Indexing & Abstracting
Full Text
Review ArticleDOI Number : 10.36811/ijrmsh.2020.110009Article Views : 14Article Downloads : 19
Exploring the Applications of Phosphodiesterase Inhibitors Beyond Erectile Dysfunction
Ihab Mansoor1, Mark Mikhail2, Abigail Wiss2, Diana Huynh2, Monica Oakes2, Heba Eassa3 and Mohamed Ismail Nounou2,*
1Pharmaceutics Department, Faculty of Pharmacy, Alexandria University, Alexandria, Egypt
2Department of Pharmaceutical Sciences, School of Pharmacy & Physician Assistant Studies (SOPPAS), University of Saint Joseph (USJ), Hartford, CT 06103, USA
3Pharmaceutics and Industrial Pharmacy Department, Faculty of Pharmacy, Al-Azhar University, Cairo, Egypt
*Corresponding Author: Mohamed Ismail Nounou, Department of Pharmaceutical Sciences, School of Pharmacy & Physician Assistant Studies (SOPPAS), University of Saint Joseph (USJ), Hartford, CT 06103, USA, Email: nounou@usj.edu
Article Information
Aritcle Type: Review Article
Citation: Ihab Mansoor, Mark Mikhail, Abigail Wiss, et al. 2020. Exploring the Applications of Phosphodiesterase Inhibitors Beyond Erectile Dysfunction. Int J Reprod Med Sex Health. 2: 18-29.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2020; Ihab Mansoor
Publication history:
Received date: 01 April, 2020Accepted date: 13 April, 2020
Published date: 15 April, 2020
Abstract
Phosphodiesterases (PDEs) represent a class of enzymes that act mainly on cAMP and cGMP. The value of these enzymes has been known for long time. Molecules that target PDEs have been used in various therapeutic applications. This review aims to explore the potential uses of PDE inhibitors (PDEIs) as therapeutic agents to treat conditions that extend beyond erectile dysfunction (ED), as well as highlight novel delivery methods for PDEIs.
Keywords: Phosphodiesterase inhibitors; Alzheimer’s disease; Cognition; Psoriasis; Sildenafil
Phosphodiesterases (PDEs): Introduction
Phosphodiesterase (PDEs) are enzymes that hydrolyse intracellular signalling molecules, namely cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), which are produced in response to different stimuli [1]. Therapeutic actions of these enzymes were first identified by Henry Hyde Salter in 1886 who was interested in studying asthma. He noticed that his breathing improved after drinking coffee which was later attributed to PDE inhibition by caffeine. Later on, theophylline (a caffeine analogue) was utilized as bronchodilator for treatment of asthma [2,3]. PDEs potential to hydrolyze cAMP was first identified in 1972 [4]. They are classified into 11 families and over 50 isoforms [5]. PDE4, 7, and 8 are cAMP-specific, while PDE5, 6, and 9 are cGMP-specific. Other PDEs can process both cyclic nucleotides or be slightly selective towards one of them. Accordingly, PDEIs can selectively inhibit certain PDE or non-selectively inhibit all or some PDEs. For example, theophylline is non selective inhibitor which inhibits PDE1 and PDE5. Other more potent and selective PDEIs were developed such as Nimodipine (PDE1 inhibitor), cilostamide (PDE3 inhibitor), Rolipram (PDE4 inhibitor) and sildenafil (PDE5 inhibitor) [6].
PDEIs as therapeutic agents
PDE inhibitors (PDEIs) increase the intracellular levels of cAMP and cGMP. In the last decades, researchers and pharmaceutical companies have been able to develop selective and potent PDEIs. Many of these inhibitors have similar structure to the endogenous substrates [7]. Theophylline was described as PDEI in 1972 and since that time, many inhibitors have been discovered and approved for use [6]. Sildenafil citrate was developed by Pfizer in 1989 to inhibit PDE5, an enzyme that is present in platelets and vascular smooth muscle and was thought to be a good target for angina [8]. Penile erection was a common side effect of sildenafil which led to its approval as a treatment for ED in 1998. Targeting PDE isoforms has been employed in treating various disease states. Figure 1 summarizes numerous application of PDEIs beyond male enhancement.
