Indexing & Abstracting
Full Text
Review ArticleDOI Number : 10.36811/ijpsh.2022.110038Article Views : 0Article Downloads : 3
Effect of Plant Hormones & Micro nutrients on Fruit Production: A Review
Ashok Kumar1, BD Bhuj2 and Shri Dhar3
1Professor Horticulture, Department of Horticulture, IIMT University, Meerut, UP, India
2Professor Horticulture, College of Agriculture, G.B.P.U.A & T-Pant Nagar, UP, India
3Principal Scientist, Division of Vegetable Science, Pusa Campus, New Delhi, India
*Corresponding Author: Ashok Kumar, Professor Horticulture, Department of Horticulture, IIMT University, Meerut, UP, India, Tel: 7983232585, 7300511143; Email: yadavakdr@gmail.com; drkumaryadav@rediffmail.com
Article Information
Aritcle Type: Review Article
Citation: Ashok Kumar, BD Bhuj, Shri Dhar. 2022. Effect of Plant Hormones & Micro nutrients on Fruit Production: A Review. Int J Plant Sci Hor. 4: 62-90.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2022; Ashok Kumar
Publication history:
Received date: 08 August, 2022Accepted date: 17 August, 2022
Published date: 20 August, 2022
Abstract
The plant growth regulators are chemical compounds, which can regulate some important metabolic activities in plants. They influence on growth and development of plants, which influence the increase in yield, quality of product, flowering and some other parameters. Plant growth regulators include auxins, gibberellins, cytokinin’s, ethylene, growth retardants and growth inhibitors. Production of low-quality fruits is a common experience therefore, to improve the yield and quality of fruit crops application of growth regulators is one of the important production strategy. Application of micronutrients and growth regulators applied fifteen days after fruit set was more effective in improving fruit quality as compared to thirty days after fruit set. The pomegranate plant continues to bear flowers irregularly once, twice or thrice in a year, depending upon germplasm, agro-climatic conditions and management practices. It produces a low yield of inferior quality with non-synchronized maturity. To avoid this, flower regulation is practiced to encourage prolific harvest at specific time depending upon rainfall/irrigation facilities, pests and diseases incidence and market demand. Investigated research areas - Moisture stress, plant growth regulators, nutrient and canopy management (training, pruning and thinning) are major horticultural interventions which influence flowering. Although, many studies have been conducted in different countries to induce profuse flowering with improved sex ratio, fruit set, retention and ultimately high-quality fruiting in desired season.
Keywords: Growth Regulator; Micronutrient; Fruit Quality; Pomegranate
Introduction
Plant growth regulators or phytohormones are organic substances produced naturally in higher plants, controlling growth or other physiological functions at a site remote from its place of production and active in minute amounts. PGRs can influence plant height, leaf number, leaf area index, dry mass, chlorophyll content, photosynthetic parameters, seed yield, oil yield, nutritional status etc. Among different elite horticultural practices, growth regulators have been advantageously used in the recent time to increase the fruit production and to improve the quality of several other fruit crops. Plant growth regulators include auxins, gibberellins, cytokinins, ethylene, growth retardants and growth inhibitors. Pomegranate (Punica granatum L.) is an important fruit crop of the tropical and subtropical regions of the world. It can be grown from plains to an elevation of up to 2000 meter. Under temperate environment, it behaves as a deciduous plant while in subtropical and tropical climate it behaves as evergreen or partially deciduous plant. In India, it is cultivated in over 1.20 lakh ha area with an annual production of 7.5 lakh tonnes and with a productivity of about 6.60 tonnes per ha. Maharashtra state is the largest producer of this fruit crop in India. The edible part of the fruit is called arils which are eaten fresh and can be preserved as syrup or used for making jam. The fruit peel, stem, root bark and leaves are a good source of secondary products such as tannins, dyes and alkaloids. Anthocyanin in pomegranate arils is a rich source of antioxidants. The edible parts of fruit contain considerable amount of proteins, carbohydrates, minerals, sugars, vitamins, polysaccharides and polyphenols. The reducing sugars, non-reducing sugars, total sugars, acidity, ascorbic acid and total soluble solids etc. are important components determining quality of fruit juice in pomegranate. The quality of the pomegranate fruits is manageable through maintaining soil moisture and avoiding wide variation in soil moisture, cultivation of recommended cultivars and application of adequate and regular irrigation during fruit growth stages. Also, use of growth regulators and micronutrients has also been reported effective in managing fruit quality in pomegranate [1,2]. Although several workers have evaluated the effect of nutrients and growth regulators on fruit quality of pomegranate in different parts of the world; the present study was undertaken to complement the available information on this aspect under Haryana conditions.
Plant hormone
It is restricted to naturally occurring plant substances, there fall into five classes. Auxin, Gibberellins, Cytokinin, ABA and ethylene. Plant growth regulator includes synthetic compounds as well as naturally occurring hormones.
1. Plant growth hormone:
The primary site of action of plant growth hormones at the molecular level remains unresolved. Reasons each hormone produces a great variety of physiological responses. Several of these responses to different hormones frequently are similar. The response of a plant or a plant part to plant growth regulators may vary with the variety of the plant. Even a single variety may respond differently depending on its age, environmental conditions and physiological state of development (especially its natural hormone content) and state of nutrition. There are always exceptions for a general rule suggesting the action of a specific growth regulator on plants. There are several proposed modes of action in each class of plant hormone, with substantial arguments for and against each mode. The importance of PGRs was first recognized in the 1930s. Since that time, natural and synthetic compounds that alter function, shape and size of crop plants have been discovered. Today, specific PGRs are used to modify crop growth rate and growth pattern during the various stages of development from germination through harvest and post-harvest preservation. Growth regulating chemicals that have positive influences on major agronomic crops can be of value.
Classes of plant growth regulators:
Growth promoters:
The plant-bio regulators or hormones which have catalytical effect, i.e. take a vital role in plant growth are called growth promoter e.g. Auxins, Gibberellins and Cytokinins.
1. Auxins:
Auxins are a group of phytohormones produced in the shoot and root apices and they migrate from the apex to the zone of elongation. Auxins promote the growth along the longitudinal axis of the plant and hence the name (auxeins: to grow or to increase). The term, auxins was introduced by Kogl and Haagen-Smit (1931) designating those plant hormones which are especially concerned with cell enlargement or the growth of the shoots. Went (1926 and 1928) isolated auxins from the Avena coleoptile tips by a method called Avena coleoptile or curvature test and concluded that no growth can occur without auxins. Auxins are widely distributed throughout the plant however, abundant in the growing tips such as coleoptile tip, buds, root tips and leaves. Indole Acetic Acid (IAA) is the only naturally occurring auxin in plants. An auxins may, thus, be defined as an organic substance which promotes growth (i.e., irreversible increase in growth) along the longitudinal axis when applied in low concentrations to shoots of the plants freed as far as practicable from their own inherit growth promoting substances. Auxins may, and generally do, have other properties but this one is critical? [3]. At low concentration, they (auxins) stimulate growth while at high concentration they retard growth. They are characterized by causing cell enlargement and stem elongation in plants. They are also active in development of branches in plants and are associated with apical dominance. Physiological effects of auxins.
It was previously thought that the sole function of auxins was to promote cell enlargement. But the work done in later years has proved them to be deeply associated with a variety of functions. In some cases they act as a stimulating agent, in others as an inhibitory agent and in still others as a necessary participant in the growth activity of other phytohormones such as gibberellins and cytokinins. The various growth processes in which the auxins (both natural and synthetic) play their role are in listed below.
The discovery of auxins can be traced back to work done by Charles Darwin in the 1880s when he was trying to find the reason why plants bend toward light. He found that the tip of a coleoptile sensed light and there was a diffusible substance produced there that caused the shaded side to grow more rapidly than the illuminated side [4]. Many others such as Boysen-Jensen, Paal, and Went in the early 1900s contributed to the body of information which eventually resulted in the discovery of the first plant hormone group auxins. IAA was identified from lower plants in the 1930s and finally from higher plants in the early 1940s [5]. This pathway to auxins discovery spanned 50 years. Valuable lessons were learned related to extraction, purification and detection which, coupled with methodology advancements, made the pathway to discovery and identification much shorter for the other plant hormones. The research with auxins in the 1930s can be considered the start of PBR research.
Physiological effects of Auxins:
1. Cell division and elongation: The primary physiological effects of auxins are cell division and cell elongation in the shoots. It is important in the secondary growth of stem and differentiation of xylem and phloem tissues.
2. Apical dominance: In many plants, if the terminal bud is intact and growing, the growth of lateral buds just below it remains suppressed. Removal of the apical bud results in the rapid growth of lateral buds. This phenomenon in which the apical bud dominates over the lateral buds and does not allow the lateral buds to grow is known as apical dominance [6].
3. Root initiation: The higher concentration of auxins inhibits the elongation of roots but the number of lateral roots is considerably increased i.e., higher concentration of auxins induces more lateral branch roots. Application of IAA in lanolin paste (lanolin is a soft fat prepared from wool and is good solvent for auxins) to the cut end of a young stem results in an early and extensive rooting. This fact is of great practical importance and has been widely utilized to promote root formation in economically useful plants which are propagated by cuttings.
4. Prevention of abscission: Natural auxins prevent the formation of abscission layer which cause the fall of leaves, flowers and fruits in tree plant.
5. Parthenocarpy: Auxins can induce the formation of parthenocarpic fruits (fruit formation without pollination and fertilization). In parthenocarpic fruits, the concentration of auxins in the ovaries is higher than in the ovaries of plants which produce fruits only after fertilization. In the later cases, the concentration of the auxins in ovaries increases after pollination and fertilization.
6. Respiration: Auxin stimulates respiration and there is a correlation between auxins induced growth and respiration. Auxins may increase the rate of respiration indirectly through increased supply of ADP by rapidly utilizing ATP in the expanding cells.
7. Callus formation: Besides cell elongation, auxins may also be active in cell division. In many tissue cultures, where the callus growth is quite normal, the continued growth of such callus takes place only after the addition of auxins.
8. Eradication of weeds: Some synthetic auxins especially 2, 4-D and 2, 4, 5-T are useful in eradication of weeds at higher concentrations.
9. Flowering and sex expression: Auxins generally inhibit flowering but in pine apple and lettuce it promotes uniform flowering.
2. Gibberellins:
The gibberellins were discovered in an interesting and incidental way. In early part of the twentieth century, Japanese farmers Kurosawa noted that some plants in rice fields were taller, thinner and paler than the normal plants; had longer and narrower leaves markedly overgrowing their unaffected neighbours; and were sometimes devoid of fruits too. They named this disease as bakanae, meaning foolish seedlings. [7] suggested that the disease is due to a substance‘secreted by a parasitic as Ascomycetous fungus, Gibberella fujikuroi (the perfect form, occurring only occasionally; the imperfect form is Fusarium moniliforme, in infecting the diseased plants. This suggestion was experimentally supported by [8] who demonstrated that sterile filtrates of the fungus could initiate symptoms of bakanae disease in healthy rice seedlings. Later in [9,10] isolated this growth promoting substance in crystalline form and named it as gibberellins A, which has now been shown as a mixture of many growth promoters, collectively known as gibberellins.
