Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ijpsh.2021.110027Article Views : 120Article Downloads : 96
Phytochemical Screening and Mass Spectrometric Analysis to Unveil the Pharmacological Properties of Weed Euryops pectinatus
Anju Meshram
Department of Biotechnology, Kalinga University, Naya Raipur, Chhattisgarh, India
*Corresponding Author: Dr. Anju Meshram, Assistant Professor, Department of Biotechnology, Kalinga University, Kotni, Naya Raipur-490121, Chhattisgarh, India, Tel: +91-7740962170; Email: anju.meshram@kalingauniversity.ac.in
Article Information
Aritcle Type: Research Article
Citation: Anju Meshram. 2021. Phytochemical Screening and Mass Spectrometric Analysis to Unveil the Pharmacological Properties of Weed Euryops pectinatus. Int J Plant Sci Hor. 3: 13-19.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2021; Anju Meshram
Publication history:
Received date: 10 May, 2021Accepted date: 18 May, 2021
Published date: 22 May, 2021
Abstract
Euryops pectinatus ‘Viridae’ weed belonging to the family Asteraceae is commonly known as Golden Euryops or African daisy. Present investigation includes phytochemical analysis of methanol extract of Euryops pectinatus weed for the presence of various secondary metabolites. Presence of various secondary metabolites was observed including phenols, flavonoids, alkaloids, steroids, saponins, glycosides, etc. These secondary metabolites are well known for their biological activities like antioxidation, anti-cancerous, anti-microbial, cardiovascular, and anti-aging. After determination of rich secondary metabolites in the methanol extract, mass spectrometric analysis was carried out for the identification of compounds present in Euryops pectinatus. Eight compounds were identified in the methanol extract reported for nematicide, antioxidant, analgesic, antitumor, anticancerous and several other important activities. Thus phytochemical and mass spectrometric analysis would open new doors to natural product research that would be cost effective and safe for the mankind.
Keywords: Euryops Pectinatus; Phytoconstituents; Bioactives; Mass-Spectroscopy; Anticancer
Introduction
Weeds compete with crops for sunlight, water, nutrients, and space. In addition, they harbor insects and pathogens, which attack crop plants. Furthermore, they destroy native habitats, threatening native plants and animals [1]. Euryops pectinatus ‘Viridae’ belonging to the family Asteraceae is a weed and grows easily in full sun on well drained soil. Due to its spreading nature, it affects the growth of other agricultural crops by utilizing available nutrition and space. However, special attention is given nowadays to unwanted weeds as they possess some special properties that make them grow easily under the abiotic stress conditions. Plants are a rich source of phytoconstituents that provide unlimited opportunities for the development of new drug leads. Therapeutic drugs identified from natural products are now acknowledged throughout the world.
Weeds are a serious problem in agriculture and tremendously reduce the productivity of agricultural land by competing with crop plants for space and nutrition. Raipur District is highly occupied with weeds belonging to Asteraceae family, of which several are reported to have medicinal value. Natural products from plants provide unlimited opportunities for new drug leads thus the demand for therapeutic drugs from natural products has grown throughout the world. To overcome the problem of growing pest in agricultural fields and waste lands, and to utilize the locally available flora of the state, it is proposed to identify the bioactive metabolites of these unwanted crop plants and opening new doors for their pharmacological applications. In this proposed work new method is designed using the existing information concerning the use of Asteraceae weeds of Raipur region for identification of their bioactive metabolites that will be cost effective and safe for pharmacological applications.
Plant extracts are rich source of alkaloids, steroids, terpenes, antioxidant phenolics, flavonoids and other biologically active compounds. In modern medicine these compounds have been investigated for their medicinal applications [2]. Botanicals and herbal preparations for medicinal usage contain various types of bioactive compounds [3]. Natural bioactive compounds like phenols and flavonoids are the important secondary metabolites in plants and posses biological properties like antioxidant, anti-aging, anti-carcinogen, protection from cardiovascular, immune/autoimmune diseases and brain dysfunctions viz. Parkinson’s, Alzheimer’s, Huntington’s diseases, etc [4,5]. These active metabolites especially from herbs are the interest subject of research, and their phytochemical or biological investigations open new doors to the natural product research. The aim of present is to analyze the phytoconstituents and bioactive metabolites of Asteraceae weeds collected from the Raipur region with the objective of characterization of secondary metabolites using mass spectrometry.
Euryops pectinatus is endemic to rocky, sandstone slopes in the Western Cape of South Africa [6]. Chhattisgarh is commonly known as Bowl of rice, and in a study of the weed flora of dry land crops in the Chunkatta and Bhilai areas, Asteraceae was found to be second largest in species distribution, most of which may possess useful properties [7]. Another study have shown that highest number of weeds present in Durg district of Chhattisgarh state belongs to Acanthaceae, Asteraceae, Malvaceae and so on [8].
Materials and Methods
Collection and preparation of plant material
Plant material was collected from Raipur and identified as Euryops pectinatus ‘Viridae’ commonly known as Golden euryops. The samples were washed thoroughly in running tap water to remove soil particles and other adhered debris and finally washed with sterile distilled water. The whole plant was air dried for 24 hours and further used for extraction.