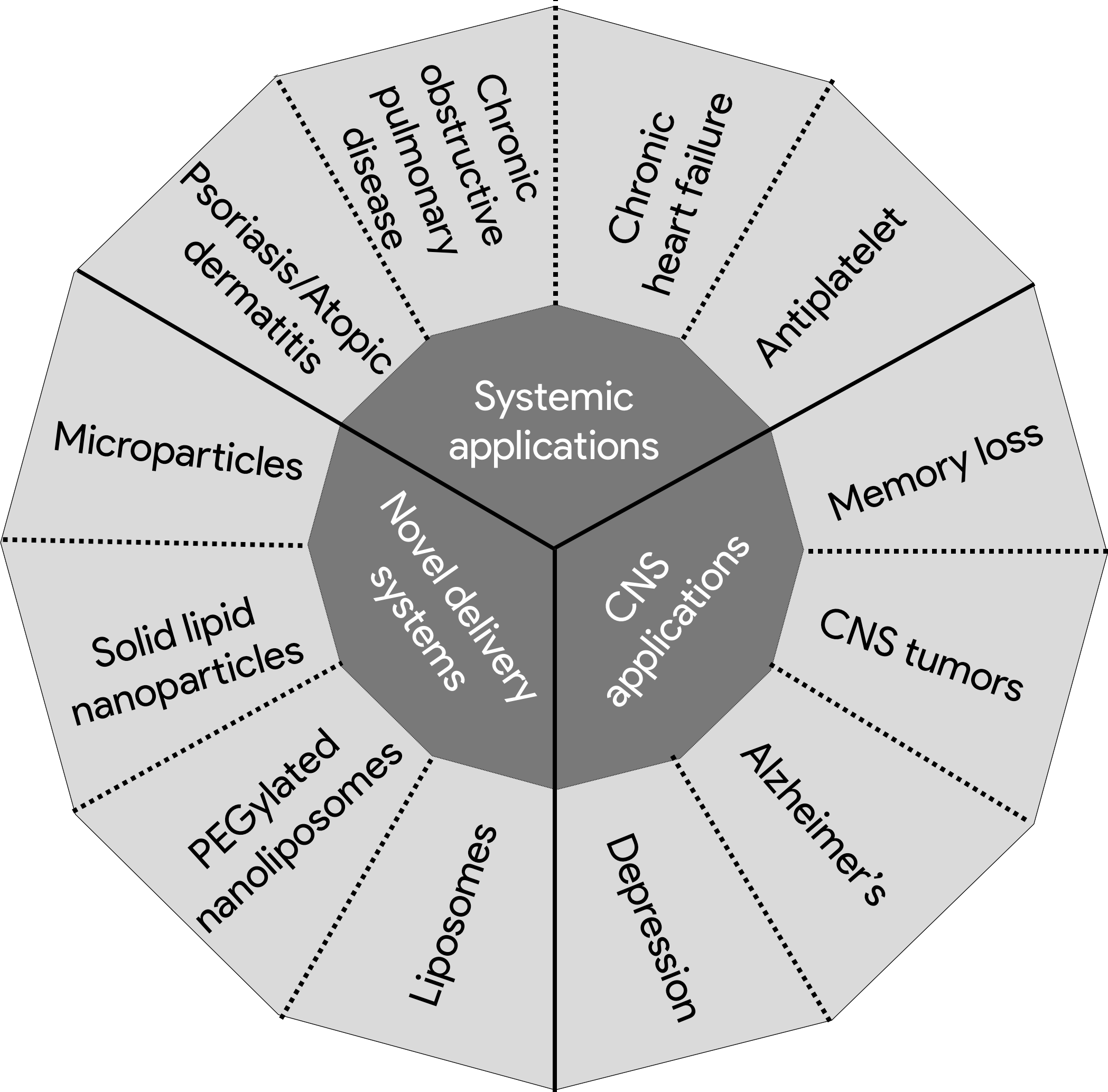
Figure 1: Different therapeutic application of PDEIs beyond male enhancement.
Systemic application
Antiplatelet effects
Platelets play an important role in the homeostasis process; they are responsible for preventing hemorrhage through triggering the coagulation cascade [9,10]. Pathological conditions can abnormally trigger platelet aggregation leading to thrombi formation which cause high morbidity and mortality due to conditions such as myocardial infarction and pulmonary embolism [9-11]. Platelet aggregation can be inhibited via multiple mechanisms and by multiple medications such as the blockage of membrane-bound receptors by clopidogrel and prasugrel [12-13]. PDE2,3, and 5 are present in platelets and are responsible for catalyzing the hydrolysis of cAMP and cGMP which act as secondary messengers for platelet modulation; thus, allowing agents such as cilostazol which is a PDE3 inhibitor to be utilized as an antiplatelet medication for patients with peripheral arterial disease and following thromboembolic stroke [12-16]. PDE5 inhibitors (PDE5is), such as sildenafil, potentiate nitric oxide (NO) activity; this synergistic effect has been shown to influence in-vitro platelet aggregation. Gudmundsdottir et al [17]. measured platelet aggregation in human peripheral blood in the presence of sildenafil and 2 NO donors and found that both NO donors caused concentration-dependent inhibition of platelet aggregation, and that sildenafil potentiated the effects of both donors in various proportions [17]. In another study, Berkels et al [18]. found that sildenafil administered to healthy men may inhibit platelet aggregation. Moreover, the addition of a NO donor to sildenafil resulted in further inhibition of platelet aggregation [18]. These studies provide evidence of the potential antiplatelet applications of PDE5is.
Chronic obstructive pulmonary disease (COPD)
COPD is an inflammatory disease of the lungs that is characterized by reduced and obstructed airflow [19]. Exposure to smoke and environmental pollution can lead to pulmonary hypertension (PH) [20]. PH, a possible complication of COPD, reduces lung function, exercise ability, and quality of life. As the disease progresses, symptom exacerbation increases until death [21]. COPD is an irreversible condition and the goals of treatment are to reduce inflammation and induce smooth muscle relaxation [19]. PDEIs have been investigated as potential treatments for COPD and PH. Inhibition of PDE4 and 5 in pulmonary arteries has been shown to produce vasodilation. Inhibitors of PDE4 and 5 have been proven to reduce airway reactivity to histamine, therefore inflammation and subsequently PH [22]. A randomized trial conducted on 278 patients with PH found that patients who received sildenafil displayed a 6-minute increase in walking distance; this trial led to the FDA approval of sildenafil in treating pulmonary arterial hypertension (PAH). A similar study found that tadalafil had similar efficacy, but the effect was transient when compared to sildenafil [23]. Sildenafil has also been examined for effect on stroke volume during exercise. The results did not show significant improvement after 3 months of treatment [24]. Combining tadalafil with roflumilast, a PDE4 inhibitor (PDE4i) has demonstrated a significant decrease in airway resistance and reactivity in animal studies [22].
Chronic heart failure (CHF)
CHF can be defined as a clinical defect in the structure or function of the myocardium [25]. PDE5 expression in the myocardium stimulates the heart [26]. A study conducted on 34 patients suffering from CHF found that compared to placebo, sildenafil reduced changes in VO2 from baseline and reduced pulmonary vascular resistance while increasing cardiac output with exercise, which translated into improved exercise capacity and quality of life (QoL) [26-28]. Another study that was conducted on 106 patients demonstrated that sildenafil improved heart failure without decreasing blood pressure [29]. Overall, PDE5is show promising results in symptoms management and improvement of QoL in patients suffering from CHF.