Since that time, gibberellins and allied substances have been found in higher plants also by [11-13]. A gibberellins (abbreviated as GA, for gibberellic acid) may be defined as a compound which is active in gibberellin bioassays and possesses a gibbane ring skeleton. There are, however, other compounds (like kaurene) which are active in some of the assays but do not possess a gibbane ring. Such compounds have been called gibberellin-like rather than gibberellins. The best known of gibberellins is Gibberellic acid (i.e. GA3). The discovery of gibberellins can be traced back to Japanese pathologist in the 1920s who studied the foolish rice disease, a disease that caused rice plants to grow so rapidly that stems were too weak resulting in lodging. The cause of this exaggerated growth was determined to be the production of a growth stimulating substance produced by the fungus Gibberella fujikuroi. Impure crystals containing a mixture of active ingredients were isolated in the late 1930s [14]. This information, and the significance of the discovery, did not surface until the 1950s because the information was published in Japanese in Japanese journals and there was a lack of exchange of information with the western world that was associated with World War-II. Gibberellic acid was identified, crystallized and synthesized in the 1950s [15].
Physiological effects of gibberellins:
1. Seed germination: Certain light sensitive seeds of Lettuce and tobacco show poor germination in dark. Germination starts vigorously if these seeds are exposed to light or red light. This requirement of light is overcome if the seeds are treated with gibberellic acid in dark.
2. Dormancy of buds: In temperate regions the buds formed in autumn remain dormant until next spring due to severe cold. This dormancy of buds can be broken by gibberellins treatments. In potato also, there is a dormant period after harvest, but the application of gibberellins sprouts they refer vigorously.
3. Root growth Gibberellins have little or no effect on root growth. At higher concentration, some inhibition of root growth may occur. The initiation of roots is markedly inhibited by gibberellins in isolated cuttings.
4. Elongation of internodes the most pronounced effect of gibberellins on the plant growth is the elongation of the internodes. Therefore in many plants such as dwarf pea, dwarf maize etc gibberellins may overcome the genetic dwarfism.
5. Bolting and flowering in many herbaceous plants, the early period of growth shows rosette habit with short stem and small leaves. Under short days, the rosette habit is retained while under long days bolting occurs i.e. the stem elongates rapidly and is converted into polar axis bearing flower primordia. This bolting can also be induced in such plants by the application of gibberellins even under non-inductive short days. In Hyoscyamusniger (a long day plant) gibberellin treatment causes bolting and flowering under non-inductive short days. While in long day plants the gibberellin treatment usually results in early flowering.
6. In short day plants, its effect sare quite variable. It may either have no effect or inhibit or may activate flowering. Parthenocarpy Germination of the pollen grains is stimulated by gibberellins; likewise, the growth of the fruit and the formation of parthenocarpic fruits can be induced by gibberellin treatment. In many cases, e.g. pome and stone fruits where auxins have failed to induce parthenocarpy, the gibberellins have proven to be successful. Seedless and fleshly tomatoes and large sized seedless grapes are produced by gibberellin treatments on commercial scale.
7. Synthesis of the enzyme one important function of gibberellins is to cause the synthesis of the enzyme amylase in the aleurone layer of the endosperm of cereal grains during germination. This enzyme brings about hydrolysis of starch to form simple sugars which are then translocated to growing embryo to provide energy source.
3. Cytokinins:
The word for Cytokinins is a generic name for all naturally occurring substances that are known to promote cell division. The term, Cytokinins was proposed by [16]. They are also known to delay senescence. The first naturally occurring cytokinin was found in corn and is referred as zeatin. The most widely distributed cytokinins are the synthetic benzyl adenine and kinetin. Kinetin was discovered from the tobacco pith callus and the chemical substance was identified as 6-furfuryl amino purine. The natural cytokinin appears to be made principally in apical root meristem, inflorescences and developing fruits. Certain cytokinins have been found to be the constituent of certain transfer RNA molecules in a number of different organisms. They are also involved in stimulation of organ formation e.g. formation of leave, fruit, buds, and branches. They tend to contract or overcome apical dominance and break dormancy. They also enhance seed germination and uniform flowering. Cytokinins regulate the transportation of metabolites in the phloem. Cytokinins are also useful in the preservation of flowers, fruits and leafy vegetables.
The discovery of cytokinins can be traced back to activities in the lab of Folke Skoog at the University of Wisconsin in the late 1940s.Skoog and Miller were studying cell division factors using a tobacco pith callus culture system. Cell division factors were found in coconut milk and yeast extracts and analysis of the active region on chromatograms suggested that the active factor was a purine [17]. All known purines were tested and found to be inactive, but degraded herring sperm DNA yielded significant activity. The active factor was finally identified and called Kinetin, but it was found to be a breakdown product of the DNA and not a naturally occurring compound [18]. Armed with success, biological activity and a system for detecting biological activity, Zeatin was isolated from immature corn kernels by Letham in 1963 [16].
Physiological effects of Cytokinins:
1. Cell division: The most important biological effect of kinetin on plants is to induce cell division especially in tobacco pith callus, carrot root tissue, soybean cotyledon, pea callus etc.
2. Cell enlargement: Like auxins and gibberellins, the kinetin may also induce cell enlargement. Significant cell enlargement has been observed in the leaves of Phaseolus vulgaris, pumpkin cotyledons, tobacco pith culture, cortical cells of tobacco roots etc.
3. Concentration of apical dominance: External application of Cytokinins promotes the growth of lateral buds and hence counteracts the effect of apical dominance
4. Dormancy of seeds: Like gibberellins, the dormancy of certain light sensitive seeds such as lettuce and tobacco can also be broken by kinetin treatment.
5. Delay of senescence (Richmond & Lang effect): The senescence of leaves usually accompanies with loss of chlorophyll and rapid breakdown of proteins. Senescence can be postponed to several days by kinetin treatment by improving RNA synthesis followed by protein synthesis. [19] while working on detached leaves of Xanthium found that kinetin was able to postpone the senescence for a number of days.
5. Delay of senescence (Richmond & Lang effect): The senescence of leaves usually accompanies with loss of chlorophyll and rapid breakdown of proteins. Senescence can be postponed to several days by kinetin treatment by improving RNA synthesis followed by protein synthesis. [19] while working on detached leaves of Xanthium found that kinetin was able to postpone the senescence for a number of days.
7. Morphogenesis: It has been shown that high auxins and low kinetin produced only roots whereas high kinetin and low auxins could promote formation of shoot buds.
8. Accumulation and translocation of solutes Plants accumulate solutes very actively with the help of Cytokinin and also help in solute translocation in phloem.
9. Protein synthesis: [20] demonstrated the increased rate of protein synthesis due to translocation by kinetin treatment.
10. Other effects Cytokinins provide resistance to high temperature, cold and diseases in some plants. They also help in flowering by substituting the photoperiodic requirements. In some cases, they stimulate synthesis of several enzymes involved in photosynthesis. Certain light sensitive seeds e.g. Lettuce and tobacco show poor germination in dark. Germination starts vigorously if these seeds are exposed to light or red light. This requirement of light is overcome if the seeds are treated with Gibberellic acid in dark e.g. Lettuce and tobacco.
11. Commercial applications Cytokinins useful for increasing shelf life of fruits, quickening of root induction and producing efficient root system, increasing yield and oil contents of oil seeds like ground nut.
4. Growth inhibitors:
The plant bio-regulators which selectively interfere with normal hormonal promotion of growth are called Growth Inhibitor e.g. Abscisic acid and Ethylene.
5. Abscisic acid (ABA):
These were previously called Dormin or Abscisin mainly because of their regulatory effect on abscission and dormancy. This hormone is widespread in higher plants and is found in many different organs and tissues (both old and young) of plants. ABA induces abscission of the leaves of a wide variety of plants and fruits of some plant species. ABA appears to be an internal factor inducing dormancy in the buds of at least some temperature zone woody plants. ABA also prevents or delays the germination of seeds. ABA retards the growth of a large variety of plant tissues and organs including leaves, coleoptiles, stems, hypocotyls and roots. It promotes senescence through leaf abscission, degeneration of excised leaves and acceleration of decomposition of chlorophyll.

Figure 1: Stomata closure restricts the uptake of CO2 in the leaves of a drought-stressed plant leading to the production of (a) H2O2 in the peroxisome by photorespiration, which enhances (b) O2− and H2O2 production, (c) 1O2 production, by the photosynthetic electron transport chain. PSI and PSII (Photosystem 1 and Photosystem II); RuBP, Ribulose 1-5 bisphosphate; PGA, 3-Phosphoglyceric acid.
Physiological effects of Abscisic acid (ABA)
1. Stomata Closure: Water shortage brings about increase in ABA level, leading to stomata closure as a response to water stress
2. Growth Inhibiters: ABA inhibits shoot growth but has less effect on root growth
3. GA Counteracts: ABA counteracts the effect of gibberellins on alpha amylase synthesis in germinating seeds.
4. Induced Dormancy: ABA affects induction or maintenance of dormancy in seeds.
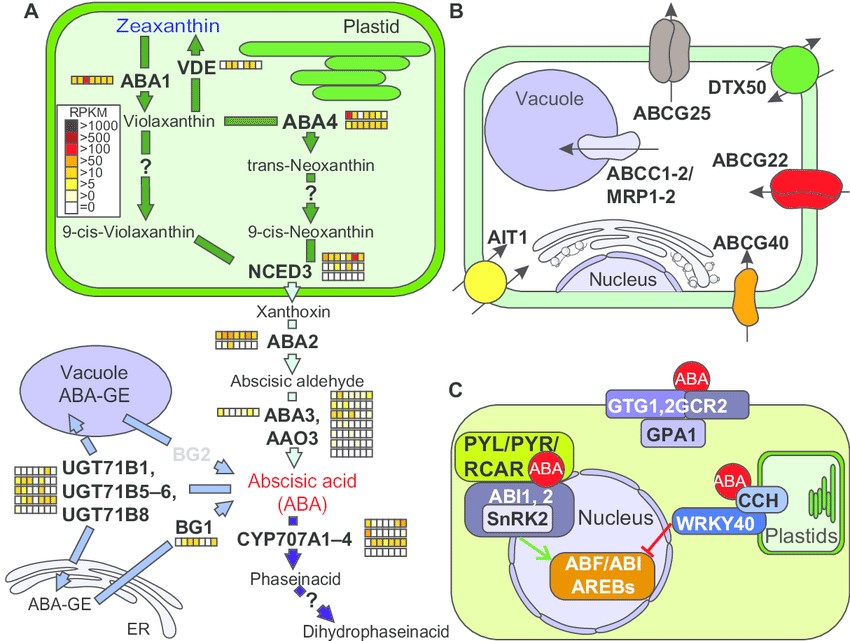
Figure 2: Pathways of abscisic acid synthesis, transport, and signaling. Notes: (a) Enzymes and intermediate products of the plastidial, endoplasmatic, and cytosolic pathways for abscisic acid production unify to the plastidal production of zeaxanthin (blue) as precursor for abscisic acid (red) synthesis in the cytosol. The storage pathway is catalyzed by Bg1 and Bg2 as well as by members of the Ugt71B gene family. For further details, see text. The arrows are colored according to the species in which the enzymes were found (fig. 1a). The expression of the identified genes in tomato is shown as explained in figure 2. Genes coding for enzyme activities not expressed by any orthologue are indicated in gray. (B) Survey and localization of main transporters involved in uptake and intracellular distribution of abscisic acid. (C) The components involved in abscisic acid signaling are represented as interaction scheme. abbreviations: Proteins: ABA, ABA deficient; VDE1, violaxanthin de-epoxidase; NCED, nine-cis-epoxycarotenoiddioxygenase; CYP707A, cytochrome P450, family 707, subfamily A; UGT71B, UDP-glucosyltransferase 71B; BG, beta-1,3-glucanase; ABCG, ATP-binding casette G25; DTX, detoxification efflux carrier; ABCG, ATPbinding cassette g; ait, aBa-importing transporter; aBcc1, atP-binding cassette c; gtg, gPcr-type g protein; gcr, g protein coupled receptor; gPa, g ProtEin alpha subunit; cch, conditional chlorina; WrKy, WrKydna-binding protein; Pyl1, Pyr1-like 1, Pyr, pyrabactin resistance; rcar, regulatory component of aBa receptor; snrK, sucrose non fermenting 1(snf1)-related protein kinase; aBi, aBa insensitive; aBf, abscisic acid responsive elements-binding factor.
