Indexing & Abstracting
Full Text
ThesisDOI Number : 10.36811/ijpsh.2021.110029Article Views : 42Article Downloads : 32
Storage Temperature and Atmosphere Alleviates Chilling Injury of Marula (Sclerocarya birrea subspecies caffra) Fruit
Agisanyang Tautsagae*, Vallantino Emongor, Seoleseng Tshwenyane and Cornelia Gwatidzo
Department of Crop and Soil Sciences, Faculty of Agriculture, Botswana University of Agriculture and Natural Resources, Private bag 0027, Gaborone, Botswana
*Corresponding Author: Agisanyang Tautsagae, Department of Crop and Soil Sciences, Faculty of Agriculture, Botswana University of Agriculture and Natural Resources, Private bag 0027, Gaborone, Botswana; Email: angwako@buan.ac.bw
Article Information
Aritcle Type: Thesis
Citation: Agisanyang Tautsagae, Vallantino Emongor, Seoleseng Tshwenyane, et al. 2021. Storage Temperature and Atmosphere Alleviates Chilling Injury of Marula (Sclerocarya birrea subspecies caffra) Fruit. Int J Plant Sci Hor. 3: 28-44.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2021; Agisanyang Tautsagae
Publication history:
Received date: 21 May, 2021Accepted date: 25 May, 2021
Published date: 27 May, 2021
Abstract
Low temperature storage is the most effective technology for keeping quality and extending the postharvest life of fresh horticultural produce. However, horticultural produce of tropical and subtropical in origin such as marula fruit are susceptible to chilling injury (CI) when stored at temperatures below their critical minimum temperatures. Therefore, low temperature storage alone is not ideal for produce of tropical and subtropical in origin. The aim of this research was to elucidate the influence of modified atmosphere packaging (MAP) on CI of marula fruits. Storage temperature below 12ºC significantly (P < 0.05) increased CI incidence and severity, and proline content of marula fruit. Marula fruit in MAP had significantly (P < 0.05) lower electrolyte leakage than fruit stored in Air. The results further showed that marula fruit stored at 12?C in MAP had significantly longer shelf-life of 21 days than fruits in Air stored at various temperatures which had a shorter shelf life. It was concluded that marula fruits be stored in MAP at 12°C plus 90-95% RH to alleviate CI incidence and severity and maintain fruit quality and extend shelf-life.
Keywords: Marula Fruit; MAP; Chilling Injury; Proline Content; Electrolyte Leakage
Introduction
Chilling injury (CI) is a storage disorder that occurs at temperatures below the critical threshold but non-freezing temperatures [1,2]. Chilling injury (CI), which is also known as cold injury or cold shock is used to describe physiological damage which is a result of exposing products to low but non-freezing temperatures [3]. This problem limits the use of low temperature to manage postharvest ripening of fruits, because temperatures that are low enough to delay ripening, decay and senescence may also be damaging to the fruit [4].CI is a major problem that affect shelf- life and marketability of both tropical and subtropical fruits such as Marula. Development of CI symptoms reduces customer acceptance and limits the storage life of the fruits. Chilling injury symptoms include skin surface pitting, sunken lesions, skin browning and pulp discoloration [5,6]. Chilling injury causes membrane damage via oxidation of membrane lipids, leading to structural changes and increased membrane permeability [5]. Various strategies that have been used to alleviate CI in fruits include high or low temperature conditioning, intermittent warming, CA storage, applications of growth regulators, hot water treatment (50-55°C) for 3-5 minutes, natural products, wax coating as well as modified atmosphere packaging (MAP) [4,7]. The objective of MAP is to change the concentrations of oxygen and carbon dioxide in the polymetric film and maintain relative humidity of the commodity. The atmosphere in MAP depends on the porosity of the polymetric film, respiration of the produce, and the storage temperature. According to [8], packaging of produce with low density polyethylene film and wrapping with heat-shrinkable film reduced pitting and scald in chilled cucumber. Packaging has also been shown to delay CI in bananas, grapefruit, pineapples, Japanese apricots and lemons [7,8].
Marula (Sclerocarya birrea subspecies caffra) belongs to the family Anacardiaceae. In Botswana it is commonly called morula, but in South Africa it is commonly known as marula (English), moroela (Afrikaans), umGanu (isiZulu), nkanyi (Xitsonga) and mfula (Tshivenda) (45). According to (44), in Kenya it is named mura (Meru) and didisa (Boran). Marula fruits is usually consumed fresh as dessert (42), and can also be used to make marula juice, jam, jelly, atchar, salad dressing as well as for local fermented alcoholic beverage called marula beer or wine (43). There is hardly any literature on storage temperature and atmosphere on CI of marula fruit, except the work of [9], who reported on effects of storage temperature on postharvest quality, ripening and marketability of marula fruits. Although various methods have been shown to alleviate CI symptoms in mango fruits which is in the same family with marula, no studies have been done on the alleviation of CI of marula fruit. Therefore, this study was undertaken to elucidate the influence of MAP on CI of marula fruits.
Materials and Methods
Experimental site
The experiment was conducted in the Department of Crop and Soil Sciences of the Botswana University of Agriculture and Natural Resources (BUAN), between January and May 2020. BUAN is located at Sebele (latitude 24 34 °S and longitude 25 57 °E, altitude of 994 m above sea level), 10 km from city of Gaborone.