Psoriasis, psoriatic arthritis, and atopic dermatitis
Psoriasis is an inflammatory condition that affects the skin and joints and is characterized by plaque formation. A significant proportion of psoriasis patients also have psoriatic arthritis [30]. PDE4, present in immune cell, is an effective target in treating psoriasis, therefore, medications that target it have been approved to treat the condition. PDE4 regulates the inflammatory pathway in several types of cells; thus, its inhibition reduces inflammation and slows down plaque formation [31,32]. Atopic dermatitis (AD) is a relapsing-remitting inflammatory skin disease with pruritic lesions. There are several hypotheses relating to the origin of the condition such water loss or imbalanced immunity [33].Treatment modalities of AD present with limiting problems of adverse events; therefore, developing topical and systemic PDE4is may be the solution [34]. A pilot study conducted on 16 adults demonstrated that apremilast, a PDE4i, reduced symptom severity in patients suffering from AD. In another study, apremilast showed a reduction in AD lesions at higher doses, but was associated with frequent adverse effects [21]. PDE4 inhibition is limited by its side effect, therefore novel topical agents such as crisaborole have been developed. In a study examining the latter therapy in AD patients aged 2 years and above, disease severity was reduced and adverse events were less frequent. In another study on the same medication, QoL was improved when compared to placebo [35]. Improvement from baseline eczema area and severity index score have been observed as early as week 1 with OPA15406, an agent that is highly selective for PDE4B. The results highlight that topical agents have favorable safety and efficacy profiles [36].
CNS application
Depression
Depression is a condition that affects 150 million people and is characterized by sadness and loss of interest. The condition is often underdiagnosed and undertreated [37].There are many etiologies associated with the condition [38]. Current therapeutic agents include selective serotonin reuptake inhibitors, tricyclic antidepressants, and monoamine oxidase inhibitors [39]. These medications act on various signaling neurotransmitters. The effects of PDE5is on depression are not fully understood yet, but newer trials have shown their efficacy in alleviating the depressive symptoms; this sheds light on potential benefits for patients who suffer from ED and depression, or patients who exhausted all means of traditional depression therapies. Results of a study that was performed on 40 male patients undergoing dialysis and suffering from ED have demonstrated that treatment with sildenafil or vardenafil have led to a statistically significant improvement in depression symptoms compared to pretreatment levels [40]. Moreover, a study that was conducted on 202 men suffering from ED and untreated depression, sildenafil citrate has demonstrated significant improvement in depression symptoms from baseline [41]. In another study conducted on 152 males, results demonstrated that patients who received sildenafil had a mean decrease of 10.6 in Hamilton depression scale scores vs. 2.3 mean score decrease in those receiving placebo [42]. Results from these trials reflect the potential benefits of PDE5is in treating depression.
Central nervous system (CNS) tumors
CNS tumors originate from exponential proliferation of mass tissue in the brain. CNS tumors are classified into primary and metastatic tumors. The latter affects around 150,000 people annually. Other conditions such as lung cancer contribute to the disease, as such, lung cancer leads to brain metastasis in ~40% of patients [43].Various treatment modalities exist for CNS tumors including surgery, radiation, and others. Investigational therapies such as gene therapy, targeted toxins, and PDE5is offer a new hope as alternative treatments for brain tumors [44].The latter have shown potential in improving blood brain barrier (BBB) permeability. PDE5Is can increase tumor sensitivity to standard treatment by preventing tumorigenesis and blocking drug efflux. They act as drug vehicles for cancer therapies to penetrate the BBB [45].Vardenafil has been shown to increase drug uptake and transport of Herceptin in-vitro and in-vivo mice, and the combination has resulted in significantly longer survival time compared to untreated and Herceptin-treated mice suffering from intracranial lung cancer [44]. The combination has also resulted in significant survival increase in mice suffering from intracranial breast tumor. The combination was beneficial to mice with high HER2+ expression only [44]. Administration of PDE5Is and an inhibitor of cGMP-specific PDE5 in rat brain tumor model has been shown to increase capillary permeability in gliosarcoma-bearing rats with no significant increase in normal brain capillaries [45]. Compared to Adriamycin only treated mice, those treated with the former and vardenafil had significantly longer survival. Another study that was conducted on a pediatric population established that adjunct chemotherapy with PDE5is performed greater when added with vincristine/etoposide/cisplatin combination to cause cell death in medulloblastoma cells [46]. The combination produced autophagy at the 12-hour mark post-treatment and enhanced the induction of chemotherapy-induced DNA damage. A patent that was published in 2009 claimed that sildenafil enhanced the permeability of the mammal BBB for a short period of time following administration, therefore allowing increased penetration of chemotherapeutic agents [40]. Current developments and investigational studies in PDE5is have shown enhanced lethality of chemotherapy on CNS tumor cells, thus highlighting their potential applications.
Alzheimer’s Disease (AD)
AD is the most common form of dementia affecting millions of patients worldwide [47]. AD is characterized by β-amyloid plaques and hyperphosphorylation of Tau. The former is protein fragment that is eliminated in healthy brains and the latter is a component of mature neurons [48]. AD is a progressive irreversible disease that affects memory and thinking skills [47]. There is no cure for AD; failure to find a cure has resulted in a shift towards non-amyloid-based approaches, one of such with promising results is the use of PDEIs [49,50]. PDE5 enzymes are present in the brain and are critical in neural control [47,50,51]. A study conducted by Teich et al [52]. to confirm the presence of PDE5 in human neurons detected its presence using various techniques [52]. PDE5is have shown efficacy in different neurological conditions due to its ability to produce anti-inflammatory and neuroprotective effects [47]. PDE5is increase NO synthases, accumulate cGMP, and activate protein kinase G. These pathways play important roles in diseases such as AD, Parkinson’s disease, and multiple sclerosis. The predicted effect of PDE5is based on the mechanism of action is to reduce the degeneration process of the neuron [47]. FDA reports and studies have demonstrated that sildenafil and vardenafil can cross the BBB making them candidates for AD [53-54]. One mechanism through which PDE5is improve AD symptoms is the upregulation of cAMP and/or cGMP. Regulation of the latter promotes increased cerebral blood flow [55]. A study that examined the effects of sildenafil on astrocytes found that sildenafil reduced Ca2+ response. Astrocytes regulate the formation and functioning of the BBB and play an important role in regulating cerebral blood flow which affects cognition and memory [56]. Another novel mechanism through which PDE5is improve AD symptoms is by promoting gene transcription through activating cAMP response element-binding (CREB). Activation of the latter signaling pathway by PDEIs may ameliorate AD symptoms through restoring synaptic function [57]. CREB phosphorylation leads to transcription of memory-associated genes and its disruption leads to memory impairment [58].