1. Growth retardants: These are the synthetic organic compounds causing retardation of cell division by inhibiting biosynthesis of plant hormones without evocating substantial growth distortions e.g. Onium compounds, pyrimidines, trizoles, tetcyclacis, morphactins, maleic hydrazideetc Abscisic acid (ABA) was the last major hormone to be identified, although its existence had been forecast for some time. Groups lead by Addicott studying hormonal relationships in cotton [21] and Wareing studying dormancy in woody perennials independently identified Abscisin II in cotton and Dormin in woody plants [22]. The two compounds were ultimately determined to be identical and were given the name Abscisic acid in 1967 at a plant hormone conference in Ottawa, Canada.
2. Maleic hydrazide (MH): It is incorporated into the nucleolus as a part of the RNA fraction and it blocks the cell division by interfering with the production of uracil. It is a general inhibitor of meristematic activity, it retards stem elongation and prevents leaf and flower initiation, as well as fruit set and enlargement.
6. Ethylene:
This is a simple gas that is produced in small quantities by many plant tissues and they serve as a very powerful regulator of growth and development. They are found very prominently in physiologically matured fruits undergoing ripening.
The growth regulation properties of ethylene were first noted by Nejublov in 1901 who reported that leaf abscission could be stimulated by coal gas. Subsequently, Denny and Miller in 1935 reported that ethylene could break dormancy, advance fruit ripening, stimulate flowering in pineapple, and was naturally produced by many organs in the plant, respectively [23,24] suggested that ethylene was an endogenous hormone. The assertion that ethylene should be elevated to hormonal status was largely dismissed by the scientific community. It was difficult to fathom how a two carbon compound floating freely in the air could be seriously considered to be a hormone. It was not until 1959 when the gas chromatograph was adopted as the primary way to quantify endogenous ethylene, that the physiological significance and importance of ethylene was recognized. Ethylene was then elevated to hormonal status [25].
Physiological effects of ethylene:
1. Fruit Ripening Ethylene in the form of gas helps ripens fruits under natural conditions. 2. Flower InitiationEthrel (Ethephon) and ACC promote flower initiation in pineapple. 3. Leaf and Fruit AbscissionAccelerates fruit abscission for mechanical harvesting in fruit crops such as grapes, cherries and citrus. 4. Inhibit Vegetative Growth Ethephon may be used for inhibiting vegetative growth of grape vines resulting in higher yield and better quality.
7. Plants Hormones inhibit GA3 biosynthesis:
Several synthetic compounds, known as growth retardants, inhibit stem elongation by inhibiting GAs biosynthesis. GAs biosynthesis inhibitors fall into three classes. Onium compounds such as quaternary ammonium (e.g.chlormequat chloride or CCC, mepiquat chloride and AMO1618) and phosphonium compounds (e.g. chlorphonium chloride) blocks the synthesis of ent-kaurene. AMO-1618 and CCC specifically inhibit the activity of copalyldiphosphate synthase and to a lesser extent that of entkaurene synthase. The second class consists of nitrogen containing heterocyclic compounds, such as ancymidol (pyrimidine), tetcyclacis (norbornanodiazetine) and trizole type compounds (e.g. paclobutrazol, uniconazole). These compounds inhibit the oxidation of entkaurene to ent-kaurenoic acid by P450 mono oxygenases. The third group includes acylcyclohexanediones, which inhibit the 2- oxoglutarate dependent dioxygenases in Gasbiosynthesis. Acylcyclohexanediones, such asprohexadione-Ca and trinexapac-ethyl (a salt and an ester, respectively) are structurally similar to 2-oxoglutarate and are inhibit dioxygenase activity by competing for the binding site for the cosubstrate, 2-oxoglutarate.
8. Plant Hormones not inhibits GAs biosynthesis:
Morphactins (fluorene, fluorene-9-carboxylic acid and chlorfluorenol): These are derivatives of fluorene-9-carboxylic acid, which has a fluorene nucleus. These compounds may exert their effect byinfluencing the plant‘s auxin metabolism, thus causing alterations in hormonal control and such consequent responses as loss of apical dominance. These compounds are useful to control the growth of woody plants, but they have been known to cause foliar distortions, retardation of stem elongation and breaking of axillary bud. It also stimulate abscission of flowers and fruits because the firmness of attachment of fruit to the plant can be decreased by application of morphactins, they may become useful aids in the mechanical harvesting of grapes and tree fruits.
9. Role of PGR in fruit production:
PBRs are used more extensively in tree fruit production than in any other horticultural or agricultural commodity, and they are essential for effective and profitable production. Several commercial uses have been selected to illustrate the evolution of the involvement of PBRs from infancy to the present and progress made in the fundamental understanding of how regulation by PBRs is achieved.
Fruit drop of Fruit Crops
Fruit abscission is considered as most important physiological response thatis regulated by PBRs. This regulation of abscission occurs at two very different times in the life and development of a fruit. The first occurs early in or at the start of fruit development and this will be referred to as the flower abscission or chemicalthinning period and second period when fruit drop prematurely or drop just as they are entering the development period when they can be harvested. Observations that auxins delayed leaf petiole abscission lead to the finding in the late 1930s by Gardner et al., (1939) that Naphthaleneacetic acid (NAA) and naphthalene acetamide (NAAm) could reduce preharvest drop. Other auxins were tested including 2,4-D (2,4-dichlorophenoxyacetic acid) but most proved to be unsatisfactory. Fenoprop (2-[2,4,5-trichlorophenoxy] propionic acid) was discovered in the 1950s and proved to be very successful [26] but the registration was dropped in the 1980s due to fear of contamination with the carcinogen dioxine. The preharvest drop control properties of daminozide (2,2-dimethylhydrazide) were recognized in the 1960s soon after its growth control properties were recognized (Edgerton and Hoffman, 1966). This compound was the dominant preharvest drop control compound for over 20 years, not only because of its effectiveness but also because it delayed ripening, increased red color, reduced ethylene production and enhanced flesh firmness. The registration of daminozide for use on apples was withdrawn in 1989 because of health concerns. NAA remained as the lone, viable drop control compound but the drop control properties were relatively short-lived, if two NAA applications were made or if the time between application and harvest was delayed, fruit softening and reduced storage life frequently occurred [27]. As a stand-alone PBR, NAA could not fill the gap vacated by daminozide.
The ethylene biosynthesis inhibitor aminoethoxyvinylglycine (AVG) was recognized as having stop drop capabilities in 1978 [28] but it was not developed for this purpose because daminozide was a very acceptable compound, it possessed several additional assets and an economical way was not known to produce this product and be competitively priced. Following the loss of daminozide Abbott Laboratories (now Valent Biosciences Corp.) initiated drop control studies with AVG and in 1997 and AVG was registered as a drop control compound on apples. It remains today as the prominent drop control PBR. The most recent candidate as a drop control PBR is 1-methylcyclopropene (1- MCP) [29]. This is a compound that is released as a gas which then binds irreversibly to ethylene binding sites within the plant. It was first used in the mid-1990s to extend the postharvest life of ornamentals. It is now used to extend the storage life of apples and the extent of its use, and the impact that it has had on commercial postharvest handling of apples, has been nothing less than remarkable [30].
This compound which normally is administered to apples as a gas in an enclosed space has been formulated so that it can be sprayed on trees. It is a most effective drop control compound on apples and the sprayable form has been registered for use as a drop control compound in several countries. Some of the most exciting work related to the control of preharvest drop on apples is just now emerging from the lab of Yuan at Virginia Tech and other locations. Combination of NAA with AVG or 1-MCP more effectively control drop than when the individual drop control compounds are used. Further, these combinations were recognized to more effectively suppress genes responsible for ethylene biosynthesis and cell wall degradation in the abscission zone. The recent and major progress being made in drop control has been achieved by combining molecular biology, good pomology and a better basic understanding of the physiology of abscission.
2. Flower abscission or Chemical thinning period:
The inherent characteristic of Pome Fruit to undergo biennial bearing has been recognized for centuries but practical and meaningful solutions emerged starting in the 1930s. Two separate approaches have been taken in crop load reduction; one is use of hormonal sprays and the second is application of caustic sprays. [31] used tar oil distillates as caustic materials to remove crop by damaging some blossoms. The compound sodium dinitro-orthocresylate (DNOC) evolved from this work and remained an important thinner of apples in arid regions until 1990 when registration was discontinued. A flurry of activity followed the loss of DNOC that continues even today. Further details and background on thinning of pome fruit with caustic materials can be found in this volume [32]. Abscission retardation was one of the early physiological responses identified with auxins. [33] Reported that NAA and NAAm could retard preharvest drop in apples. Armed with this information [34] attempted to increase fruit set on shy-bearing Starking Delicious‘using these compounds but instead these PBRs caused abscission rather than preventing it. Work by Batjer, Davidson, Southwick and Weeks, Murneek and others in the 1940s and 1950s refined the use of both NAA and NAAm as thinning agents and these compounds are still in common use today. Observations by [35] of reduced fruit set following the use of the newly introduced insecticide carbaryl (1-naphthyl methylcarbamate) led to general and widespread use of this compound as a fruit thinner that persists today.
In some regions carbaryl is the favoured thinner because it is mild, its response is not rate sensitive and over thinning is quite unlikely. Carbaryl is now under regulatory scrutiny, and in some areas including large portions of Europe, it may no longer be available for use.BA emerged as a chemical thinner candidate in the late 1970s when it was found to be a very effective thinner on Winesap‘apples, but the active ingredient was not packaged into a thinning product until the 1990s; and even then it appeared as an altered formulation of a previous product that also contained a small amount of GA. Although the amount of GA that was present was small, and seemingly unimportant, its presence altered the thinning activity of BA, making it an erratic product to use. A thinning formulation that contained only BA was introduced several years later and this has proved to be very effective. When combined with carbaryl it is a potent chemical thinner [36]. ABA has appeared on the horizon as a new and potentially useful chemical thinner. It has been shown to be an effective thinner on both apples and pears [37,38]. It has the added advantage of also being a naturally occurring plant hormone which should be useful in facilitating product registration and grower acceptance. The mode of action has not been defined but undoubtedly, closing of stomata, thus restricting carbohydrate supply will prove to be a contributing factor. What progress are we making with chemical thinning? An enormous number of field experiments have been done in an attempt to achieve consistent thinning results. Progress has been hampered because important pieces of the puzzle have been missing, but there is reason to be optimistic. The missing links have been the lack of understanding of basic control points in the abscission process, the absence of a method to predict thinning responses, and sorting out the genes primarily responsible for abscission from the background noise of no participatory genes. We as a scientific community are now making progress. [39] And other have linked light, temperature and carbohydrates to the abscission process. [40] Has linked auxins and more elaborate schemes are surfacing that implicate participation by ABA and ethylene. The development of a computer model [41] has incorporated the important environmental signals that affect abscission into a model that quite accurately predicts thinner response and provides guidance in the selection of thinning programs prior to application.