Experimental Design
The experiment was set up in a 5x2 factorial in a completely randomized design (CRD) with three treatments, each replicated three times. Storage temperatures at 6, 8, 10, 12 and 25±1ºC and atmosphere (modified atmosphere packaging-MAP and Air-20.9% O2+0.03% CO2) were applied. The fruits were stored at different treatment temperatures and at 25±1 °C room temperature as control and stored either in MAP or Air. Due to the seasonality of the fruits, the experiment was carried out from January- May 2020, when fruits were available in the forests. Fresh marula fruits were collected randomly at physiological maturity from 10 different marula trees around Sebele to make a representative sample. Selective collection of firm, green, uniform fruits free from bruises and defects were judged subjectively based on epidermal fruit colour. Fruits were then washed to remove soil and other external material. Three kilograms (kg) of fruits from a composite sample were packaged in plastic bowls (Airopen) and low density polymeric film or plastics (MAP-3000 ml) and placed in different temperatures as stated above.
Data collection
Data were collected daily for three weeks. The variables determined are indicated below.
Chilling Injury (CI) Incidence
Chilling injury incidence and severity were evaluated daily. The CI incidence was determined from a sample of 10 fruit/replicate/treatment. Fruit showing symptoms of CI were counted and expressed as a percentage.
Chilling Injury Severity
Chilling severity was evaluated on a predetermined scale 0: no injury; 1: slight injury (where 1/3 of the fruit showed some injury symptoms); 2: moderate injuries (where 2/3 of the fruit showed some injury symptoms) and 3: severe injury (determined by more than 67% of the fruit showing injury depending on the peel damage [5]. Chilling injury index was calculated by the following equation: Chilling Injury Incidence (CII)=[(no of fruits with no injury x 0)+(no of fruits with slight injuryx1)+(no of fruits with moderate injury x 2)+(no of fruits with severe injury x 3)]÷no of fruits sampled.
Electrolyte Leakage
