique received. Binary outcomes were analysed with log binomial regression models and results presented as adjusted risk ratios. Continuous outcomes were analysed with linear regression models and results presented as adjusted differences in means. Frequency and descriptive statistics were used with mean, standard deviation, minimum and maximum. In addition, to verify the relationship between the variables, tests were used for the hypotheses listed. The Anova test was used to verify the relation of single versus double layer. Statistical analysis consisted of performing an intraclass correlation. All categorical variables were compared using Pearson's chi-square method. Logistic regression was carried out to assess any association between the possible factors and to prevent mutual influence. A P-value of < 0.05 was considered statistically significant. These procedures were processed in SPSS version 19.0 for Windows.
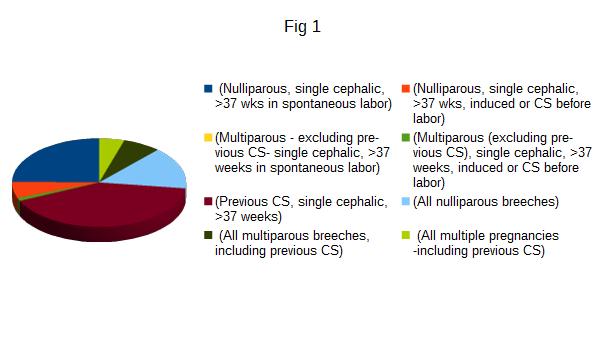
The trial recruited 100 patients, but 15 were lost at follow up because unavailable for ultrasonography, so the results are relative on 85 cases. Characteristics of patients are summarized in tab 1. Patients aged between 23 and 47 years (mean 34,5 years) and with a parity variable between 1 and 4 (average 1 delivery). The multiparae were 47 and the nulliparae were 38. Every patient underwent to CS between 35 and 41 weeks of gestational age. Preterm deliveries were 16 (18.8%) and term 69 (81.2%). The rate of RCOG category 4 CS was 71,8% (61/85) and unplanned CS (RCOG categories 1-2-3) were 28,2% (24/85): 2 patients underwent to category 1 CS (8.3%), 8 to category 2 (33.3%), 11 to category 3 (45.8%). Robson classes are summarized in Figure1: 21 patients (24.8 %) belonged to class 1 (Nulliparous, single cephalic, >37 wks in spontaneous labor), 5 (6 %) to class 2 (Nulliparous, single cephalic, >37 wks, induced or CS before labor), none to class 3 (Multiparous - excluding previous CS), single cephalic, >37 weeks in spontaneous labor), 1 (1.3%) to class 4 (Multiparous (excluding previous CS), single cephalic, >37 weeks, induced or CS before labor), 35 (41 %) to class 5 (Previous CS, single cephalic, >37 weeks), 13 (15.4 %) to class 6 (All nulliparous breeches), 6 (7%) to class 7 (All multiparous breeches, including previous CS), 4 (4.5%) to class 8 (All multiple pregnancies -including previous CS), none to class 9 (All abnormal lies -including previous CS) and class 10 (All single cephalic, <36 wks, including previous CS). During the ultrasound follow up we found 10 CSD (11,8%): 5 in the single layer (9,1%) and 5 in double layer group (16,7%) (p 0,112). The intraclass correlation between CS categories (planned versus unplanned) and CSD was 0.148. According to Robsons’s class, 8/10 CSD (80%) were found in class 5, 1 in class 1 and 1 in class 6 (p 0.008), with no correlation with single or double layer suture (p 0.141). There were 7 patients with immediate postoperative complications: 2 cases presented fever (> 38° C), 2 cases of infection of the wound, and 3 had postpartum anemia (< 8 g/dL). In this population there were 2 cases of CSD (2/7; 28.6%; p 0.153). There weren’t correlations between CSD and patient’s age after stratification of included cases in under 35 (6/10) and over 35 years old (4/10, p 0.754) or CSD and preterm delivery (p 0.92). Out of 10 CSDs found, 9 were in multiparous (p 0.019). 9/10 CSD were found in patients with uterine anteflexion (p 0.856). In the follow up period, prolonged postmenstrual spotting was the most common symptom (10.5%, 9/85), followed by pelvic pain (2.3%, 2/85), regardless the presence/absence of CSD (p 0.485). Hypertrophic scar and abdominal keloid were only present in 9 cases (10.6%), regardless presence/absence of CSD (p 0.949).