We are developing fruit measurement systems that allow prediction of thinner results in about 7 days [42]. More recently [43] have identified specific genes involved in the abscission process and they have shown that activation can be linked to specific PBRs. Abscission is a complex process that undoubtedly involves several hormones and many enzymes. Hormonal signal supregulate and downregulate genes to drive this process [44]. Critical breakthroughs in understanding and regulating abscission will only occurs increasing fundamental understanding components of the abscission process and by specifically identifying genes that are regulated into action or inaction. Role of PGRs in vegetative growth appropriate regulation of vegetative growth is important in Pome fruit production since there is an inverse relationship between growth and flowering and excessive vegetative growth negatively impacts fruit quality, postharvest life, and development of an efficient and productive tree structure [45]. Reported that daminozide could effectively inhibit growth of apple trees. It was an important discovery. Since it could also reduce fruit size, affect fruit shape and increase fruit set, its use for growth control early in the season was generally limited to directed application to the tops of vigorous trees, use on young nonbearing trees or on bearing trees where the crop was partially or completely lost. Daminozide remained a viable option for controlling tree growth until 1989 when its registration was cancelled. Ethephon was also identified as a very effective growth retardant in the 1960s but its use on bearing trees was limited because it was also a strong fruit thinner [46].
It was used quite extensively in the 1970s and 1980s in combination with daminozide for growth control and increased flowering on nonbearing trees on semi-dwarfing rootstocks [47]. Paclobutrazol and other triazole gibberellin biosynthesis inhibitors were extensively tested in the 1980s. Paclobutrazol was registered for use as a growth retardant in several countries, but its use has been limited due to long persistence in the tree, concerns about ground water contamination and a negative influence on fruit size in pome fruit [48]. There were no viable PBR options for growth control of bearing trees until the gibberellins biosynthesis inhibit or prohexadione-calcium (Pro-Ca) was identified and extensively tested in the early 1990s and eventually registered for use by BASF as the proprietary products Apogee in the US and Canada and Regalis in Europe and elsewhere [49]. Pro-Ca degrades relatively rapidly in the tree necessitating repeat application for season long growth control. This seeming short coming has a distinct advantage since it affords a high degree of growth control via metabolism and reapplication. Pro-Ca must be applied quite early, as soon as sufficient leaf area has emerged for absorption, since it requires about 10 days on Pome fruit to start to restrict vegetative growth.
10. Flower bud formation:
[50] Showed that NAA had the intrinsic ability to promote flower bud formation distinct from effects related to thinning. Early the focus on NAA was to enhance flower bud formation by chemical thinning to reduce crop load. In the mid-1960s, when daminozide came into general use, it was found that daminozide could enhance flowering when applied after bloom. High rates reduced fruit size so lower rates were used to reduce the impact on fruit size. Ethephon proved to be the most effective promoter of flower bud formation. However, its use on bearing trees was limited because ethephon also caused thinning [51]. Many investigators concluded that a combination of daminozideplus ethephon was the appropriate combination to increase flowering, because of the thinning response, most of this work focused on influencing flowering on young and non-bearing trees. Enhancement of flowering became a lower priority in the 1980s and 1990s because there was a shift to planting trees propagated on dwarfing rootstocks that tended to be much more precocious thus the need for increased flower formation was diminished.
11. Factors affecting growth promotion and inhibitions:
I. External factors: The plant growth promotion and inhibition are controlled by internal regulators that are modified according to environmental conditions. The four most important external factors affecting plant growth are light, temperature, water and mineral nutrients.
Light: It is ultimate source of energy and the most important ecological factor affecting growth promotion and inhibition in plants. Variation in quality, intensity and duration of light affect plant growth. It derives the process of photosynthesis which produces the carbohydrate that are needed to osmotically retain water in the cell for growth.
Temperature: An optimal temperature is needed for plant growth. Metabolic reactions and plant growth increases with temperature. Most plant biological activity and growth occurs between 32 ?F and 122 ?F [52].
Water: is maintain the rigidity of the plant tissue is occurring by water. Cell expansion is controlled by cell turgor pressure, which depends on water. Any deficit in the water supply reduces the cell turgor pressure and limits cell elongation, resulting in a smaller plant [53].
Mineral nutrients: Mineral nutrients are needed for the biochemical process of the plant. These constitute the raw materials required for growth of the plant. Supply the plant necessary mineral ions and organic substances such as proteins, carbohydrates and others.
II. Internal factor: These are the product of the genetic instructions carried in the plant. These influence the extent and timing of growth and are mediated by signals of various types transmitted within the cell, between cells, or all around the plant. Hence, plant growth promotion and inhibition are characterized by high degree of co-ordination and phasing. The growth of one part is closely related with the growth or activities in other parts of the plant. Such internal coordination system is maintained by naturally occurring organic compounds referred to as plant hormones or phyto-hormones.
Plant Hormone: The utilization of the nutrients for proper development of the plant is controlled by certain chemical messengers called hormones. Plant hormones are regulators produced by the plants which in low concentration regulate a physiological plant process. Hormones usually move within plant from a site of production to site of action. Phyto-hormones are not carbohydrate in the sense that carbohydrates are produced and require by the plant in large quantities while phyto-hormones are produced in small quantities but their effect are pronounced. Regulation of growth by is actually based on interaction and delicate balance amongst the various groups of phyto-hormones. A new need became very apparent starting in the 1990s when new, unique and better tasting apples were introduced and these were planted extensively. Many of these new varieties had much greater biennial bearing problems than previous standard varieties. Honey crisp‘is an excellent example of a new cultivar that is being afflicted by this problem.
Many new high density orchards were planted that were highly dependent upon continuous and consistent production to be economically viable. Consequently, the focus on flowering research is to find strategies to increase flowering that do not substantially affect either crop load or fruit maturity. The PGR options are NAA and ethephon, both of which are thinners and they have the potential to advance fruit ripening [54]. The general approach at the present time is to use multiple applications of low rates of either NAA or ethephon starting near the end of June drop. Flowering in pome fruit undoubtedly is a very complex and interactive process. Lack of consistent flowering in high density plantings remains an important problem and it needs to be addressed in a more innovative way. Breakthroughs and ultimate regulation of flowering will only come after we have achieved a better understanding of the physiology and mechanisms of flower bud formation. With this knowledge we can then achieve success similar to those we are just now realizing in the understanding in fruit abscission process.
1. Auxins: Pear fruit treated with NAA (20 ppm) have strong effect on fruit set and development [55]. The increase in fruit size as a result of foliar application of NAA might be because it had improved the internal physiology of developing fruit in terms of better supply of water, nutients and other compounds vital for their proper development which resulted in improving size [56]. The increase in cell size of
plums following auxin application at the beginning of pit-hardening, possibly indicates their ability to mobilize carbohydrates uptake and thus enlarge the cell considerably [57]. Nphenyl-phthalamic acid (at 0.4 kg/ha Nevirol 60 WP) was sprayed at full bloom.PPA application extended the flowering time of most of the cultivars. Because application was at full bloom, the spray was able to increase the duration of the main bloom and extend flowering time. It was thought that PPA application might increase fruit set in the cases of openand self-pollination [58].
2. Gibberellins: Among various growth regulators, the role of gibberellins is definitely more important in the setting of fruits because many experiment with gibberellins spray on the flowers have shown enhancement in fruit set. The gibberellins have been used in improving fruit set with greater promise as compared toauxins in pome fruits. GAs induced fruit set either with mature or immature embryo sacs and differences in fruit-set were related to the stage of nucleus development at the time of application. Single or multiple applications of GA3resulted in similar or increased fruit set compared with pollination, and increased fruit set compared with no pollination. GA3 application decrease fruit mass and increased the fruit development period in blueberry [59]. Fruit sprayed with GA3(100 ppm) have retained maximum number of fruits in Amrapali due to the beneficial effect of GA3 in delaying the formation of abscission layer. GA spray might have triggered auxin level and nullified the action of ABA consequently retained more fruit [60]. The exogenous application of GA3might have stimulated cell division and cell elongation. Consequently, rate of growth and development of fruit was enhanced resulting in larger size of fruits. Gibberellins stimulate the stalk length of seedless grapes to grow longer, thereby alleviating compaction and it promotes elongation of the fruit. A mixture of GA4+7and BA (Promalin) increase fruit shape, size and quality in Starking Delicious apple. In citrus fruits, gibberellins delay senescence, Gas sprays might have triggered auxin level and nullified the action of ABA consequently retained more fruits and allowing the fruits to be left on the tree longer to extend the marked period.
3. Growth retardant: Daminozide (SADH), chlormequat and paclobutrazol have been found effective in improving fruit set in temperate fruits [48]. A class of inhibitors of gibberellins biosynthesis, thecyclohexanetriones has the ability to control the vegetative growth. This class includes the Prohexadione calcium (PCa), which is particularly effective and has the potential to increase the productivity of apple trees and reduce the need for pruning. Although the apple tree needs 5 to 10% of flowers fertilized to obtain high production, in adverse pollination conditions or when the flowering intensity is small, it may be necessary the growth regulators application to improve fruit set. Plants treated with PCa+TDZ in petal fall showed the highest values of fruit set and average number of fruits with floral clusters, followed by the plants treated with PCa+TDZ in phenological stage. The results obtained show the mixture PCa+TDZ more effective on increase apple fructification than PCa sprayed alone.The time of application influence the PCa response, being the best results obtained when PCa were sprayed I full bloom stage; Applications of prohexadione calcium with thidiazuron had the best results of number of fruits per tree and production per tree when applied in the petal fall stage [61]. To assess the effect of chemicals and growth regulators on physical characters of ParbhaniBhusan mango, an experiment was conducted with treatments consisted of NAA at 40, 60, 80 ppm, KNO3at 2.0, 4.0 and 6.0%, urea at 1, 1.5, 2.0%, triacontanol at 300, 500, and 700 ppm, and distilled water sprayed at flowering, pea and marble stage of fruit development. Triacontanol at 700 ppm showed significantly maximum length (10.91 cm), breath (8.91 cm), volume (336.58 cm3), weight (330.41 g) and mesocarp (69.92%). In the case of endocarp (stone), the lowest proportion of endocarp (12.00%) was recorded in triacontanol at 700 ppm [62]. Several workers conducted the experiments on fruit crops to observe the effects of different method on thinning are summarized.
4. Fruit quality and yield: Application of plant bio-regulators resulting in better quality and yield in fruit crops because it improved the internal physiology of developing fruit in terms of better supply of water, nutrients and other compounds vital for their proper growth and development which resulted in improved size, quality and ultimately greater yield [63]. The effect of urea and growth substances like NAA, Ethrel (ethephon) and Paclobutrazol on the yield and quality of mango cv. Langra was studied. Paclobutrazol at 7.5 g/tree applied as soil treatment produced the highest yield on both 'off' and 'on' years. Urea 2%+Ethrel 200 ppm was also found very beneficial. These treatments also improved fruit quality significantly during both years of the study compared with the control [64]. The plant growth regulators (PGRS) are very important in the integration of developmental activities of plant. It play important role in metabolism and distribution solutes within the plant. Apart from it, they also regulate expression of intrinsic genetic potential of plants. The use of growth regulators has become an important component of agro-technical practices especially for fruit plants. So far in fruit crops, optimum fruit setting can be maintained by exogenous application of plant growth regulators. The auxin and gibberellins are widely used to control the fruit drop and to improve the quality of fruit.