Electrolyte leakage was determined according to the method of [10], with slight modifications. Ten discs from the peel and pulp tissue were taken using a 10 mm diameter cork borer, then sample tissues were rinsed with distilled water to eliminate the electrolyte at the cut surface. The samples were placed in a flask containing 25 ml of 0.4 M mannitol. Incubation for 30 minutes at 25?C was done where the electrical conductivity (EC) was measured in a suspending solution with an EC meter as an initial reading. The samples in a flask were reheated at 98°C for 15 minutes and the electrical conductivity re-measured after cooling. Membrane permeability was calculated using the formula given below:
Proline Content
The skin and pulp from 20 fruits marula fruits per replicate were cut and mixed to form a composite sample. Zero point five (0.5g) grams the samples were weighed and homogenized in 10ml of 3% aqueous sulfosalicylic acid. The homogenate was filtered through Whatman filter paper (grade 1). Then 2 ml of the filtrate was reacted with 2ml acid-nihydrin and 2ml glacial acetic acid in a test tube for an hour at 100°C in water bath to develop the colours. Soon after removal from the water bath, the test tube was cooled in an ice bath and proline extracted with 4ml toluene, mixed vigorously with a test tube stirrer for 15-20 seconds. The chromophore containing toluene was aspirated from the aqueous phase, warmed to room temperature and the absorbance was read in a UV 160 IPC spectrophotometer at 520 nm using toluene as a blank. Proline content in marula fruit pulp was determined using the formula given below:
μmole proline/g of fresh weight = (μg proline/ml×ml toluene/115.5 μg/mole)/(g sample) [11].
Weight loss
Marula fruits were weighed before and after storage to determine the percent of the weight loss using the formula below.
Shelf-life
Shelf-life was determined by the number of days’ fruits maintained their fresh physiological status at various storage temperatures and atmospheres until they become unacceptable due to developing physiological disorders.
Data analysis
The data collected was subjected to analysis of variance (ANOVA) using the Statistical Analysis System (SAS). Treatment means were separated using the Least Significant Difference (LSD) at P = 0.05.
Results
Chilling Injury Severity and Incidence
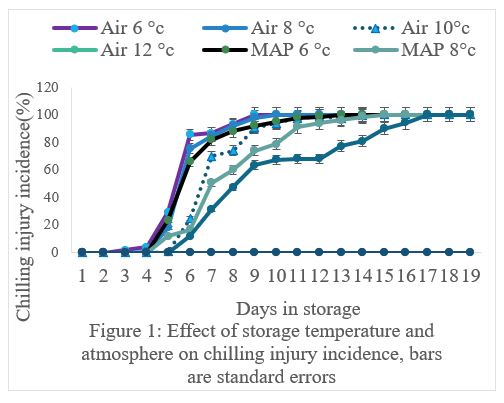
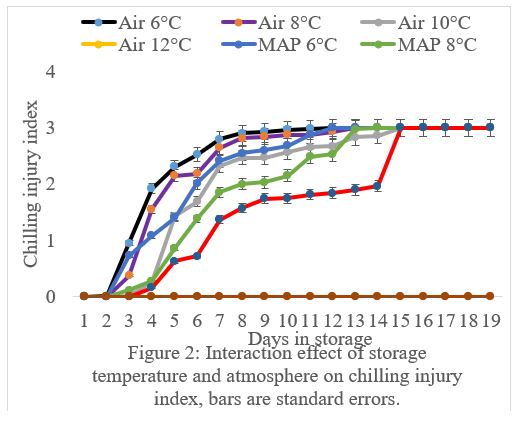