Figure 1: Classification of patients according to the class of Robson: each color corresponds to the class.
Three top-level pole dancers volunteered to participate in this study, carried out in September 2017. Some relevant biometrics are reported in table 1.
| Table 1: Characteristics of the patients. | |||
|---|---|---|---|
| Characteristics | Single layer | Double layer | T |
| Mean Age (years) | 34.7 ( 23- 47; σ 4,9) | 34 ( 25- 40: σ 4,1) | 0,518 |
| Parity | 1 ( 0-4; σ 0,9) | 1 (0- 3; σ 0,8) | 0,6611 |
| Gestational age (wks) | 38.6 ( 35- 41; σ 1,4 ) | 37.6 (35-41; σ 0,8) | 0,2411 |
| Robson class 5 | 55 (64.7%) | 30 (35.3%) | 0.453 |
Conclusion
The CSD is due to the presence of a diverticulum on the anterior wall of the uterine isthmus or of the cervical canal at the site of a previous caesarean delivery scar and the consequences of this complication for a future pregnancy are unknown. Since 1985, the international healthcare community has considered the ideal rate for CS to be between 10% and 15%. Since then, CS have become increasingly common in both developed and developing countries. When medically justified, a caesarean section can effectively prevent maternal and perinatal mortality and morbidity. However, there is no evidence showing the benefits of caesarean delivery for women or infants who do not require the procedure. As with any surgery, CS are associated with short and long term risk which can extend many years beyond the current delivery and affect the health of the woman, her child, and future pregnancies [18]. Possible known risk factors for CSD include the number of CS, uterine position, labor before CS, and the surgical technique used to close the uterine incision [19]. Bij de Vaate et al classified all risk factors into four main categories: factors related to closure technique, development of the lower uterine segment or location of the incision, wound healing and miscellaneous factors [14]. Among the existing systems used to classify CS, the 10-group classification (also known as the ‘Robson classification’) has become widely used in many countries in recent years [20]. To our knowledge, no previous studies evaluated the correlation with Robson classification and CSD. We found a statistical correlation between Class 5 and CSD. The establishment of a repeatable cutting plane is difficult, even in cases of elective CS. The cervical-corporal junction in a term pregnancy is difficult to locate, and the development of the lower uterine segment may vary according to the pregnancy duration, size of the uterus, effacement of the cervix and other factors [21]. Colmorn et al. found that elective repeated CS nearly prevents complete uterine rupture at the second delivery, while women with a first elective versus emergency caesarean have increased risk of severe complications in the second pregnancy [22]. There is a consensus that planned VBAC is a clinically safe choice for the majority of women with a single previous lower segment caesarean delivery [23]. Such a strategy is also supported by health economic modelling and would also limit any escalation of the caesarean delivery rate and maternal morbidity associated with multiple caesarean deliveries [24]. WHO statement declares that as CS rates increased above 10% and up to 30% no effect on mortality rates was observed. Intra-operative and post-operative complications are more in the emergency CS as compared to elective CS group as previously demonstrated [25]. Instead, we didn’t found correlation between CS categories (planned versus unplanned) and CSD. In a 2014 systematic review and meta-analysis of comparative studies, single- and double-layer hysterotomy closure resulted in similar rates of overall maternal infectious morbidity, endometritis, wound infection, and blood transfusion (20 studies including almost 15,000 patients). In that meta-analysis, single-layer closure resulted in a nonstatistical increase in “uterine rupture or dehiscence” in the next pregnancy) [26]. Also in our study the choice of the type of hysterotomy, whether in single or double layer suture, does not seem to determine the next occurrence of CSD, as recently described by The CORONIS collaborative group. That study enlarged cervical dilatation and induction of labour as prognostic factors for developing a uterine niche, instead contractions before labour reduced the risk. In our study Robson class 2 and 4 resulted unrelated to CSD development. The CSD may lead to the occurrence of gynecologic symptoms such as abnormal uterine bleeding secondary to intermittent passage of retained menstrual blood within the CSD pelvic pain, and infertility [27-30]. On the contrary, in many women the improperly healed caesarean scar may be asymptomatic or stay unrecognized by gynecologists who are unaware of the condition [31]. The prevalence of symptomatic or clinically relevant CSDs reported in the literature ranges from 19.4% to 84%, maybe due to diagnostic issues and the tendency to unreport asintomatic cases [32]. Several hypotheses have been put forward to explain the etiology of abnormal uterine bleeding in women with CSD, such as poor contractility of the uterine muscle around the niche, which may result in retention of menstrual blood within it [33-35]. It has been postulated that the characteristics of the myometrium alter during labor and that, for example, a thinner myometrium may be less well vascularized, which may lead to insufficient wound healing and niche development [36]. The occurence of CSD is probably the result of many other factors. However, it is linked to various longterm problems and of different gravity, so we should do everything possible to avoid it with correct maneuvers. The gynecologic sequelae caused by deficient uterine scar healing after CS are only recently being identified and described. More research is needed to detect risk factors and to estabilish the right management. The main strengths of this study are the single surgeon, single ultrasound performer, the quasi-randomized system, the completeness of follow-up and the rigorous data collection process.