PDE5 effect on cognition
Cognitive decline is one of the most common signs and symptoms of AD [59].There is no treatment for AD and the goal of pharmacotherapy is to slow down the decline of cognitive function [60]. The results of a study conducted on 27 patients with ED to assess the effect of repeated dosing of udenafil on cognition revealed that the scores of International Index of Erectile Dysfunction, frontal assessment battery, Seoul verbal learning test, and physical health questionnaires have significantly improved from baseline after 2 months of treatment highlighting that repeated dosing of PDE5is increases congnition [61]. Cognition improvement has been also confirmed in another study. A study that was performed on 10 healthy young males who received an oral dose of 100 mg revealed that the participants showed enhanced ability to focus attention and select relevant target stimuli in auditory tasks [62]. Another study conducted on male Swiss mice who received sildenafil found that the 3mg/kg dose demonstrated a significant improvement in cognition function and memory retention [63]. Another study found that PDE5is were able to reverse age-induced retention deficits of mice and helped improve cognition. These studies highlight the potential of PDE5is in slowing down cognitive decline making it a possible pharmacological option for decreasing AD symptoms.
PDE5 effect on memory loss
Memory loss is one of the first symptoms of AD [64]. Many studies conducted on mice demonstrated how the use of PDE5is can slow it down. One study showed that sildenafil has anti-AD effects when given at advanced stages of the disease in mouse models. The results of the latter study highlighted that sildenafil reduced Tau hyperphosphorylation, decreased glycogen synthase kinase 3β activity and reduced cyclin-dependent kinase 5. Moreover, sildenafil also increased levels of brain-derived neurotrophic factor and the activity-regulated cytoskeletal-associated protein [65]. Another study conducted on mice found that sildenafil enhances phosphorylation of CREB through cGMP elevations leading to beneficial outcomes in amyloid disposition mouse model [66]. In another study, tadalafil was found to reverse memory deficits in mouse models. PDE5is main activity was towards Tau pathology [67]. Another study conducted on mice found that sildenafil influenced retention by modulating time-dependent mechanisms involving memory storage [67,68]. Therefore, the administration of sildenafil 30 minutes prior to training enhanced retention performance. PDE5is have demonstrated through studies on humans, mice, and primates promising results on memory and cognition impairment that are associated with AD highlighting their importance and potential use for AD management.
Novel delivery systems for PDE5is
Optimal uterine endometrial thickness is essential for successful pregnancy. Endometrial thickness depends on blood flow among other factors. Refai et al [69]. investigated the use of sildenafil-loaded liposomes in improving uterine blood flow through vaginal delivery of the drug in rats. The histopathological examination of the rats that received chitosan-coated sildenafil liposomes demonstrated that this group had greater dilatation and congestion of the endometrial blood vessels, as well as increased endometrial layer thickness compared to the control group. The results of this study highlighted the potential benefits obtained from using chitosan-coated liposomal formulation [69]. Pulmonary arterial hypertension (PAH) is a condition that is characterized by increased pulmonary vascular resistance and vascular remodeling. The progressive thickening of the pulmonary arteries (PAs) is mainly caused by proliferation of the smooth muscle cells. Li et al [70]. hypothesized that targeting overexpressed glucose transport-1 (GLUT-1) on PAs smooth muscle cells would result in higher delivery of apoptosis-inducing drugs; thus, inhibit the proliferation of these muscle cells and reverse monocrotaline-induced PAH in rats. To achieve the objective, the investigators associated sildenafil-loaded liposomes with glucuronic acid (GlcA) resulting in glucuronic acid modified liposome (Glc-Lip). Results of the study demonstrated that Glc-Lips loaded with sildenafil significantly inhibited PA remodeling and reduced PA pressure by 32.4%, medial thickening by 41.3%, and improved right ventricular hypertrophy by 44%. This study highlighted new opportunities for using targeted therapies in treating PAH [70]. A study conducted by Shahin et al [71]. evaluated the effectiveness of using sildenafil-loaded hydrogel microparticles inhaler in treating PAH. The investigators hypothesized that the latter formulation would provide improved lung disposition, controlled release, and superior local and systemic kinetic profile compared to oral formulation. In-vivo experiments were conducted on male albino rats. The results of the study demonstrated that compared to the oral formulation, the inhaled preparation showed fast initial drug release in the lungs, higher drug concentration, significantly higher (by 4-fold) relative drug bioavailability, and significantly higher Cmax. Additionally, the inhaled formulation displayed higher half-life and almost double mean residence time (MRT) value. Mean PA pressure was reduced by 21-42% with the inhaled formulation. In summary, the results show that the formulated inhalable spray-dried hydrogel microparticles can potentially be considered as an alternative to oral route in treating PAH [71]. Wound healing is a process that spans 3 phases including inflammation, proliferation, and remodeling. Kulshrestha et al [72]. evaluated the effectiveness of topical sildenafil formulations in promoting wound healing, as well as investigated their dermal toxicity. The study was conducted on female Sprague Dawley rats using various sildenafil concentrations. The results demonstrated that none of the concentrations caused adverse events and skin primary irritation index (PII) decreased with