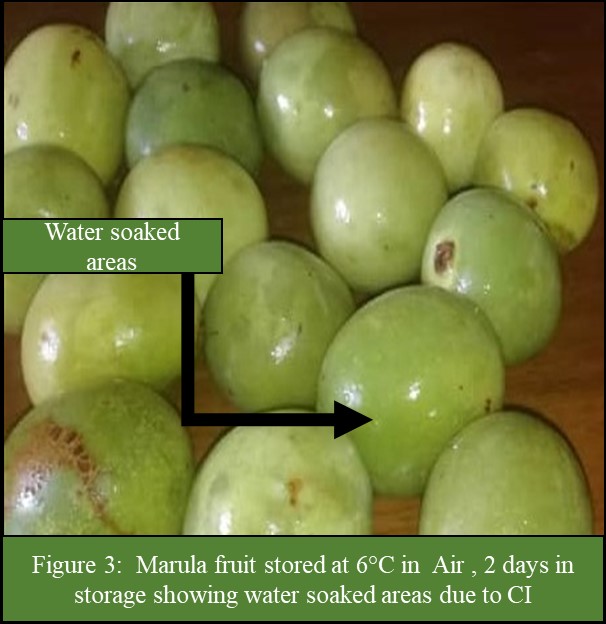
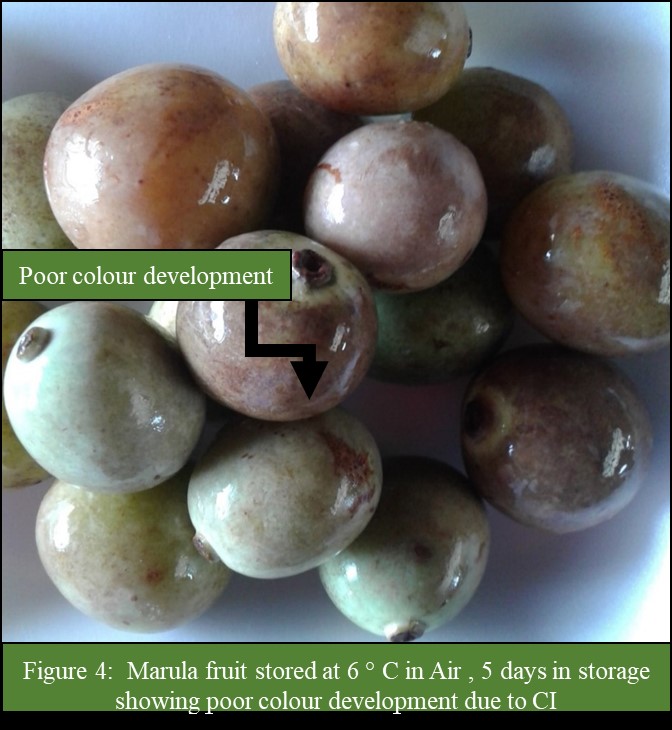
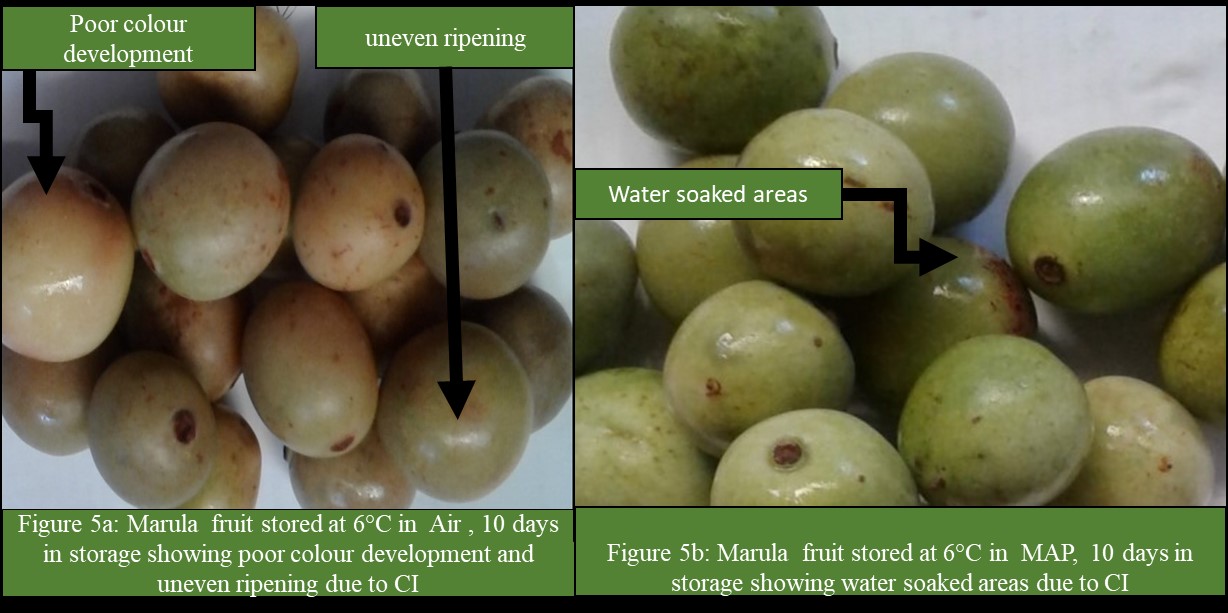
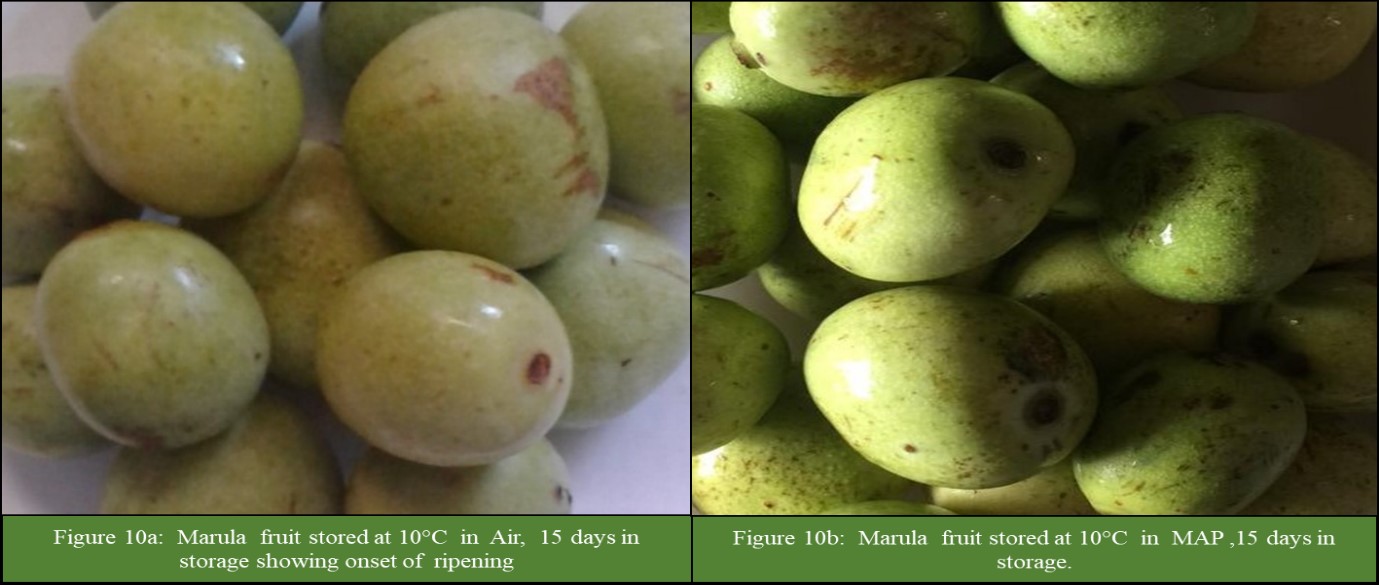
The results showed that there was a significant (P<0.05) interaction between storage temperature and atmosphere on CI incidence and severity (Figure 1 and 2). The CI incidence and severity increased with a decrease in storage temperature, irrespective of storage atmosphere and duration of storage (Figure 1 and 2). At temperatures less than 12°C, the incidence and severity of CI significantly (P<0.05) increased (Figure 1 and 2). Fruit in MAP stored at 6, 8 and 10°C had significantly (P<0.05) lower incidence and severity of CI than fruit stored at the same temperatures but in Air (Figure1 and 2). The CI symptoms observed on marula fruit stored at 6, 8 and 10°C were water-soaked areas, sunken depressions, uneven ripening, and poor colour development (Figure 3, 4, 5a, 5b, 6a, 6b, 7a,7b, 8a, 8b, 9a, 9b, 10a, 10b). The CI symptoms were noticeable on fruits after 10 and 15 days of storage at 6, 8 and 10°C, respectively (Figure 3,4,5a, 5b, 6a, 6b, 7a,7b, 8a, 8b, 9a, 9b, 10a, 10b). Marula fruits stored at 12°C both in Air and MAP did not develop CI (Figure 11).





Proline content
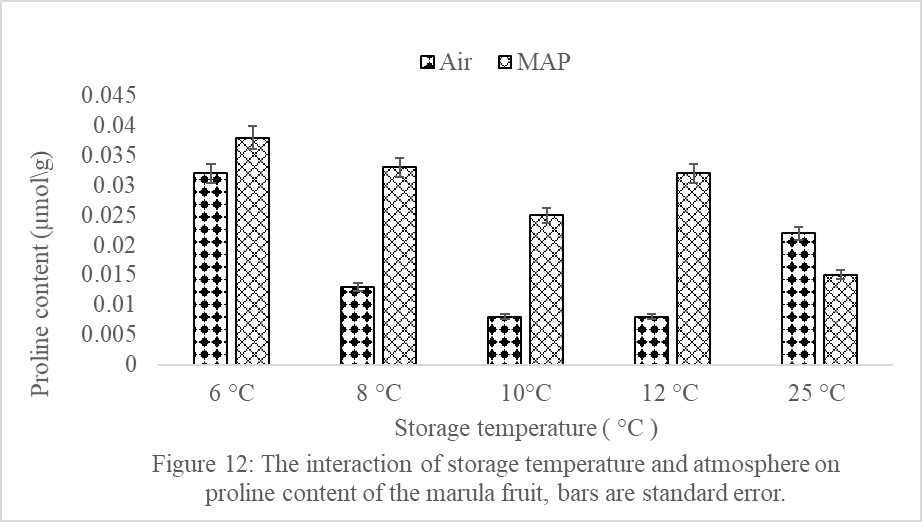
There were no significant (P>0.05) interactions of storage temperature and atmosphere on fruit proline content (Figure 12). Marula fruits in MAP tended to have non-significantly (P>0.05) lower proline content than fruit in Air, irrespective of the storage temperature (Figure 12). Storage atmosphere alone had no influence on fruit proline content. However, storage temperature significantly (P<0.05) affected proline content of marula fruit (Figure 13). As storage temperature decreased, the fruit proline content significantly (P<0.05) increased (Figure 13). Fruits stored at 6°C had the highest proline content of 0.04 μmoles/g, but fruit stored at 8, 10, 12 and 25°C did not significantly (P>0.05) differ in their proline contents (Figure 13). Fruit stored at 6ºC (had severe CI) had significantly (P<0.05) higher proline content than fruit stored at 12 and 25ºC which did not develop CI (Figure 13). The MAP alone had no significant (P<0.05) effect on fruit proline content.