12. Use of plant growth regulators in fruit crops production:
1. Seed germination: Seed germination may require gibberellins for the activation of vegetative growth of the embryo. Gibberellin application also activates the alpha amylase synthesis in the aleurone layer where it converts starch to sugar which translocated to growing embryo to provide energy source. Reports on pre-sowing seed treatments of different fruit crops on germination and seedling growth is presented in Table 1. Pre-sowing treatment with GA3 at 200 ppm was found effective in increasing germination and extending seed longevity of ber cv. Gola. Maximum germination 98.76 and 77.82% were obtained with GA3 at 200 ppm pre-treated seeds sown at one and 17thmonths after treatment whereas untreated seeds recorded 86.23 and 24.58% germination. Days to first germination were fewest with 1.0% thiourea treatment, while the shortest span of germination was obtained with 1000-2000 ppm Cycocel. Incidence of polyembryony was highest with 400 ppm GA3and 1000 ppm Cycocel [65].
2. Control of vigour: Various growth retardants have been used to restrict the vegetative growth of plant such as AMO 1618, ancymidal, pacloburazol, B-9 (Phosphon D), chlormequatetc (Table 2). The reduction in vegetative growth by growth retardants is due to the systemic inhibition of GAs biosynthesis pathway at the sub-apical meristem, which ultimately reduced cell elongation and rate of cell division and decreased the shoot growth. These growth retardants also inhibit the gibberellin precursor by blocking the oxidation of ent-kaurene to ent-kaurenoic acid. Prohexadione-Ca (ProCa) has been evaluated as a growth inhibitor in the vigorous red apple cultivars 'Fuji' and 'Royal Gala'. Greatest inhibition of shoot growth was obtained when ProCa was first sprayed at about 200 mg L1from full bloom (FB) up to 12 days after full bloom (DAFB). Shoot regrowth often occurred later in the growing season, and a second application of ProCa was then needed to maintain growth inhibition. Inhibition of shoot growth was due mainly to a reduction in internode length. No effects on yield have been found, except for cv. 'Royal Gala', where an increase in crop-load and a decrease in fruit size were recorded. Fruit quality parameters were not affected; but, in cv. 'Fuji', the red colouration of fruit was promoted by ProCa, particularly when repeated sprays of the chemical (at 125 or 250 mg L-1) were made. No effects on fruit colour were seen in ProCatreated cv. 'Royal Gala' trees [66].
3. Root initiation: A low concentration (10-10to 10-9M) of auxin promotes the growth of intact roots, whereas a higher concentration (10-6M) inhibits root growth. Adventitious roots can arise in a variety of tissue locations from clusters of mature cells that renew their cell division activity. These dividing cells develop into a root apical meristem in a manner somewhat analogous to the formation of lateral roots. In horticulture, the stimulatory effect of auxin on the formation of adventitious roots has been very useful for the vegetative propagation of plants by cuttings (Table 3). Application of 1000 ppm p-hydroxybenzoic acid+5000 ppm IBA results in the maximum rooting percentage (85%), number of roots per cutting (56.9), length of longest root (23.2 cm) and survival percentage of cuttings (80.4%) in pomegranate cv. Kandhari [67].
4. Flowering: In many woody plants including fruits GA inhibits flower formation. In these cases growth retardants viz. paclobutrazol, SADH which inhibits GA biosynthesis are used to promote flowering such as in pears and mango. Application of paclobutrazol helps in restricting the vegetative phase and increasing the reproductive phase of mango [68]. The effects of soil application of 10 ml paclobutrazol and foliar application of 20 ppm NAA, 1% urea, 1% potassium nitrate, 200 ppm ethrel and 5000 ppm mepiquat chloride on the flowering of 10-year-old mango cv. Alphanso trees were determined in a field experiment conducted in Coimbatore, Tamil Nadu, India. Soil application with 10 ml paclobutrazol resulted in the longest panicle (31.57 cm), number of branches per panicle (13), total number of flowers per panicle (620), number of hermaphrodite flowers (189.67) number of male flowers (425) and hermaphrodite flowers (30.59%). The male: hermaphrodite flower ratio was highest with application of 1% potassium nitrate [69].
5. Crop regulation: Plant bio-regulators have been found very effective in thinning flowers and manipulating the cropping season e.g. two sprays of 600-800 ppm NAA done in April - May at 15 days interval in tarai condition at 50% flowering is effective in regulating the crop in guava. Reduction of crop load of rainy season crop of guava through foliar application of different crop regulating chemicals like urea, 2,4-D, potassium iodide and NAA to increase the yield and quality of winter season crop have been successfully standardized for different agro-climatic zones. The studies were earned out on 20 years old Sardar guava trees in which urea (10, 15 and 20%), potassium iodide (0.5, 1.0 and 2.0%), maleic hydrazide (1000, 2000 and 3000 ppm), ethephon (600, 1200 and 1800 ppm) and NAA (200, 400 and 600 ppm) were sprayed during May for crop regulation. All the chemicals reduced the yield of rainy season crop significantly over control and subsequently increased the yield of winter season crop. NAA (600 ppm) application produced maximum winter crop yield (359.3 q/ha) followed by the application of 15 per cent urea (356.2 q/ha). The higher concentrations of urea were found very effective in improving the fruit quality and resulted in maximum TSS (14.0%), ascorbic acid (342.2 mg/100 g), total sugars (10.20%) and pectin (1.59%) content in the winter crop. Acidity was significantly reduced by 1800 ppm ethephon during both the seasons. Maximum profit (Rs.1,42,600/ha) was obtained with NAA 600 ppm followed by 15 and 20 per cent urea treatments [70].
6. Fruit set and development: It is well known that PBRs are used on many fruit tree to manipulate immature fruit drop, fruit set or size and loosen or remove fruits. The plant bioregulators (PBRs) that contribute in increasing fruit set in different fruit crops have been subject of research and field testing since long. The exogenous application of auxin and gibberellins in most of cross-pollinated fruit crops help in preventing early fruit abscission by substituting to some extent, the normal endogenous production of the same. A part from auxins and gibberellins, the growth retardants, ethylene inhibitors, polyamines and mixture of bio regulators have been found to increase fruit set. However, the mode of action for the above PBRs is yet to be understood clearly. The role of different bio regulators in fruit production is mentioned here under. Prepollination applications of putrescine (1.0 and 0.01 mM) positively affect fruit set in House after hand-pollination [71-92]. Pollination stimulates the development of the ovary and the surrounding tissues leading to the formation of fruit. The changes taking place from the flower to the fruit stage is known as the fruit set. The auxin stimulus leading to fruit set may not only from the pollen but also from the ovary. Pollination stimulates auxin formation in the ovary. Unpalliated ovaries have only small amounts of auxin. Application of auxin to the fruit increases its size and date of maturity. Application of auxin, late in the life of fruit, hastens maturity and gives fruit of smaller size. The exogenous application of GA3 might have stimulated cell division and cell elongation. Consequently, rate of growth and development of fruit was enhanced resulting in larger size of fruits. Gibberellins stimulate the stalk length of seedless grapes to grow longer, thereby alleviating compaction and it promotes elongation of the fruit. A mixture of GA4+7and BA (Promalin) increase fruit shape, size and quality in Starking Delicious apple. In citrus fruits, gibberellins delay senescence, Gas sprays might have triggered auxin level and nullified the action of ABA consequently retained more fruits and allowing the fruits to be left on the tree longer to extend the marked period [92-105].
13. Micronutrients Use Efficiency of Fruit Plants under Changing Climate:
Micronutrients play a pivotal role in the growth, development, and function in humans and plants. Despite their beneficial effects, micronutrient deficiencies pose serious threats to crop yield and nutritional quality, which, in turn cause deleterious effects on the health and well-being of humans. Many staples are inherently poor sources of micronutrients caused by insufficient amounts and or poor bioavailability. Climate change further exacerbates the already existing problem of micronutrient deficiency. Climate change, particularly rising [CO2] concentration, temperature, and water availability modifies the plant's micronutrient uptake and utilization patterns in different magnitude and directions. Understanding the effects of these environmental signals on plant micronutrients and its use efficiency is important to underpin crop breeding for improved nutritional quality and to formulate suitable crop management strategies under changing climate [106-120]. In this chapter, we discuss how key climate variables individually and interactively influence micronutrient uptake and utilization in crop species. We discuss the physiological perspectives in nutrient acquisition and utilization under a changing climate with a special focus on wheat, rice, and legumes.
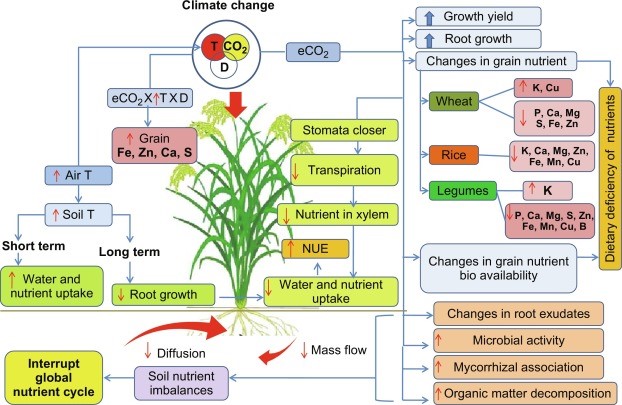
Figure 3: Micronutrients Use Efficiency of Crop-Plants under Changing Climate.
Micronutrients are essential elements that are used by plants in small quantities. For most micronutrients, crop uptake is less than one pound per acre. In spite of this low requirement, critical plant functions are limited if micronutrients are unavailable, resulting in plant abnormalities, reduced growth and lower yield. In such cases, expensive, high requirement crop inputs such as nitrogen and water may be wasted. Because of higher yields, higher commodity prices and higher costs of crop inputs, growers are reviewing all potential barriers to top grain production, including micronutrient deficiencies [121-135]. This Crop Insights will discuss general micronutrient requirements, deficiency symptoms, soil and plant sampling, and fertilization practices. Future Crop Insights articles will discuss specific crops, their micronutrient or secondary nutrient requirements and management considerations.
1. Detecting Micronutrient Deficiencies:
Micronutrient deficiencies can be detected by visual symptoms on crops and by testing soils and plant tissues. To understand visual symptoms, it is useful to know the role each micronutrient plays in plant growth and development.
2. Functions of Micronutrients:
Micronutrients differ in the form they are absorbed by the plant, their functions and mobility in the plant, and their characteristic deficiency or toxicity symptoms table 1 and 2).
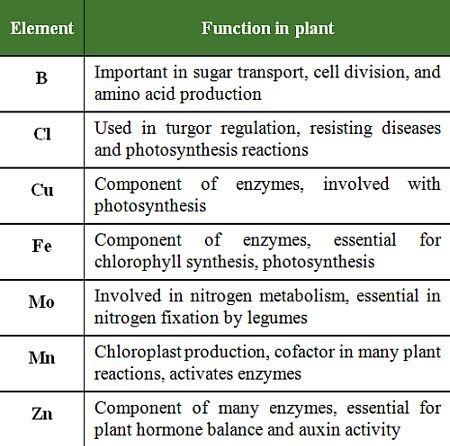
Table 1: Functions of micronutrients in plants.