References
- The CORONIS Collaborative Group. 2013. Cesarean section surgical techniques (CORONIS): a functional, factorial, unmasked, randomized controlled trial. Lancet. 382: 234-248. [Ref.]
- Tower AM, Frishman GN. 2013. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 20: 562-572. [Ref.]
- Magan EF. 2002. Intraoperative haemorrage by blunt vs sharp expansion of the uterine incision at the cesarean delivery. 109: 448-452. [Ref.]
- Morris H. 1995. Surgical pathology of the lower uterine segment cesarean section scar: is the scar a source of clinical symptoms? Int J Gynecol Pathol. 14: 16-20. [Ref.]
- Ofili-Yebovi D, Ben-Nagi J, Sawyer E, et al. 2008. Deficient lower-segment Cesarean section scars: prevalence and risk factors. Ultrasound Obstet Gynecol. 31: 72-77. [Ref.]
- Surapaneni K, Silberzweig JE. 2008. Cesarean section scar diverticulum: appearance on hysterosalpingography. AJR Am J Roentgenol. 190: 870-874. [Ref.]
- Fabres C, Aviles G, De La Jara C, et al. 2003. The cesarean delivery scar pouch: clinical implications and diagnostic correlation between transvaginal sonography and hysteroscopy. J Ultrasound Med. 22: 695-700. [Ref.]
- Borges LM, Scapinelli A, de Baptista Depes D, et al. 2010. Findings in patients with postmenstrual spotting with prior cesarean Section. J Minim Invasive Gynecol. 17: 361-364. [Ref.]
- Osser OV, Jokubkiene L, Valentin L. 2009. High prevalence of defects in Cesarean section scars at transvaginal ultrasoundexamination. Ultrasound Obstet Gynecol. 34: 90-97. [Ref.]
- Fitzpatrick KE, Kurinczuk JJ, Alfirevic Z, et al. 2012. Knight M. Uterine rupture by intended mode of delivery in the UK PLoS Med. 9. [Ref.]
- Roberge S, Chaillet N, Boutin A, et al. 2011. Single versus double -layer closure of the hysterotomy incision during cesarean delivery and risk of uterine rupture Int J Gynaecol Obstet. 115: 5-10. [Ref.]
- Roberge S,Boutin A,Chaillet N, et al. 2012. Systematic review of Cesarean scar assessment in the non pregnant state: imaging techniques and uterine scar defect. Am J Perinatol. 29: 465-471. [Ref.]
- Kikhareva Osser O, Valentin L. 2011. Clinical importance of appearance of cesarean hysterotomy scar at transvaginal ultrasonography in non pregnant women. Obstet Gynecol. 117: 552-553. [Ref.]
- Bij De Vaate A. J. M, Van Der Voet L. F, Naji O, et al. 2014. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following Cesarean section: systematic review. Ultrasound Obstet Gynecol. 43: 372-382. [Ref.]
- RCOG Good Practice No. 2010. “Classification Of Urgency Of Caesarean Section -A Continuum Of Risk”[Ref.]
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. 2012 Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 39: 252-259. [Ref.]