Electrolyte leakage
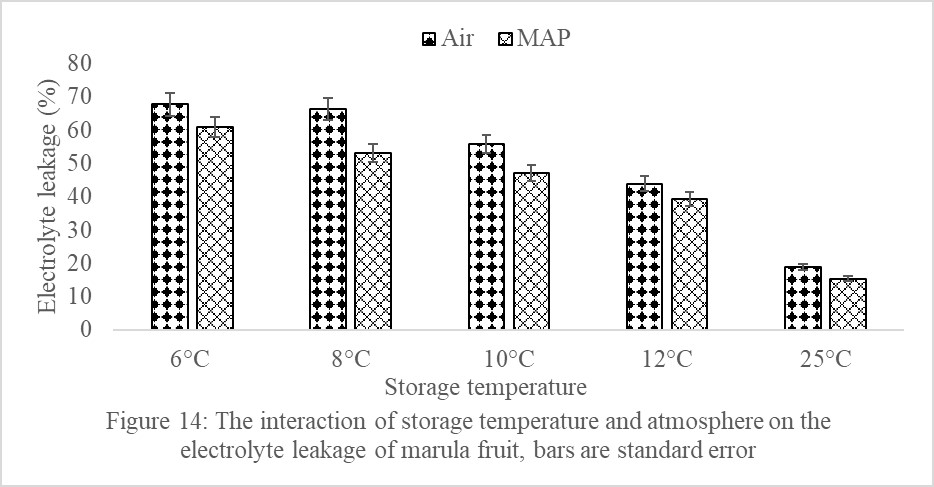
In this study, there was a significant (P<0.05) interaction between storage temperature and atmosphere on fruit electrolyte leakage (Figure 14). Marula fruit in MAP and stored in various temperatures had significantly lower electrolyte leakage than fruit stored in Air, except in fruit stored at 25ºC (Figure 14). Fruit stored in Air and held at 6ºC had the highest electrolyte leakage of 67.7% (Figure 14). The lowest electrolyte leakage was on fruit in MAP but stored at 25ºC (Figure 14).
Weight loss
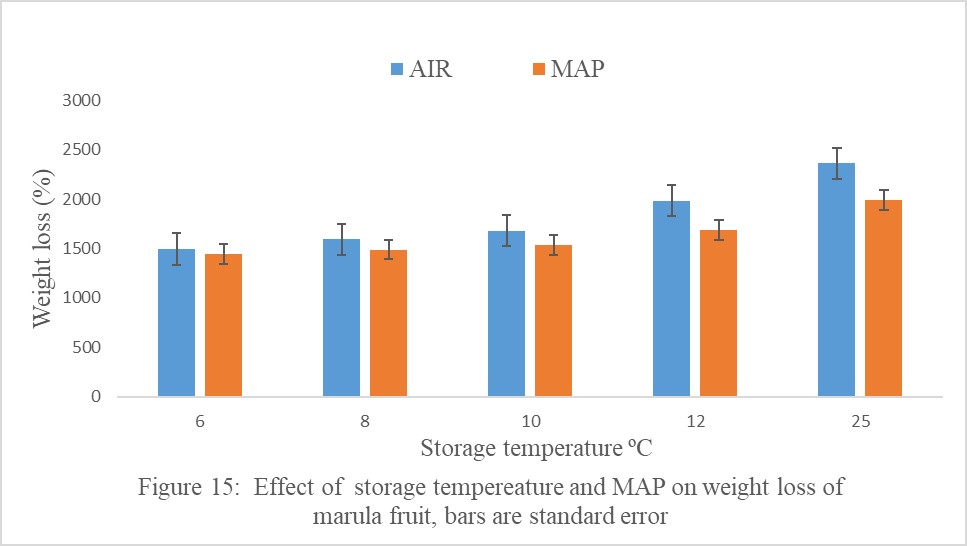
Storage temperature and atmosphere significantly interacted to influence fruit weight loss (Figure 15). Marula fruit in MAP and stored at lower temperatures had significantly lower weight loss than fruit stored in Air but stored at the same temperatures. As storage temperature increased from 6 to 25ºC, fruit weight loss increased irrespective of the storage atmosphere (Figure 15). Fruits in MAP and at 6ºC had the lowest weight loss of 1400 g and fruit stored in Air and stored at 25ºC had the highest weight loss of 2362 g at the end storage life (Figure 15,16).

Shelf- life
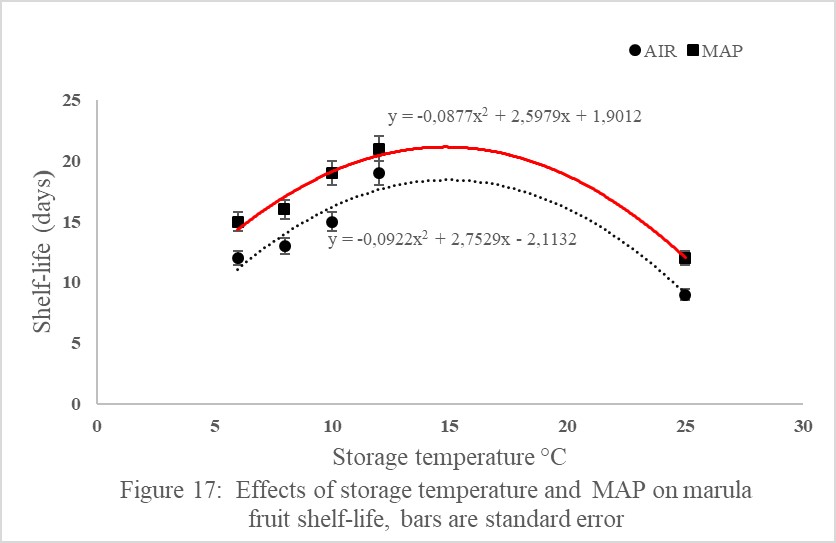
Storage temperature and atmosphere interacted significantly to influence marula fruit shelf-life (Figure 17). Marula fruit stored at 12?C in MAP had significantly higher shelf-life of 21 days than fruits stored in Air stored at 6, 8, 10 and 12?C which had a shelf-life of 12, 13, 15 and 19 days, respectively (Figure 17). Marula fruit held in MAP had significantly higher shelf-life than fruit stored in Air and stored at the same temperatures under study (Figure 17). Marula fruits stored at 25?C as control had the shortest shelf-life of 9 days when stored in Air (Figure 17,18).