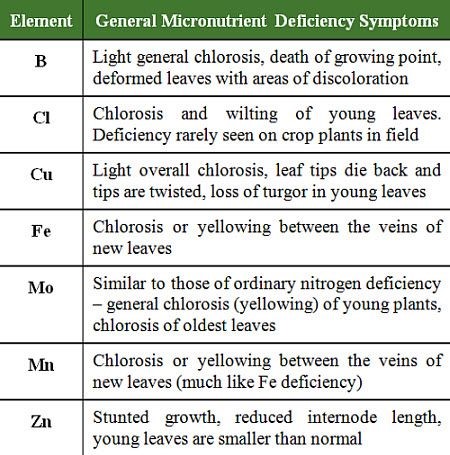
Table 2: General micronutrient deficiency symptoms.
Iron or zinc deficiency symptoms in corn. Soil and tissue testing can help determine which of these nutrients is deficient.
3. Micronutrient Deficiency Symptoms:
Except for Mo, the micronutrients are considered weakly mobile or immobile in plants. This means that deficiency symptoms appear first or most severely on newest plant tissues (assumes plants started with some supply of the nutrient but ran out as plants developed.) For molybdenum, deficiency symptoms appear first on oldest plant tissues. Symptoms vary according to crop, but generalized symptoms are shown in Table 2.
Micronutrient deficiencies usually have a patchy distribution in fields due to variation in soil properties that affect availability (e.g., pH, drainage, and salinity) and management history such as manure applications. Learning to identify deficiencies visually is important in recognizing problem areas and planning remediation for future crops. However, it is often too late for corrective action in the current crop by the time visual symptoms appear.
4. Common Micronutrient Deficiencies:
The probability of a micronutrient deficiency is greatly increased on specific soils types and in certain crops (Table 3).
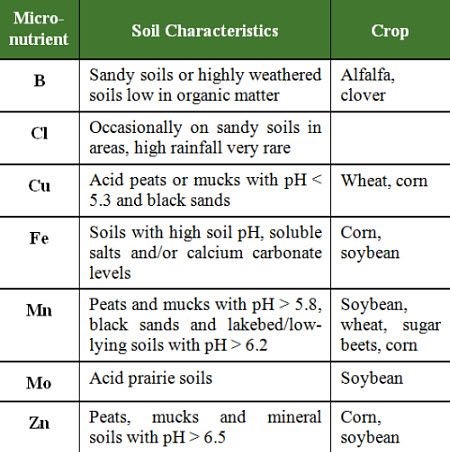
Table 3: Soil conditions which may lead to micronutrient deficiencies for various crops.
5. Soil Tests to Detect Micronutrient Deficiencies:
Many plant symptoms associated with micronutrient deficiencies, including stunting and chlorosis, may have a variety of causes, including disease, insect or herbicide damage, or environmental conditions. Soil and plant analysis are both useful in determining if the cause is truly nutritional. Though adequate for this purpose, micronutrient soil tests are not as precise as soil pH, phosphorus, and potassium tests [136-143]. The most reliable micronutrient soil tests are for zinc, boron, copper, and manganese. Because interpretations are soil specific, it is best to use locally calibrated recommendations. Soil tests for iron and molybdenum are considered to be of little value in predicting the supply of these nutrients in soils. When sampling for micronutrients, sample the root zone from 0 to 8 inches deep.
6. Plant Analysis to Detect Micronutrient Deficiencies:
Plant tissue analysis is more reliable than soil testing for identifying many micronutrient problems, and can also supplement soil test information. Tissue testing is especially valuable in cases where reliable soil tests are unavailable. However, molybdenum and chlorine levels cannot be determined by this method. Plant analysis can be used in two ways; one is to monitor the crop's micronutrient status, and the other is to diagnose a problem situation. By quantifying the nutrient content of tissues, plant analysis can point out an existing or potential problem before visual symptoms develop. If in-season micronutrient deficiencies are suspected, plant samples should be taken as early as practical; treatments, when needed, should be made in a timely manner. Research has shown that once a micronutrient deficiency is detected, the plant has already suffered irreversible yield loss. Because plant nutrient composition varies with the crop, age of the plant, part of the plant sampled and other factors, it is important to follow the standard sampling procedures provided by your plant diagnostic laboratory. In order to obtain a representative sample, take multiple plants from areas randomly distributed throughout the affected field area. Avoid border plants and those contaminated with dust, soil or foliar sprays. Taking samples of non-symptomatic plants to compare with apparent nutrientdeficient plants can increase the usefulness of plant analysis. Be aware that interpreting results is complex and may require expert advice.
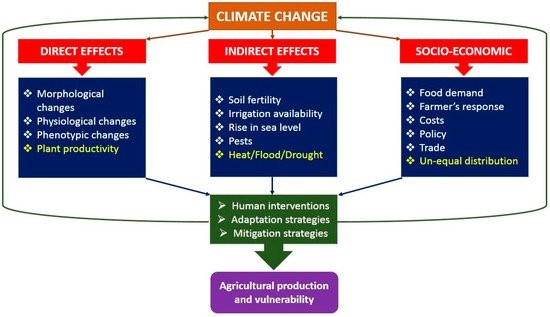
Figure 4: Direct, indirect and socio-economic effects of climate change on agricultural production.
References
1. Malhotra HK, Khajuria H, Jawanda JS. 1983. Studies on physico-chemical characteristics of pomegranate cultivars. Punjab Horiculture Jourrnal. 23: 153-161.
2. Reddy P, Prasad DM. 2012. Effect of plant growth regulators on fruit characters and yield of pomegranate (Punica granatum L.). International Journal of Plant, Animal and Environmental Sciences. 2: 91-93.
3. Thimann K. 1963. Plant Growth Substances: Past, Present and Future. Ann Rev Plant Physiol. 14: 1-18.
4. Jacobs W. 1979. Plant hormones and plant development. Cambridge University Press, New York.
5. Thimann KV. 1969. The auxins P 1-45. In: M.B. Wilkins (ed.), Physiology of plant growth and development. McGraw-Hill, New York.
6. Thimann KV, Skoog F. 1933. Studies on the growth hormone of plants. Proc Nat Acad Sci. Wash. 19: 714. Ref.: https://pubmed.ncbi.nlm.nih.gov/16577553/ DOI: https://doi.org/10.1073/pnas.19.7.714
7. Sawada K. 1912. Diseases of agricultural products in Japan. Form Agric Rev. 63: 10-16.
8. Kurosawa E. 1926. Experimental studies on the nature of the substance excreted by the bakanae‘fungus. Trans Nat Hist Soc Formos, 16: 213-227.
9. Yabuta T, Hayashi T. 1939. Biochemical studies on Bakanaefungus of the rice. Part 2. Isolation of Gibberellin, the active principle which makes the rice seedlings grow slenderly. J Agric Chem Soc Japan. 15: 257-266.
10. William MW. 1978. Adverse weather and post bloom thinning chemicals can affect seed content and size of Red Delicious apples. Biochemistry of the bakanae‘fungus of rice. Agric Hort (Tokyo) 10: 17-22.
11. Mitchell JE, Angel CR. 1951. The growthstimulating properties of a metabolic product of Fusariummoniliforme. Phytopathology. 41: 26- 27.
12. West CA. 1973. Biosynthesis of gibberellins. In: Milborrow BV (ed) Biosynthesis and its control in plants. Academic Press, London, New York. 473-482.
13. Sumiki YA, Kawarada H, Kitamura Y. 1953. The biochemistry ofGibberellafujikuroi. The chemical structure of gibberellin. International Congress for Microbiology, Rome. 101-102.
14. Phinney BO. 1983. The history of gibberellins. p. 19-52. In: A. Crozier (ed.) The biochemistry and physiology of gibberellins. 1.
15. Brian P, Hemming H. 1955. The effect of gibberellic acid on shoot growth of pea seedlings. Physiol Plant. 8: 669-681.
16. Letham D. 1963. Zeatin, afaction inducing cell division from Zea mays. Life Sci. 2: 569-573.
17. Jablonski JR, Skoog F. 1954. Cell enlargement and cell division in excised tobacco pith tissue. Physiol Plant. 7: 16-24.
18. Miller CO, Skoog F, Okumura FS. 1955. Structure and synthesis of kinetin. J Am Chem Soc. 77: 2662.
19. Richmond AE, Lang A. 1957. Effect of kinetin on protein content and survival of detached Xanthium leaves. Science. 125: 650-651.
20. Osborne DJ. 1962. Effect of kinetin on protein and nucleic acids metabolism in Xanthium leaves during senescence. Plant Physiol. 37: 595-602. Ref.: https://pubmed.ncbi.nlm.nih.gov/16655700/ DOI: https://doi.org/10.1104/pp.37.5.595
21. Ohkuma K, Lyon JL, Addicott FT. 1963. Abscisin II, an abscission acceleratin substance from young cotton. Fruit Science. 142: 1592- 1593. Ref.: https://pubmed.ncbi.nlm.nih.gov/17741533/ DOI: https://doi.org/10.1126/science.142.3599.1592
22. Cornforth JW, Milborrow BV, Ryback G. 1965. Chemistry and physiology of Dormin‘in sycamore: Identity of sycamore Dormin with Abscisin II. Nature. 205: 1269-1270.
23. Abeles F. 1973. Ethylene in plant biology. Academic Press, London. 302.
24. Crocker W, Hitchcock AE, Zimmerman PW. 1935. Similarities in the effects of ethylene and the plant auxins. Contrib. Boyce Thompson Inst. 7: 231-248.
25. Burg SP, Thimann KV. 1959. The physiology of ethylene formation in apples. Proc Nat Acad Sci. 45: 335-344. Ref.: https://pubmed.ncbi.nlm.nih.gov/16590387/ DOI: https://doi.org/10.1073/pnas.45.3.335
26. Southwick FW, Demoranville ID, Anderson JF. 1953. The influence of some growth regulating substances on preharvest drop, color, and maturity of apples. Proc Amer Soc Hort Sci. 61: 155-162.
27. Smock RM, Edgerton LJ, Hoffman MB. 1954. Some effects of stop drop auxins and respiratory inhibitors on maturity of apples. Proc Amer Soc Hort Sci. 63: 211-219.
28. Bangerth F. 1978. The effect of a substituted amino acid on ethylene biosynthesis, respiration, ripening, and preharvest drop of apple fruits. J Amer Soc Hort Sci. 103: 401- 404.
29. Yuan R, Carbaugh DH. 2007. Effects of NAA, AVG, and 1-MCP on ethylene biosynthesis, preharvest drop, fruit maturity, and quality of Golden Supreme and Golden Delicious apples. Hort Science. 42: 101-105.
30. Watkins CB. 2006. The use of 1-methyl cyclopropene (1-MCP) on fruits and vegetables. Biote Chnol Adv. 24: 389-409. Ref.: https://pubmed.ncbi.nlm.nih.gov/16530376/ DOI: https://doi.org/10.1016/j.biotechadv.2006.01.005
31. Auchter EC, Roberts JW. 1933. Experiments in spraying apples for the prevention of fruit set. Proc Amer Soc Hort Sci. 30: 22-25.
32. Fallahi E, Greene DW. 2010. The impact of blossom and post-bloom thinners on fruit set and fruit quality in apples and stone fruit. Acta Hort. 884: 179-187.