- Sanders RC, Parsons AK. 2014. Anteverted retroflexed uterus: a common consequence of cesarean delivery. AJR Am J Roentgenol. 117-124. [Ref.]
- WHO Statement on Caesarean Section Rates 2015.[Ref.]
- Glavind J, Madsen L, Uldbjerg N, et al. 2013. Ultrasound evaluation of Cesarean scar after single and double layer uterotomy closure: a cohort study. Ultrasound Obstet Gynecol. 42: 207-212. [Ref.]
- Betran AP, Vindevoghel N, Souza JP, et al. 2014. A Systematic Review of the Robson Classification for Caesarean Section: What Works, Doesn’t Work and How to Improve It. PLoS One. 9: 97769. [Ref.]
- Micha? Pomorski, Tomasz Fuchs, Anna Rosner-Tenerowicz, et al. 2017. Morphology of the cesarean section scar in the non-pregnant uterus after one elective cesarean section. Ginekologia Polska. 174-179. [Ref.]
- Colmorn LB, Krebs L, Klungsøyr K, et al. 2017. Mode of first delivery and severe maternal complications in the subsequent pregnancy. Acta Obstet Gynecol Scand. [Ref.]
- RCOG Green-top Guideline No. 45, October 2015, Birth After Previous Caesarean Birth.[Ref.]
- Fawsitt CG, Bourke J, Greene RA, et al. 2013. At what price? A cost-effectiveness analysis comparing trial of labour after previous caesarean versus elective repeat caesarean delivery. 8: 577-585. [Ref.]
- Raees M, Yasmeen S, Jabeen S, et al. Maternal morbidity associated with emergency versus elective caesarean section. J Postgrad Med Inst. 27: 55-62. [Ref.]
- Voet LLFV, Vaate AMJB, Heymans MW, et al. Prognostic Factors for Niche Development in the Uterine Caesarean Section Scar. Eur J Obstet Gynecol Reprod Biol. 213: 31-32. [Ref.]
- Belinda Centeio L, Scapinelli A, Depes D, et al. 2010. Findings in patients with postmenstrual spotting with prior cesarean section. J Minim Invasive Gynecol. 17: 361-364. [Ref.]
- Wang CB, Chiu WW, Lee CY, et al. 2009. Cesarean scar defect: correlation between cesarean section number, defect size, clinical symptoms and uterine position. Ultrasound Obstet Gynecol. 34: 85-89. [Ref.]
- Gubbini G, Centini G, Nascetti D, et al. 2011. Surgical hysteroscopic treatment of cesarean-induced isthmocele in restoring fertility: prospective study. J Minim Invasive Gynecol.18: 234-237. [Ref.]
- Thurmond AS, Harvey WJ, Smith SA. 1999. Cesarean section scar as a cause of abnormal vaginal bleeding: diagnosis by sonohysterography. J Ultrasound Med. 18: 13-16.[Ref.]
- Murat Api, Aysen Boza, Husnu Gorgen, et al. 2015. Should Cesarean Scar Defect Be Treated Laparoscopically?.[Ref.]
- A Case Report and Review of the Literature. [Ref.]
- Florio P, Gubbini G, Marra E, et al. 2011. A retrospective case-control study comparing hysteroscopic resection versus hormonal modulation in treating menstrual disorders due to isthmocele. Gynecol Endocrinol. 27: 434-438. [Ref.]
- Raimondo G, Grifone G, Raimondo D, et al. 2015. Hysteroscopic Treatment of Symptomatic Cesarean-induced Isthmocele: A Prospective Study. Journal of Minimally Invasive Gynecology. 22: 297-301. [Ref.]
- Gyamfi C, Juhasz G, Gyamfi P, et al. 2006. Single- versus double-layer uterine incision closure and uterine rupture. J Matern Fetal Neonatal Med. 19: 639-643. [Ref.]
- Babu KM, Magon N. 2012. Uterine Closure in Cesarean Delivery: A New Technique N Am J Med Sci. 4: 358-361. [Ref.]
- Buhimschi CS, Buhimschi IA, Yu C, et al. 2006. The effect of dystocia and previous cesarean uterine scar on the tensile properties of the lower uterine segment. Am J Obstet Gynecol. 194: 873-883.[Ref.]




