increasing sildenafil concentration with the 3% formula exhibiting similar PII to that of placebo. Additionally, the 5% and 10% concentrations demonstrated equivalent and higher healing rates than the 3% concentration. The re-epithelization rate was found to be highest in the 5% and 10% concentration groups compared to the control group. The effects of formulations on NO levels were significantly high in the 5% and 10% concentration groups. Similarly, the 5 and 10% concentrations were found to exhibit high and significant effects on increasing the content of collagen, hydroxyproline, and total protein. In conclusion, the results demonstrate that the hydrogel formulations of sildenafil were safe and effective in promoting skin healing in traumatic wounds.72 Thyroid tumors represent a rising global challenge. Despite the majority being well differentiated and can be treated with radiation and surgery, there is a minority that is less differentiated with poor response to radiation and surgery. De Rose et al [73]. demonstrated that blocking overexpressed PDE-5 with sildenafil or tadalafil inhibited the proliferation of thyroid cancer cells. In this study, the investigators, with regard to free drug, tested the antiproliferative properties of PEGylated nanoliposomal preparations of sildenafil and tadalafil on TPC-1 and BCPAP human thyroid cancer cell lines. Encapsulated drugs demonstrated significant antiproliferative activity even at 1 μM in TPC-1 cell line. However, in the BCPAP cell line, much more significant inhibition of growth was obtained with tadalafil nanolipsome at a concentration of 0.1 μM. In summary, the study demonstrated that nanoformulations of sildenafil and tadalafil enhanced the antiproliferative properties of these drugs [73]. Avanafil is a drug that has been approved by the FDA and the European Medicines Agency (EMA) for treating erectile dysfunction. Kurakula et al. investigated methods to improve the solubility and bioavailability profiles of avanafil through using solid-lipid nanoparticles (SLNs) technique. Ex-vivo permeation study was conducted on rats’ skin. The results of the study demonstrated that after 24 hours, the proportion of the drug that permeated from the HPMC formula was higher compared to the chitosan formula. In addition, SLNs were efficiently delivered to the deeper layers of the skin as seen through the confocal laser scanning microscopy; this finding suggests that avanafil SLNs penetration into dermal tissue is expected to improve bioavailability of the drug. In conclusion, the study highlights the potential benefits that could be obtained from using this route as an alternative for oral administration [74]. Cancer is a global problem. Multidrug treatment represents a valuable strategy to improve efficacy. Combination therapy comes with various problems ranging from molecule-molecule interaction to severe adverse events. de Melo-Dogo et al [75]. evaluated the impact of incorporating 2 chemotherapeutic agents (crizotinib and palbociclib) with sildenafil in a nanocarrier made of amphiphilic polymeric micelles on the cytotoxic efficacy of the 2 drugs. The formulation was intended to tackle the hallmarks of non-small cell lung cancer (NSCLC) and drug-resistance mechanisms. The cytotoxic efficacy of the formulation was tested on non-small human lung adenocarcinoma cell line (A549). Results demonstrated that the cytotoxic effects of the triple combination resulted in a significant decrease in tumor cell viability (1.78-fold decrease). Moreover, the triple combination produced a synergistic effect and exhibited extensive cytotoxic activity. In summary, the study demonstrated the cytotoxic efficacy of the nanocarrier encapsulated triple therapy in lung cancer cells highlighting its potential use in treating NSCLC [75]. In another study involving cancer that was conducted by Marques et al [76]. the cytotoxic efficacy of micellar vehicles containing crizotinib and sildenafil on breast cancer cells was evaluated. The authors hypothesized that incorporating the 2 latter agents in a PEG-PLA micellar carrier would produce a synergistic cytotoxic effect on MCF-7 breast cancer adenocarcinoma cell line. Results revealed that after 48 hours, the viable breast cancer cells were reduced by 96% with the sildenafil-crizotinib combination micellar formula, which highlights the synergistic effects between the 2 drugs. Moreover, the results of the study revealed a significant improvement in intracellular bioavailability as well as hemo- and biocompatibility of the formulation. In summary, incorporating sildenafil and crizotinib in nanomicelles resulted in synergistic cytotoxic activity on breast cancer cells highlighting the potential benefits of the formulation in cancer therapy [76].
Conclusion
PDEs have been extensively studied as targets for treating of a wide range of disease states. Their potential application in condition such as AD, COPD, and CHF has been demonstrated in various in-vitro and in-vivo studies. With the promising results of these studies, one can conclude that new potential applications of PDEIs are likely to arise.
References
1. Sage A, Mogg TD. 2010 Chapter 7 - Pharmacology. of drugs used to treat cardiac disease. In: Marr CM, Bowen IM, eds. Cardiology of the Horse (Second Edition). Edinburgh: W.B. Saunders. 75-87.
2. Banner KH, Page CP. 1995. Theophylline and selective phosphodiesterase inhibitors as anti-inflammatory drugs in the treatment of bronchial asthma. The European respiratory journal. 8: 996-1000. Ref.: https://bit.ly/34rj15k
3. Boswell-Smith V, Spina D, Page CP. 2006. Phosphodiesterase inhibitors. Br J Pharmacol. 147: 252-257. Ref.: https://bit.ly/34oFArg
4. Rotella DP. 2007. 2.23 - Phosphodiesterases. In: Taylor JB, Triggle DJ, eds. Comprehensive Medicinal Chemistry II. Oxford: Elsevier. 919-957.
5. Weil PA. 2018. Hormone Action & Signal Transduction. In: Rodwell VW, Bender DA, Botham KM, Kennelly PJ, Weil PA, eds. Harper's Illustrated Biochemistry, 31e. New York, NY: McGraw-Hill Education.