33. Gardner FE, Marth PC, Batjer LP. 1939. Spraying with plant growth substances to control pre-harvest drop of apples. Proc Amer Soc Hort Sci. 37: 415-428.
34. Burkholder CL, Mc Cown M. 1941. Effect of scoring and of α-napthyl acetic acid and amide sprays upon fruit set and of the spray on preharvest fruit drop. Proc Amer Soc Hort Sci. 38: 117-120.
35. Batjer LP, Westwood MN. 1960. 1-napthal Nmethylcarbonate, a new chemical for thinning apples. Proc Amer Soc Hort Sci. 75: 1-4.
36. Greene DW, Autio WR. 1994. Combination sprays with benzyladenineto chemically thin spur-type Delicious apples. Hort Science. 29: 887-890.
37. Greene DW. 2009. Effect of abscisic acid on thinning and return bloom of Bartlett pears. Hort Science. 44: 1128.
38. Greene DW. 2007. Effect ofabscisic acid (ABA) and benzyladenine (BA) on fruit set and fruit quality of McIntosh apples. Hort Science. 42: 908.
39. Byers RE, Carbaugh DH, Presley CN. 1991. The influence of low light on apple fruit abscission. J Hort Sci. 66: 7-17.
40. Bangerth FK. 2004. Internal regulation of fruit growth and abscission. Acta Hort. 636: 235- 248.
41. Lakso AN, Robinson TL, Greene DW. 2008. Using and apple tree carbohydrate model to understand thinning responses to weather and chemical thinners. Compact Fruit Tree. 41: 17- 20.
42. Greene DW, Lakso AN, Robinson TL. 2005. Predicting chemical thinner response on apples. Compact Fruit Tree. 38: 17-20.
43. Zhu H, Beers EP, Yuan R. 2008. Aminoethoxyglycine inhibits fruit abscission induced by naphthaleneacetic acid and associated relationships with expression of genes for ethylene biosynthesis, perception, and cell wall degradation in delicious apples. J Amer Soc Hort Sci. 133: 727-734.
44. Costa G, Dal Cin V, Ramina A. 2006. Physiological, molecular and practical aspects of fruit abscission. Acta Hort. 727: 301-309.
45. Batjer LP, Williams MW, Martin GC. 1964. Effects of Ndimethylaminosuccinamic acid (B9) on vegetative and fruit characteristics of apples, pears and sweet cherries. Proc Amer Soc Hort. Sci. 85: 11-19.
46. Edgerton LJ, Greenhalgh WJ. 1969. Regulation of growth, flowering and fruit abscission with 2-chloroethylphosphonic acid. J Amer Soc Hort Sci. 94: 11-13.
47. Byers RE, Barden JA. 1976. Chemical control of vegetative growth and flowering of nonbearing Delicious apple trees. Hort Science. 11: 506-507.
48. Miller SS. 1989. Plant Bioregulators in apple and pear culture. Horticultural Reviews. 10: 380-402.
49. Rademacher W, Van SK, Garuz P. 2004. Impact of prohexadione-Ca on the vegetative and reproductive performance of apple and pear trees. Eur J Hort Sci. 69: 221-228.
50. Harley CP, Moon HH, Regeimbal LO. 1958. Evidence that post-bloom applethinning sprays of naphthaleneacetic acid increase blossom-bud formation. Proc Amer Soc Hort Sci. 72: 52-56.
51. Byers RE. 2003. Flower and Fruit thinning and vegetative: fruit balance. In: D.C. Ferree and I.J. Warrington (eds.), Apples botany production and uses. CABI Publishing, Wallingford, UK. 409-436.
52. Barbour MG, Burk JH, Pitts WD. 1987. Terrestrial plantecology. The Benjamin/Cummings Publishing Co., Melnopark, California. 634.
53. Brown RW. 1995. The water relationship of range plants: adaptations of water deficits. In: Bedunah DH, Sosebee RE. Wildl and plants: physiological ecology and developmental morphology. Society of range management. Denver, CO. 291-413.
54. Cline J. 2008. The return bloom of apples as affected by ethephon and naphthalene acetic acid. Compact Fruit Tree. 38: 40-45.
55. Chen X, Bao J, Chen Y. 2012. Effect of hormone treatment on deformed fruit development in pear. African journal of biotechnology. 11: 10207-10209.
56. Jain MC, Dashora LK. 2010. Effect of different plant bio-regulators in relation to fruit quality and yield of guava (PsidiumguajavaL.) cv. Sardar. Progressive Horticulture. 42: 50-53.
57. Stern RA, Flaishman M, Arie RB. 2007. Effect of synthetic auxins on fruit size of five cultivars of japanese plum (Prunus salicina L.). Scientia Horticulturae. 112: 304-309.
58. Racsko J, Szabo M, Nyeki J. 2006. Direct and indirect effect of N-phenyl-phthalamic acid and fertilization on fruit setting and fruit quality parameters of apple (Malus domestica Borkh). Acta Horticulturae. 727: 209-215.
59. Cano MR, Darnell RL. 1998. Cell numbe and cell size in pathenocarpic vs. pollinated blueberry (Vacciniumashei) fruit. Ann Bot. 80: 419-425.
60. Rani R, Brahmachari VS. 2004. Effect of growth substances and calcium compounds on fruit retention, growth and yield of Amrapali mango. Orissa Journal of Horticulture. 32: 15- 18.
61. Leite GB, Petri JL, Couto M. 2010. Increasing Apple fruit set on Condessa ‘using Growth regulators. Acta Horticulturae, 884: 537-543.
62. Shinde AK, Patil BP, Pujari KH. 2008. Investigations on the control of fruit drop in Alphonso mango. Indian Journal of Plant Physiology. 11: 93-99.
63. Pandey V. 1999. Effect of NAA and GA spray on fruit retention, growth, yield and quality of ber cv. Banarsi Karaka. Orissa Journal of Horticulture. 27: 69-73.
64. Karuna K, Mankar A, Singh J. 2007. Effect of urea and growth substances on yield and quality of mango cv. Langra. Orissa Journal of Horticulture. 35: 67-70.
65. Hore JK, Sen SK. 1994. Role of presowing seed treatment on germination, seedling growth and longevity of Ber (ZizyphusmauritianaL.) seeds. Indian Journal of Agriculture Research. 28: 285-289.
66. Medjdoub R, Val J, Blanco A. 2005. Inhibition of vegetative growth in red apple cultivars using prohexadione-calcium. Journal of Horticultural Science and Biotechnology. 80: 263-271.
67. Tripathi SN, Shukla HS. 2004. Propagation of pomegranate (Punica granatum L.) cultivars by stem cuttings with indole butyric acid and phydroxybenzoic acid. Indian Journal of Horticulture. 61: 362-365.
68. Bagel BS, Tiwari R, Gupta N. 2004. Effect ofcultar and NAA on flowering and fruiting of mango (Mangifera indica L.) cv Langra. South Indian Horticulture. 52: 302-304.
69. Vijayalakshmi D, Srinivasan PS. 2002. Impact of chemicals and growth regulators on induction of flowering in 'off' year mango cv. Alphonso. Orissa Journal of Horticulture. 30: 32-34.
70. Suleman M, Sharma JR, Kumar R. 2006. Effect of different chemicals on cropping pattern, and quality of guava cv. Sardar. Haryana Journal of Horticultural Sciences. 35: 226-227.
71. Mora OF, Tanabe K, Tamura F. 2004. Effects of putrescineapplication on fruit set in Housui Japanese pear (Pyrus pyrifolia Nakai). Scientia Horticulturae. 104: 265-273.
72. Adi PR, Prasad DM. 2012. Effect of plant growth regulators on fruit characters and yield of pomegranate (Punica granatum L.) cv. Ganesh. Int J of Plant Animal and Environmental Scie. 2: 91-93.
73. Anawal VV, Narayanaswamy P, Ekabote SD. 2016. Effects of plant regulators on fruit-set and yield of pomegranate cv. Bhagwa. Int. J of Current Res. 8: 2908-2910.
74. Anawal VV, Narayanaswamy P, Kumar YHS, 2015. Effects of plant regulators on induction of flowering in pomegranate (Punica granatum L.) cv. Bhagwa. Int J of Sci Res. 4: 7-9.
75. Bagel BS, Tiwari R. 2003. Individual and integrated effect of urea and NAA on flowering and fruiting of mango (Mangifera indica L.). South Indian Horticulture. 51: 1-6.
76. Batjer LP, Marth PC. 1945. New materials for delaying fruit abscission of apple. Science. 101: 363-364. Ref.: https://pubmed.ncbi.nlm.nih.gov/17755605/ DOI: https://doi.org/10.1126/science.101.2623.363
77. Bhat A, Kher R, Gupta SP. 2006. Effect of growth regulators and nutrients on fruit cracking and fruit quality in lemon. Haryana J of Hort Scie. 6: 35:267.
78. Black BL, Bukovac MJ, Hull J. 1995. Effect of spray volume and time of NAA application on fruit size and cropping of red chief Delicious apple. Scientia Horticulturae. 64: 253-264.
79. Brassins. A new family of plant hormones from rape pollen. Nature. 225: 1065-1066. Ref.: https://pubmed.ncbi.nlm.nih.gov/16056912/ DOI: https://doi.org/10.1038/2251065a0
80. Chanana YR, Gill KS. 2007. Effect of soil application of paclobutrazol on growth of Earli Grande peach tree. Indian Journal of Horticulture. 64: 211-212.
81. Chanana YR, Kaur B, Kaundal GS. 1998. Effect of flowers and fruit thinning on maturity, yield and quality in peach. Indian J Hort. 55: 323-326.
82. Chandra R, Govind S. 1990. Gibberellic acid, thio-urea, ethrel and acid treatments in relation to seed germination and seedling growth in guava (Psidiumguajava L.). Progressive Horticulture. 22: 40-43.
83. Chaudhary BR, Sharma MD, Shakya SM. 2006. Effect of plant growth regulators on growth, yield and quality of chilly (Capsicum annuum L.). Journal of the Institute of Agriculture and Animal Science. 27: 65-68.
84. DW. 2003. Endogenous hormones and bioregulator use on apples. p. 437-457. In: DC Ferree and IJ Warrington (eds.), Apples botany production and uses. CABI Publishing, Wallingford, UK.
85. Das B, Nath V, Jana BR. 2007. Evaluation of different methods of crop regulation in guava grown under rainfed plateau conditions of eastern India. Indian Journal of Horticulture. 64: 294-299.
86. Debnath A, Vanajalatha K, Momin U. 2011. Effect of NAA, GA3, kinetin and ethrel on yield and quality in phalsa (Grewia sub-inaequalis DC). The Asian J of Hort. 6: 474-477.
87. Denny FE, Miller LP. 1935. Storage temperature and chemical treatments for shortening the rest period of small corms and cormels of gladiolus. CBTI. 3: 267-294.
88. Devnath S, Kundu S. 2001. Effect of bioregulators and nutrients on growth and differentiation of mango (Mangiferaindica L.) shoots. Environment and Ecology. 19: 829-832.
89. Dhillon WS, Bindra AS, Brar BS. 2002. Effect of nitrogen fertilization on fruit yield, quality and nutrient status of grapevine cv. Perlette. Indian J Hort. 60: 208-213.
90. Digrase SS, Tambe TB, Kadam AS. 2016. Effect of different plant growth regulators and chemicals on growth and yield of pomegranate (PunicagranatumL.) cv. Bhagwa. Advance Research Journal of Crop Improvement. 7: 96- 99.