Discussion
Chilling Injury and Incidence
The results of the current study showed that as storage temperature decreased below ≤12°C, the incidence and severity of CI increased which was attributed to stress induced by the low temperatures hence leading to the development of CI symptoms such as watersoaked areas, skin pitting, poor colour development and aroma, uneven ripening, and sunken depressions. Results of the current study agree with those of [9] and [12] in marula fruit, [2,13] and [14] in mango fruit, respectively. [15] Reported that storage temperature significantly influenced CI incidence and severity of wild plum (Ximmenia americana L.) fruits. As storage temperature decreased below 15°C, the incidence and severity of chilling significantly increased in wild plum fruits [15]. [9] reported that the optimum temperature for marula fruit storage was 12ºC, below which CI developed. Marula fruits like any other tropical and subtropical fruits, are susceptible to CI when stored at temperature below their critical minimum temperatures [9,16]. Although low temperature storage is considered the most effective method of extending postharvest life and maintaining the quality of most fruits [14,17], low temperature storage may be detrimental to the storage of tropical and subtropical fruits. These problems limit the use of low storage temperature to manage postharvest ripening of chilling sensitive horticultural produce because temperatures that are low enough to delay ripening, decay and senescence may also be damaging to the fruit [2,9].
Various physiological, biochemical alteration and cellular dysfunction occur in chilling sensitive species in response to chilling stress [18]. These alterations include increased membrane permeability and alteration of activities of membrane proteins. If chilling stress is prolonged, these alterations will result in development of chilling injury symptoms such as skin surface pitting, sunken or surface lesions, uneven ripening, pulp discoloration, greyish-scald discoloration of the skin, watersoaking of tissues, off-flavour, susceptibility to fungal decay, reduced aroma and carotenoids [9]. Marula fruits stored at 12°C, in Air or MAP did not develop chilling injury.
In the current study, MAP alleviated CI incidence and severity. This was attributed to the role of plastic film in helping to maintain high relative humidity and modify the concentrations of O2 and CO2 in the atmospheres surrounding the marula fruit. [19,20] reported that the major effects of MAP are the maintenance of high humidity and modification of atmospheric composition surrounding the horticultural produce. The reduction of water loss from the tissues in MAP was reported to inhibit the collapse of epidermal and underlying cells and prevents pitting formation [21]. Packaging with low density polyethylene film has been reported to alleviate CI in cucumber fruit [8]. Wrapping fruit individually in heat-shrinkable film has also been reported to reduce pitting and scalding in chilled grapefruit [22]. The MAP has also been shown to delay CI in bananas, pineapples, Japanese apricots, lemons, and tomatoes [21], loquat [23,24] and litchi fruits [25].
The reduction in CI incidence and severity by MAP observed in the current study could also be attributed to the role of MAP in acting as a barrier to marula fruits hence protecting it from CI. Induction of chilling tolerance by physical treatments or exposure to other stresses such as high and low temperature, becomes a great potential approach for protecting harvested fruit from CI and enhancement of membrane integrity by regulating plasma membrane proteins and lipids [26], improvement of antioxidant system and suppression of reactive oxygen species [27,28], proline accumulation by modulating its synthesis and degradation [29], and maintenance of higher ATP content and energy charge [30] are considered as the mechanisms being involved in the acquisition of chilling tolerance. Dysfunction of cell membrane and excess production of the reactive oxygen species are two primary events involved in CI development [31,32].
Proline Content
As the storage temperature decreased, the proline content in marula fruits increased significantly irrespective of duration of storage. Marula fruits stored at 6?C, had the highest proline content while those stored at 12 and 25?C had the lowest proline content which may be due to lack of chilling stress on the fruit. The high proline content in fruit stored at 6ºC in the current study was attributed to low temperature stress. Proline has been thought to enhance plant and/or plant organ resistance to environmental stresses [33]. Some studies have attributed high proline content in chilled mango fruit to either enhanced protein degradation at chilling temperatures and/or proline synthesis [29,14].
Electrolyte Leakage
In the current study, there was a significant interaction between storage temperature and atmosphere on fruit electrolyte leakage. Marula fruit in MAP and stored in various temperatures had significantly lower electrolyte leakage than fruit stored in Air. Fruit stored in Air and stored at 6ºC had the highest electrolyte leakage of 67.7%. It is reported in literature that MAP could be useful method in controlling CI development in various kinds of fruits [20,34]. Loquat fruit kept in LDPE bags and stored at 1 and 5°C for 30 days had higher fruit quality and lower CI than unwrapped fruit [24]. Litchi fruit packaged in LDPE bags and stored at 13°C for 9 days had higher sugar and organic acids contents and lower browning of pericarps induced by CI than control fruit [25]. The major effects of MAP are the maintenance of high humidity and modification of atmospheric composition [19,20].
Phase transitions and lateral phase separation of membrane lipids are the primary molecular events leading to development of CI symptoms [35]. Evidence for membrane damage and loss of cell membrane integrity as the cause of CI includes increased electrolyte leakage [36]. The stage of fruit ripeness, duration to exposure to low temperatures, storage temperature and their interaction may influence the fruit electrolyte leakage. Membrane damage can be measured by the ion leakage, which in the present study, the electrolyte leakage was significant in marula fruit stored in temperatures of ≤10°C than fruit stored at 25°C. In the current study, electrolyte leakage increased with a decrease in storage temperature from 12 to 6°C. According to [37], electrolyte leakage is a useful index of CI as it is affected by lower storage temperatures.[38] reported that electrolyte leakage was an effective index of CI in grapefruit.