6. DeNinno MP. 2012. Future directions in phosphodiesterase drug discovery. Bioorganic & Medicinal Chemistry Letters. 22: 6794-6800. Ref.: https://bit.ly/39U9YuV
7. Lugnier C. 2006. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 109: 366-398. Ref.: https://bit.ly/2RHB7en
8. Osterloh IH. 2004. The discovery and development of Viagra®(sildenafil citrate). In: Sildenafil. Springer. 1-13. Ref.: https://bit.ly/2Rqv9OM
9. Rumbaut RE, Thiagarajan P. 2010. Integrated Systems Physiology: from Molecule to Function to Disease. In: Platelet-Vessel Wall Interactions in Hemostasis and Thrombosis. San Rafael (CA): Morgan & Claypool Life Sciences Copyright (c) 2010 by Morgan & Claypool Life Sciences. Ref.: https://bit.ly/2XrEF7Y
10. Periayah MH, Halim AS, Mat Saad AZ. 2017. Mechanism Action of Platelets and Crucial Blood Coagulation Pathways in Hemostasis. Int J Hematol Oncol Stem Cell Res. 11: 319-327. Ref.: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5767294/
11. Gresele P, Falcinelli E, Momi S. 2008. Potentiation and priming of platelet activation: a potential target for antiplatelet therapy. Trends Pharmacol Sci. 29: 352-360. Ref.: https://bit.ly/2UUPKwC
12. Rondina MT, Weyrich AS. 2012. Targeting phosphodiesterases in anti-platelet therapy. Handb Exp Pharmacol. 210: 225-238. Ref.: https://bit.ly/2Xw0yTT
13. Gresele P, Momi S, Falcinelli E. 2011. Anti-platelet therapy: phosphodiesterase inhibitors. Br J Clin Pharmacol. 72: 634-646. Ref.: https://bit.ly/2y0jUFQ
14. Dunkern TR, Hatzelmann A. 2005. The effect of Sildenafil on human platelet secretory function is controlled by a complex interplay between phosphodiesterases 2, 3 and 5. Cell Signal. 17: 331-339. Ref.: https://bit.ly/34oZlz3
15. Eikelboom JW, Hirsh J, Spencer FA, et al. 2012. Antiplatelet drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 141: 89-119. Ref.: https://bit.ly/2VhHfL9
16. Higashi Y. 2016. Antiplatelet Drugs and Endothelial Function. J Atheroscler Thromb. 23: 1147-1149. Ref.: https://bit.ly/2RsjOxt
17. Gudmundsdottir IJ, McRobbie SJ, Robinson SD, et al. 2005. Sildenafil potentiates nitric oxide mediated inhibition of human platelet aggregation. Biochem Biophys Res Commun. 337: 382-385. Ref.: https://bit.ly/39T5Wmh
18. Berkels R, Klotz T, Sticht G, et al. 2001. Modulation of human platelet aggregation by the phosphodiesterase type 5 inhibitor sildenafil. J Cardiovasc Pharmacol. 37: 413-421. Ref.: https://bit.ly/2UVEgsT
19. Celli BR, MacNee WA, Agusti AA, et al. 2004. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. European Respiratory Journal. 23: 932-946. Ref.: https://bit.ly/3cbuQPP
20. Fromer L. 2011. Diagnosing and treating COPD: understanding the challenges and finding solutions. Int J Gen Med. 4: 729-739. Ref.: https://bit.ly/3ecvoXu
21. Chaouat A, Naeije R, Weitzenblum E. 2008. Pulmonary hypertension in COPD. European Respiratory Journal. 32: 1371-1385. Ref.: https://erj.ersjournals.com/content/32/5/1371.short
22. Mokry J, Urbanova A, Medvedova I, et al. 2017. Effects of tadalafil (PDE5 Inhibitor) and roflumilast (PDE$ inhibitor) on airway reactivity and markers of inflammation in ovalbumin-induced airway hyperresponsiveness in guinea pigs. Journal of Physiology and Pharmacology. 68: 721-730. Ref.: https://bit.ly/2JWmuzr
23. Butrous G. 2014. The role of phosphodiesterase inhibitors in the management of pulmonary vascular diseases. Global Cardiology Science & Practice. 42: 257-290. Ref.: https://bit.ly/3c7Pa4o
24. Rietema H, Holverda S, Bogaard HJ, et al. 2008. Sildenafil treatment in COPD does not affect stroke volume or exercise capacity. European Respiratory Journal. 31: 759-764. Ref.: https://bit.ly/2UVNNA0
25. Inamdar AA, Inamdar AC. 2016. Heart Failure: Diagnosis, Management and Utilization. J Clin Med. 5: 62. Ref.: https://bit.ly/2yNcZ37
26. Perez NG, Piaggio MR, Ennis IL, et al. 2007. Phosphodiesterase 5A inhibition induces Na+/H+ exchanger blockade and protection against myocardial infarction. Hypertension. 49: 1095-1103. Ref.: https://bit.ly/2y1J2Mo
27. De Vecchis R, Cesaro A, Ariano C, et al. 2017. Phosphodiesterase-5 Inhibitors Improve Clinical Outcomes, Exercise Capacity and Pulmonary Hemodynamics in Patients With Heart Failure With Reduced Left Ventricular Ejection Fraction: A Meta-Analysis. J Clin Med Res. 9: 488-498. Ref.: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5412522/
28. Lewis GD, Shah R, Shahzad K, et al. 2007. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 116: 1555-1562. Ref.: https://bit.ly/2x9XQZv
29. Amin A, Mahmoudi E, Navid H, et al. 2013. Is chronic sildenafil therapy safe and clinically beneficial in patients with systolic heart failure? Congestive heart failure (Greenwich, Conn). 19: 99-103. Ref.: https://bit.ly/2XoqHDS