91. Dubey AK, Singh DB, Dubey N. 2002. Crop regulation in guava (Psidium guajavaL.) cv. Allahabad Safeda. Progressive Horticulture. 34: 200-203.
92. Edgerton LJ, Hoffman MB. 1966. Inhibition of fruit drop and color stimulation with Ndimethylaminosuccinamic acid. Nature. 299: 314-315.
93. El Khawaga AS. 2003. Effect of paclobutrazol and zinc sulphate on splitting and fruit quality of Manfalouty pomegranate trees under upper Egypt conditions. J Agric Sci. 28: 6289-6294.
94. Gautam DR, Jindal KK. 2003. Flowering regulation in pollinizing cultivars of Apple. Haryana J Hort Science. 32: 156-158.
95. Ghosh SN, Bera B, Ray S. 2009. Effect of plant growth regulators in yield and quality in pomegranate cv. Rubi. Journal of Horticultural Sciences. 4: 158-160.
96. Gill DS, Daulta BS. 1996. Effect of plant growth regulators on rooting of cuttings in plum (PrunusdomesticaL.). Haryana Journal of Horticultural Sciences. 25: 19-22.
97. Greene, Goswami JD, Patel NM. 2013. Effect of plant growth substances on growth, fruit setting and yield of pomegranate cv. Sindhuri. International Journal of Agricultural Sciences. 1: 332-334.
98. Goswami JD, Patel NM, Bhadauria HS. 2013. Effect of plant growth regulators on quality traits of pomegranate cv. Sindur. The Asian Journal of Horticulture. 8: 361-363.
99. Gupta RK, Brahmachari VS. 2004. Effect of foliar application of urea, potassium nitrate and NAA on fruit retention, yield and quality of Mango cv. Bombai. Orissa Journal of Horticulture. 32: 7-9.
100. Gupta NK, Bist LD. 2005. Effect of different planting systems and paclobutrazol on vegetative growth of Bagugosa pear. Indian Journal of Horticulture. 62: 20-23.
101. Khalil A, Aly SH. 2013. Cracking and fruit quality of pomegranate (Punicagranatum L.) as affected by pre-harvest sprays of some growth regulators and mineral nutrients. Journal of Horticultural Science & Ornamental Plants. 5: 71-76.
102. Khalil HA, Aly SHS. 2013. Cracking and fruit quality of pomegranate as affected by pre-harvest spray of some growth regulators and mineral nutrients. Journal of Horticultural Science and Ornamental Plants. 5: 71-76.
103. Knight JN, Browning G. 1986. Regulation of Conference Pear Cropping with Gibberelic Acid and Ethephon or Paclobutrazol. Acta Horticulturae. 179: 337- 342.
104. Rahemi M, Atahosseini A. 2004. Effect of plant growth regulators on fruit characteristics and leaf area of pomegranate cv. Shisheh Cup. Acta Horticulturae. 662: 313-318.
105. Manivannan MI, Irulandi S, Thingalmaniyan KS. 2015. Studies on the effect of preharvest application of plant growth regulators and chemicals on yield and quality of guava (Psidium guajava L.) cv. L.-49. International Journal of Agricultural Sciences. 11: 138-140.
106. Medrano RC, Darnell RL. 1998. Effect of GA3 and pollination on fruit set development in Rabbiteye Blueberry. Hort Science. 33: 632- 635.
107. Meland M, Sekse L, Kaiser C. 2011. Ethephon as a Blossom and Fruitlet Thinner. Affects Crop Load, Fruit Weight, Fruit Quality and Return Bloom of Summerred‘ Apple (Malus x domestica) Borkh. Hort Science. 46: 432-438.
108. Miller SS, Swietlik D. 1986. Growth and fruiting responses of deciduous fruit trees treated with paclobutrazol. Acta Hort. 179: 563- 566.
109. Momenpour A, Taghavi TS, Manocher S. 2009. Effect of benzyl adenin and gibberellin on runner production and some vegetative traits of three strawberry cultivars. African Journal of Agricultural Research. 6: 4357-4361.
110. Mukunda LL, Ramana KTV, Krishna VNP. 2014. Effect of growth regulators and chemicals on fruit yield and quality of hasta bahar flowering in acid lime (Citrus aurantifoliaSwingle) cv. Balaji. Journal of Agriculture and Allied Sciences. 3: 11-13.
111. Murti GSR, Upreti KK, Kurian RM. 2001. Paclobutrazolmodifies tree vigour and flowering in mango cv. Alphonso. Indian Journal of Plant Physiology. 6: 355-360.
112. Perez DCM, Mogollon N, Ojeda M. 2009. The effect of GA3 on the growth and flowering of strawberry cv. Chandler vitroplants. Acta Horticulture. 842: 793-796.
113. Roussos PA, Denaxa NK, Damvakaris T. 2009. Strawberry fruit quality attributes after application of plant growth stimulating compounds. Scientia Horticulture. 119: 138- 146.
114. Racsko J, Soltesz M, Szabo Z. 2009. The physiological role of the seed set on the flowering and fruit development in some temperate fruit crops. Journal of Plant Reproductive Biology. 1: 33-42.
115. Rashmi K, Sindhu SS, Sehrawat SK. 2007. Germination studies in Aonla (Emblica officinalis G.). Haryana Journal of Horticultural Sciences. 36: 9-11.
116. Singh RP, Sharma YP, Awasthi RP. 1990. Influence of different cultural practices on premature fruit cracking of pomegranate. Progressive Horticulture. 22: 92-96.
117. Robinson TL. 2006. Interaction of Benzyladenine and Naphtalene acetic acid on fruit set, fruit size and Crop value of Twelve Apple cultivars. Acta horiculturae. 727: 283- 289.
118. Saied E, Mohammad RS, Babak J. 2012. Influence of foliar application of Volk oil, Dormex, GA3 and Potassium Nitrate on vegetative growth and reproductive characteristic of strawberry cv. Merak. Journals of Environmental Biology. 6: 35-38.
119. Saini RS, Singh G, Dhaliwal GS. 2003. Effect of crop regulation in peaches with urea and ammonium thiosulphate on yield and physic-Chemical characteristics of fruits. Haryana J Hort Science. 32: 187-191.
120. Sharma G, Ananda SA. 2004. Effect of pre-bloom foliar application of plant bioregulators on growth, fruiting and quality of apple under warmer agroclimatic conditions. Acta Horticulture. 662: 353-357.
121. Sharma G. 2004. Effect of environmental conditions, nutrient, plant growth regulator applications and orchard floor management practices on flowering, fruit set, yield and quality in apple. PhD. Thesis. Dr YS Parmar University of Horticulture and Forestry, Nauni, Solan, HP, India.
122. Sharma N, Belsare C. 2011. Effect of plant bio-regulators and nutrients on fruit cracking and quality in pomegranate (Punicagranatum L.) ‘G-137’ in Himachal Pradesh. Acta Horticulture. 890: 347-352.
123. Sharma P, Singh AK, Sharma RM. 2005. Effect of plant bio-regulators (PBRs) and micro-nutrients on fruit set and quality of litchi cv. Dehradun. Indian Journal of Horticulture. 62: 24-26.
124. Sheikh MK. 2015. Effect of growth regulators and hand thinning of flowers/fruits on size of pomegranate (Punica granatum L.) ‘Ganesh’. Acta Horticulture. 1089: 407-410.
125. Singh K, 2004. Effect of plant growth regulators on vegetative growth, flowering, fruit set and fruit quality of Baggu ghosha pear. MSc. Thesis. Dr YS Parmar University of Horticulture and Forestry, Nauni, Solan. HP, India, 2004.
126. Singh A, Singh JN. 2005. Flowering, fruiting and yield responses to plant bio regulators of strawberry cv. Sweet Charlie. Environment and Ecology. 23: 714-716.
127. Singh G. 2008. Effect of irrigation, foliar fertilization and plant bioregulators on growth, yield and quality of pomegranate cv. G137. PhD. Thesis. Dr Y S Parmar University of Horticulture and Forestry, Nauni, Solan, HP, India, 2008.
128. Singh DB, Ranganath HR. 2006. Induction of regular and early fruiting in mango by paclobutrazol under tropical humid climate. Indian Journal of Horticulture. 63: 248-250.
129. Singh HJ, Bal JS. 2007. Effect of pruning and growth regulators on vegetative and fruiting characters of guava planted at different spacing. Haryana Journal of Horticultural Sciences. 36: 224-227.
130. Singh DK, Maurya VN, Singh AR. 1996. A note on regeneration of guava (PsidiumguajavaL.) cultivars by stooling with aid of IBA. Haryana Journal of Horticultural Sciences. 25: 34-36.
131. Singh VR, Mandal BK, Singh C. 2011. Effect of growth regulators and their concentration on fruiting yield, size and quality of mango cv Langra. Environment-andEcology. 29: 306-309.
132. Singh DK, Bhattacharya B, Mondal K. 2002. Role of pre-sowing seed treatment with different chemicals on germination behavior and seedling growth of Jackfruit (Artocarpus heterophyllus L.). Environment and Ecology. 20: 741-743.
133. Singh G, Singh AK, Verma A. 2000. Economic evaluation of crop regulation treatments in guava (PsidiumguajavaL.). Indian Journal of Agricultural Sciences. 70: 226-230.
134. Stern RA, Flaishman M, Steve A. 2007. Effect of synthetic auxins on fruit development of Bing cherry (Prunus avium L.). Scientia Horticulturae. 114: 275-280.
135. Suman M, Sangma PD, Meghawal DR. 2017. Effect of plant growth regulators on fruit crops. Journal of Pharmacognosy and Phytochemistry. 6: 331-337.
136. Supe VS, Marbhal SK. 2008. Effect of plant growth substances on fruit size of pomegranate (Punicagranatum L.) cv. Mridula. The Asian Journal of Horticulture. 3: 18-20.
137. Tejpal SB, Laxmi R, Binayak C. 2018. A Recent Advances in Use of Plant Growth Regulators (PGRs) in Fruit Crops -A Review, nternational Journal of Current Microbiology and Applied Sciences. 7.
138. Ueda J, Kato J. 1980. Isolation and identification of a senescence-promoting substance from worm wood (Artemisia absinthium). Plant Physiol. 66: 246-249. Ref.: https://pubmed.ncbi.nlm.nih.gov/16661414/ DOI: https://doi.org/10.1104/pp.66.2.246
139. Van OKJ, Conklin ME, Blakeslee AF. 1941. Factors in coconut milk essential for growth and development of Datura embryos. Science. 94: 350. Ref.: https://pubmed.ncbi.nlm.nih.gov/17729950/ DOI: https://doi.org/10.1126/science.94.2441.350
140. Vejendla V, Maity PK, Bank BC. 2008. Effect of chemicals and growth regulators on fruit retention, yield and quality of mango cv. Amrapali. Journal of Crop and Weed. 4: 45-46.
141. Went FW. 1926. On growth accelerating substances in the coleoptile of Avenasativa. Proceedings Koninklijke Nederlandse Akademie van Wetenschappen. 30: 10-19.
142. Went FW. 1928. Wuchsstoff und Wachstum. Receuil des Travaux Botaniques Neerlandais. 25: 1-116.
143. Yadav PK. 2002. Effect of urea, borax and NAA on yield parameters of guava (PsidiumguajavaL.) cv. L-49 in rainy season. Progressive Agriculture. 2: 195-196.




