Waxing and coating are a form of MAP widely used in various kinds of fruits during postharvest treatment [20]. Edible coating combined with MAP is beneficial in reducing respiration, delay oxidative reaction and therefore, improved the CI tolerance of apple and banana [39].
Weight loss
Storage temperature and atmosphere significantly interacted to influence fruit weight loss. Marula fruit in MAP and stored at lower temperatures had significantly lower weight loss than fruit in air but stored at the same temperatures. As storage temperature increased from 6 to 25ºC, fruit weight loss increased irrespective of the storage atmosphere. The interaction of low temperature storage and MAP has shown to minimize water loss in fruits and vegetables [40]. Modified atmosphere packaging is reported to affect physicochemical and physiological processes in fruits positively or negatively [40]. Some of the beneficial effects of MAP include delayed ripening by reduced water loss, reduced decay and physiological changes such as oxidation and reduced respiration [41].
Shelf-life
The results of the current study showed that MAP had significantly longer shelf-life of 21 days than fruits in air stored at 6, 8, 10 and 12?C. Marula fruits stored at 25?C as control had the shortest shelf-life of 9 days when stored in Air. The increase in marula shelf-life in MAP irrespective of temperature was attributed to the role of MAP in delayed ripening, reducing transpiration and water loss, reducing softening and compositional changes, reducing decay and physiological changes such as oxidation and reducing respiration [41]. Low storage temperature of 12ºC and MAP prolonged marula shelf-life to 19 days compared to fruit stored in air at 25ºC (9 days) because low temperature lowers respiration rate, decreases sensitivity to ethylene therefore reduced fruit ripening, reduces the growth of rot causing micro-organisms and reduces water loss [9,14].
Conclusion
Postharvest management of marula fruits is important for their successful postharvest storage and maintenance of fruit quality during marketing. This study showed that a combination of low temperature storage of 12°C and MAP were effective in maintaining the quality of marula fruits ripening. Modified atmosphere packaging and storage temperature of 12ºC significantly reduced respiration rate, electrolyte leakage and weight loss, increase fruit shelf-life hence delayed fruit ripening. It was recommended that in order to maintain fruit quality, and extend shelf-life and the marketing period of the fruit; the fruit should be stored in MAP and held at 12°C. It was also recommended that this study be repeated with other MAP technologies such as waxing and low-density polymeric films with different porosities.
Acknowledgements
The authors thank The Botswana University of Agriculture and Natural Resources for the use of their facilities and chemicals for the present research.
References
1. Kader AA, Rolle RS. 2004. The role of post-harvest management in assuring the quality and safety of horticultural produce. 1.
2. Emongor VE. 2015. The effects of temperature on storage life of mango. American Journal of Experimental Agriculture. 5: 252-261.
3. Raison JK, Orr GR. 1990. Proposals for a better understanding of the molecular basis of chilling injury. 145-164.
4. Gross KC, Wang CY, Saltveit M, et al. 2002. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Crops. An Adobe Acrobat pdf of a draft version of the forthcoming revision to U.S. Department of Agriculture, Agriculture Handbook 66 on the website of the USDA, Agricultural Research Service, Beltsville Area.
5. Zhao Z, Jiang W, Cao J, et al. 2006. Effect of cold-shock treatment on chilling injury in mango (Mangifera indica L cv ‘Wacheng’) fruit. Journal of the Science of Food and Agriculture. 86: 2458-2462.
6. Ding ZS, Tian SP, Zheng X L, Zhou ZW, Smith Y et al. 2007. Response of reactive oxygen metabolism and quality in mango fruit to exogenous oxalic acid or salicyclic acid under chilling temperature stress. Postharvest Biology and Technology, 61: 103-109.
7. Wang CY. 1993. Approaches to reduce chilling injury of fruits and vegetables. Horticultural Reviews 15: 63-95.
8. Wang CY, Qi L. 1997. Modified atmosphere packaging alleviates chilling injury in cucumbers. Journal of Postharvest Biology and Technology. 10: 195-200.
9. Emongor VE, Tautsagae A. 2016. Effect of Storage Temperature on Postharvest Quality, Ripening and Marketability of Marula Fruits (Sclerocarya birrea subsp. caffra). British Journal of Applied Science & Technology 14: 1-12.
10. Chan HT JR, Sanxter S, Couey HM. 1985. Electrolyte leakage and ethylene production induced by chilling injury of papayas. HortScience, 20: 1070-1072.
11. Bates LS, Walderen RP, Teare ID. 1973. Rapid determination of free proline for water-stress studies. Plant soil. 39: 205- 207.
12. Nerd A, Mizrahi Y. 1993. Domestication and introduction of marula (Sclerocarya birrea subsp. Caffra) as a new crop for the Negev desert of Israel, pp. 496-499. In: J. Janick and J. E. Simon (Eds.), New Crops, Wiley, New York.
13. Tasneem A. 2004. Postharvest treatments to reduce chilling injury symptoms in stored mangoes. Master of Science Thesis, Department of Bioresource Engineering.
14. Pholoma SB. 2016. Effects of Storage Temperature and Hot Water Treatment Duration on Chilling Injury and Quality of Mango (Mangifera indica L). MSc Thesis, Botswana College of Agriculture, Faculty of Agriculture, Crop Science and Production Department.