30. Menter AG, Feldman S, Van Voorhees A, et al. 2008. Guidelines of care for the management of psoriasis and psoriatic arthritis. American Academy of Dermatology. 58: 826-849. Ref.: https://bit.ly/2RsMh6l
31. Page CP, Spina D. 2011. Phosphodiesterase inhibitors in the treatment of inflammatory diseases. Handbook of experimental pharmacology. 204: 391-414. Ref.: https://bit.ly/2XB2BGj
32. Heng Li JQ, Wei Tang. 2018. Phosphodiesterase-4 Inhibitors for the Treatment of Inflammatory Diseases. Frontiers in Pharmacology. 9: 1-21. Ref.: https://bit.ly/39UUqqB
33. Nygaard U, Deleuran M, Vestergaard C. 2017. Emerging Treatment Options in Atopic Dermatitis: Topical Therapies. Dermatology (Basel, Switzerland). 233: 333-343. Ref.: https://bit.ly/34nEex2
34. Ahluwalia J, Udkoff J, Waldman A, et al. 2017. Phosphodiesterase 4 Inhibitor Therapies for Atopic Dermatitis: Progress and Outlook. Drugs. 77: 1389-1397. Ref.: https://bit.ly/2y7TdyQ
35. Simpson EL, Paller AS, Boguniewicz M, et al. 2018. Crisaborole Ointment Improves Quality of Life of Patients with Mild to Moderate Atopic Dermatitis and Their Families. Dermatology and therapy. 8: 605-619. Ref.: https://link.springer.com/article/10.1007/s13555-018-0263-0
36. Suga H, Sato S. 2019. Novel topical and systemic therapies in atopic dermatitis. Immunological medicine. 1-10. Ref.: https://bit.ly/2JZEskl
37. Smith K, De Torres IJN. 2014. A world of depression. 515. Ref.: https://go.nature.com/3c4x9UH
38. Council NR. 2009. Depression in parents, parenting, and children: Opportunities to improve identification, treatment, and prevention. National Academies Press. Ref.: https://bit.ly/3e9uZoH
39. Feighner JP. 1998. Mechanism of action of antidepressant medications. Paper presented at: Assessing Antidepressant Efficacy: A Reexamination., Jan, Phoenix, AZ, US1999. Ref.: https://bit.ly/2y5DikC
40. Solak Y, Atalay H, Kan S, et al. 2011. Effects of sildenafil and vardenafil treatments on sleep quality and depression in hemodialysis patients with erectile dysfunction. 23: 27-31. Ref.: https://go.nature.com/3aTAHsJ
41. Kennedy SH, Dugré H, Defoy IJIcp. 2011. A multicenter, double-blind, placebo-controlled study of sildenafil citrate in Canadian men with erectile dysfunction and untreated symptoms of depression, in the absence of major depressive disorder. Wen PYJCor. 26: 151-158. Ref.: https://bit.ly/2XnrCEv
42. Seidman SN, Roose SP, Menza MA, et al. 2001. Treatment of erectile dysfunction in men with depressive symptoms: results of a placebo-controlled trial with sildenafil citrate. 158: 1623-1630. Ref.: https://bit.ly/2Xw4eFb
43. Nayak L, Lee EQ. 2012. Epidemiology of brain metastases. Wen PYJCor. 14: 48-54. Ref.: https://bit.ly/3c81Vw2
44. Hu J, Ljubimova JY, Inoue S, et al. 2010. Phosphodiesterase type 5 inhibitors increase Herceptin transport and treatment efficacy in mouse metastatic brain tumor models. 5. Ref.: https://bit.ly/2xm0cnQ
45. Black KL, Yin D, Ong JM, et al. 2008. PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. 1230: 290-302. Ref.: https://bit.ly/2Vgls6B
46. Roberts JL, Booth L, Conley A, et al. 2014. PDE5 inhibitors enhance the lethality of standard of care chemotherapy in pediatric CNS tumor cells. 15: 758-767. Ref.: https://bit.ly/2Rtstjh
47. García-Osta A, Cuadrado-Tejedor M, García-Barroso C, et al. 2012. Phosphodiesterases as therapeutic targets for Alzheimer's disease. ACS Chem Neurosci. 3: 832-844. Ref.: https://bit.ly/3aZTFOv
48. Iqbal K, Liu F, Gong CX, et al. 2010. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 7: 656-664. Ref.: https://bit.ly/39WKE7A
49. Geldenhuys WJ, Darvesh AS. 2015. Pharmacotherapy of Alzheimer's disease: current and future trends. Expert Rev Neurother. 15: 3-5. Ref.: https://bit.ly/2JWds5A
50. Mao F, Wang H, Ni W, et al. 2018. Design, Synthesis, and Biological Evaluation of Orally Available First-Generation Dual-Target Selective Inhibitors of Acetylcholinesterase (AChE) and Phosphodiesterase 5 (PDE5) for the Treatment of Alzheimer's Disease. ACS Chem Neurosci. 9: 328-345. Ref.: https://bit.ly/2XqJsXs
51. Puzzo D, Staniszewski A, Deng SX, et al. 2009. Phosphodiesterase 5 Inhibition Improves Synaptic Function, Memory, and Amyloid-β Load in an Alzheimer's Disease Mouse Model. The Journal of Neuroscience. 29: 8075. Ref.: https://bit.ly/2wstsJe
52. Teich AF, Sakurai M, Patel M, et al. 2016. PDE5 Exists in Human Neurons and is a Viable Therapeutic Target for Neurologic Disease. J Alzheimers Dis. 52: 295-302. Ref.: https://bit.ly/39T9yER
53. de Santana Nunes AK, Raposo C, Bjorklund U, et al. 2016. Sildenafil (Viagra®) prevents and restores LPS-induced inflammation in astrocytes. Neurosci Lett. 630: 59-65. Ref.: https://bit.ly/2Xw5atb
54. Black KL, Yin D, Ong JM, et al. 2008. PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. Brain Res. 1230: 290-302. Ref.: https://bit.ly/39X5rYs
55. Peixoto CA, Nunes AKS, Garcia-Osta A. 2015. Phosphodiesterase-5 Inhibitors: Action on the Signaling Pathways of Neuroinflammation, Neurodegeneration, and Cognition. Mediators Inflamm. 940207. Ref.: https://bit.ly/2JSOq7f
56. Koehler RC, Roman RJ, Harder DR. 2009.Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 32: 160-169. Ref.: https://bit.ly/2x7CjR7
57. Yin JC, Tully T. 1996. CREB and the formation of long-term memory. Curr Opin Neurobiol. 6: 264-268. Ref.: https://bit.ly/3aZ7EE3
58. Teich AF, Nicholls RE, Puzzo D, et al. 2015. Synaptic therapy in Alzheimer's disease: a CREB-centric approach. Neurotherapeutics. 12: 29-41. Ref.: https://bit.ly/3ebG6gQ
59. McMahon CG, Samali R, Johnson H. 2000. Efficacy, safety and patient acceptance of sildenafil citrate as treatment for erectile dysfunction. J Urol. 164: 1192-1196. Ref.: https://bit.ly/2XtmKOf
60. Bature F, Guinn B-A, Pang D, et al. 2017. Signs and symptoms preceding the diagnosis of Alzheimer's disease: a systematic scoping review of literature from 1937 to 2016. BMJ Open. 7: e015746. Ref.: https://bit.ly/2y1MvKU
61. Allain H, Bentue-Ferrer D, Tribut O, et al. 2003. Alzheimer's disease: the pharmacological pathway. Fundam Clin Pharmacol. 17: 419-428. Ref.: https://bit.ly/3ecBbfu
62. Shim YS, Pae CU, Kim SW, et al. 2011. Effects of repeated dosing with Udenafil (Zydena) on cognition, somatization and erection in patients with erectile dysfunction: a pilot study. Int J Impot Res. 23: 109-114. Ref.: https://go.nature.com/2UZmMfb
63. Schultheiss D, Muller SV, Nager W, et al. 2001. Central effects of sildenafil (Viagra) on auditory selective attention and verbal recognition memory in humans: a study with event-related brain potentials. World J Urol. 19: 46-50. Ref.: https://bit.ly/3caCASb
64. Clark AS, Meerts SH, Guarraci Zaprinast FA. 2009. a phosphodiesterase type-5 inhibitor, alters paced mating behavior in female rats. Physiol Behav. 96: 289-293. Ref.: https://bit.ly/2JSAVV5
65. Cuadrado-Tejedor M, Hervias I, Ricobaraza A, et al. 2011. Sildenafil restores cognitive function without affecting beta-amyloid burden in a mouse model of Alzheimer's disease. Br J Pharmacol. 164: 2029-2041. Ref.: https://bit.ly/2VlgDcf
66. Puzzo D, Staniszewski A, Deng SX, et al. 2009. Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-beta load in an Alzheimer's disease mouse model. J Neurosci. 29: 8075-8086. Ref.: https://bit.ly/3cbA7a5
67. Garcia-Barroso C, Ricobaraza A, Pascual-Lucas M, et al. 2013. Tadalafil crosses the blood-brain barrier and reverses cognitive dysfunction in a mouse model of AD. Neuropharmacology. 64: 114-123. Ref.: https://bit.ly/3aZV4Vh
68. Baratti CM, Boccia MM. 1999. Effects of sildenafil on long-term retention of an inhibitory avoidance response in mice. Behav Pharmacol. 10: 731-737. Ref.: https://bit.ly/3b0j8aj
69. Refai H, Hassan D, Abdelmonem R. 2017. Development and characterization of polymer-coated liposomes for vaginal delivery of sildenafil citrate. Drug Deliv. 24: 278-288. Ref.: https://bit.ly/39UXEdH
70. Li B, He W, Ye L, et al. 2019. Targeted Delivery of Sildenafil for Inhibiting Pulmonary Vascular Remodeling. Hypertension. 73: 703-711. Ref.: https://bit.ly/2JUmMXr
71. Shahin HI, Vinjamuri BP, Mahmoud AA, et al. 2019. Design and evaluation of novel inhalable sildenafil citrate spray-dried microparticles for pulmonary arterial hypertension. J Control Release. 302: 126-139. Ref.: https://bit.ly/2yQmrCX
72. Kulshrestha S, Chawla R, Alam MT, et al. 2019. Efficacy and dermal toxicity analysis of Sildenafil citrate based topical hydrogel formulation against traumatic wounds. Biomed Pharmacother. 112: 108571. Ref.: https://bit.ly/3b1Lxx0
73. De Rose RF, Cristiano MC, Celano M, et al. 2016. PDE5 Inhibitors-Loaded Nanovesicles: Physico-Chemical Properties and In Vitro Antiproliferative Activity. Nanomaterials (Basel). 6: 92. Ref.: https://bit.ly/2UY9AqU
74. Kurakula M, Ahmed OA, Fahmy UA, et al. 2016. Solid lipid nanoparticles for transdermal delivery of avanafil: optimization, formulation, in-vitro and ex-vivo studies. J Liposome Res. 26: 288-96. Ref.: https://bit.ly/3cbAuS1
75. de Melo-Diogo D, Gaspar VM, Costa EC, et al. 2014. Combinatorial delivery of Crizotinib-Palbociclib-Sildenafil using TPGS-PLA micelles for improved cancer treatment. Eur J Pharm Biopharm. 88: 718-729. Ref.: https://bit.ly/39ZPMrg
76. Marques JG, Gaspar VM, Markl D, et al. 2014. Co-delivery of Sildenafil (Viagra®) and Crizotinib for synergistic and improved anti-tumoral therapy. Pharm Res. 31: 2516-2528. Ref.: https://bit.ly/2xa2o21




