15. Emongor VE, Ramagonono G. 2019. Storage temperature influences postharvest quality of wild plum (Ximmenia americana L.) fruit. Ghana Journal of Science. 60: 1- 10.
16. Dube S, Dlamini NR, Shereni I, et al. 2012. Extending the shelf Life of Fresh marula (S. berria): Juice by Altering Its Physico- Chemical Parameters, Biochemical Testing.
17. Ramagonono G. 2018. Effects of storage temperature on postharvest quality of wild plum (Ximenia americana L.). MSc Thesis, Botswana University of Agriculture and Natural Resources, Faculty of Agriculture. 82.
18. Wang CY. 1982. Physiological and biochemical responses of plants to chilling stress. Journal of Horticultural Science. 17: 173-186.
19. Wang CY. 2013. Managing chilling injury in vegetables. Acta Horticulturae. 1012: 1081-1085.
20. Peng J, Cao SF. Zheng, YH, et al. 2013. Managing chilling injury in fruits. Acta Horticulturae. 1012: 1087-1095.
21. Wang CY. 2010. Alleviation of chilling injury in tropical and subtropical fruits. Acta Horticulturae. 864: 267-273.
22. Miller WR, Chun D, Risse LA, et al. 1990. Conditioning of florida grapefruit to reduce peel stress during low-temperature storage. Horticultural Science. 25: 209-211.
23. Zheng YH, Xi YF. 2000. Study on the effects of the modified atmosphere packaging on loquat fruit. Food Science. 21: 56-58.
24. Ding CK, Wang CY, Gross KC, Smith DL, et al. 2002a. Jasmonate and salicylate the expression of pathogenesis-related protein genes and increase resistance to chilling in tomato fruit. Planta. 214: 895-901.
25. Somoboonkaew N, Terry LA. 2010. Physiological and biochemical profiles of imported litchi fruit under modified atmosphere packaging. Postharvest Biology and Technology. 56: 246-253.
26. Li BQ, Zhang CF, Cao BH, et al. 2012. Brassinolide enhances cold stress tolerance of fruit by regulating plasma membrane proteins and lipids. Amino Acids. 43: 2469- 2480.
27. Aghdam MS, Asghari MR, Moradbeygi H, et al. 2012. Effects of postharvest salicyclic acid treatment on reducing chilling injury in tomato. Romanian Biotechnological Letters. 17: 7466-7474.
28. Chen BX, Yang HQ. 2012. 6- Benzylaminopurine alleviates chilling injury of postharvest cucumber fruit through modulating antioxidant system and energy status. Journal of the Science of Food and Agriculture.
29. Cao S, Cai Y, Yang Z, et al. 2012. MeJA induces chilling tolerance in loquat fruit by regulating proline and γ-aminobutyric acid contents. Food Chemistry. 133: 1466-1470.
30. Zhu MY, Bai H, Liang LS, et al. 2012. Mechanism of energy metabolism on cold acclimation treatment for alleviating chilling injury of peach fruit during low temperature storage. Transactions of the Chinese Society of Agricultural Engineering, 28: 257-264.
31. Chongchatuporn U, Ketsa S, van Doorn WG, et al. 2013. Chilling injury in mango (Manngifera indica) fruit peel: relationship with ascorbic acid concentrations and antioxidant enzyme activities. Postharvest Biology and technology, 86: 409-417.
32. Luo Z, Wu X, Xie Y, Chen C, et al. 2012. Alleviation of chilling injury and browning of postharvest bamboo shoot by salicyclic acid treatment. Food Chemistry. 131: 456- 461.
33. Kinnersley AM, Turano FJ. 2000. Gamma aminobutyric acid (GABA) and plant responses to stress. Critical Review in Plant Science 19: 479-509.
34. Bodbodak S, Moshfeghifar M. 2016. Advances in modified packaging of fruits and vegetables. In: S. Bodbodak (Ed.). 127- 183.
35. Mirdehghan SH, Rahemi M, Martinez- Romero D, et al. 2007. Reduction of pomegranate chilling injury during storage after heat treatment: role of polyamines. Postharvest Biology and Technology 44: 19-25.
36. Junmatong C, Uthaibutra J, Boonyakiat D, et al. 2012. Reduction of chilling injury of ‘Nam Dok Mai No.4’ mango fruit by treatments with salicyclic acid and methyl jasmonate. Journal of Agricultural Science. 4: 126-136.
37. Forney CF, Lipton WJ. 1990. Influence of controlled atmospheres and packaging on chilling sensitivity. 257-268.
38. McCollum TG, McDonald RE. 1991. Electrolyte leakage, respiration, and ethylene production as indices of chilling injury in grapefruit. HortScience. 26: 1191- 1192.
39. Vilas-Boas EV, Kader AA. 2006. Effect of atmospheric modification, 1-MCP and chemicals on quality of fresh-cut banana. Postharvest Biology and Technology. 39: 155-162.
40. Valero D, Serrano M. 2010. Postharvest biology and technology for preserving fruit quality.162-173.
41. Kader AA. 2013. Postharvest technology of horticultural crops - An overview from Farm to Fork, 1: 1-8.
42. Mojeremane W, Tshwenyane SO. 2004. The resource role of morula (Sclerocarya birrea): A multipurpose Indigenous Fruit Tree of Botswana. Journal of Biological Sciences. 4: 771-775.
43. Colin L 2007. Marula tree varied attributes. Britannica online encylopedia.
44. Mateke SM 1995.The horticultural annual report. Veld Products Research and Development. Gaborone. Botswana. 1-38.
45. Venter F, Venter J. 1996. Making the most of indigenous trees. Briza, Pretoria. Part of the South African National Biodiversity Institutes plant information website.




