Plant sample extraction
Fresh leaves of Euryops pectinatus ‘Viridae’ (10g) were shade dried for 3-4 days and crushed using mortar-pestle. Extraction was done with methanol thrice for three consecutive days at 28oC (100 ml x 3). The extract was filtered twice with Whatman filter No. 1. Extract was combined and concentrated at 40oC in water bath. The crude methanol extract of E. pectinatus was stored at 8oC for further analysis.
Qualitative phytochemical analysis
Euryops pectinatus ‘Viridae’ methanol extract were tested for the presence of different classes of compounds [9-11]. Qualitative chemical test were used regarding the nature of phytoconstituents present in the methanolic extract (Table 1).
|
Table 1: Phytochemical analysis of explants of Golden Euryops. |
|
|
Compounds |
Test |
|
Alkaloids |
Dragendroff’s Test |
|
Glycosides |
Salkowski’s Test |
|
Polyphenols and Tannins |
Ferric Chloride Test |
|
Flavonoids |
Lead acetate Test |
|
Proteins |
Ninhydrin Test |
|
Carbohydrates |
Molisch’s Test |
|
Saponins |
Foam Test |
|
Steroids |
Steroids Test |
|
Quinones |
Quinones Test |
|
Coumerin |
Coumerin Test |
|
Terpenoids |
Salkowski Test |
|
Phlobatannins |
Phlobatannins Test |
|
Phytosterol |
Libermann’s Test |
Gas Chromatography- Mass Spectrum analysis (GC-MS)
Characterization of crude methanol extract of Euryops pectinatus ‘Viridae’ was done by Gas Chromatography- Mass Spectrometry. GC-MS was recorded in a GCMS-2010 Shimadzu instrument operating in EI mode at 70ev. A Restek-5MS column (30m x 0.25mm x 0.25μm) was used. The oven temperature program was 1000 to 2500C at 50C min-1 and held for 5 min at 2500C and from 2500C to 2800C at 100C min-1 and held for 10 min at 2800C. The injector temperature was 2500C with normal injection mode. The flow rate of carrier gas helium was 1.21ml min-1. The identification of methanol extract was confirmed by comparing the mass spectral data with those of authentic compounds and with data obtained from the literature.
Identification of components
Interpretation on mass spectrum GC-MS was conducted using the database of National Institute Standard and Technology (NIST) having more than 62,000 patterns. The spectrum of the unknown component was compared with the spectrum of the known components stored in the NIST library. The name, molecular weight and structure of the components of the test materials were ascertained.
Results and Discussion
The preliminary phytochemical screening of the methanolic extract of leaves of Euryops pectinatus ‘Viridae’ showed the presence of various secondary metabolites of which alkaloids, flavonoids, terpenoids, saponin glycosides, tannin and phenolics compounds were the most prominent (Table 2).
|
Table 2: Preliminary phytochemical analysis of the methanol extract of Golden Euryops. |
|
|
Compounds |
Golden Euryops (MeOH) |
|
Alkaloids |
+++ |
|
Glycosides |
++ |
|
Polyphenols and Tannins |
+++ |
|
Flavonoids |
+++ |
|
Proteins |
+ |
|
Carbohydrates |
++ |
|
Saponins |
+ |
|
Steroids |
++ |
|
Quinones |
- |
|
Coumerin |
+ |
|
Terpenoids |
++ |
|
Phlobatannins |
- |
|
Phytosterol |
+ |
|
+++ Maximum, ++ Moderate, + Least presence, -Absence of the compound |
|
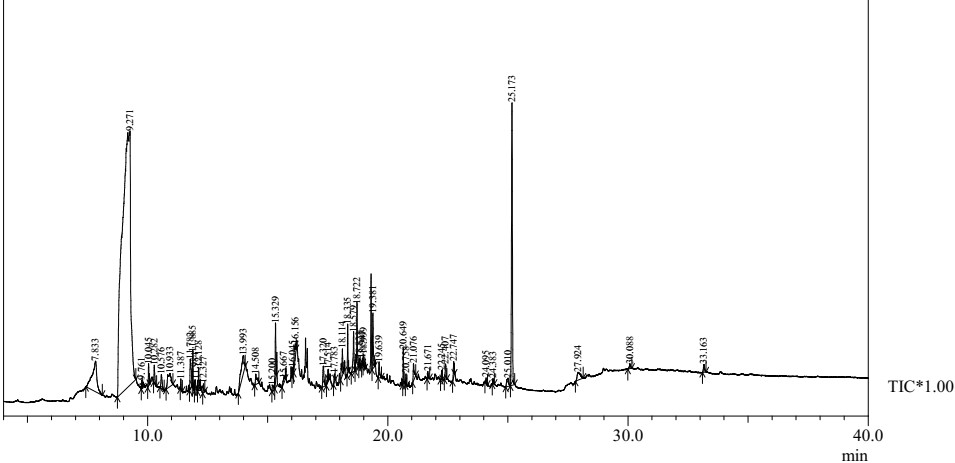
Figure 1: Mass spectrometric analysis of methanol extract of Euryops pectinatus ‘Viridae’.
|
Table 3: Bioactive compounds identified in the methanol extract of leaves of Golden Euryops. |
|||||
|
S. No. |
R. Time |
Name |
Mol. Formula |
Mol. Weight |
Area% |
|
1 |
10.576 |
1,4-Diacetyl-3-acetoxymethyl-2,5-methylene-l-rhamnitol |
C14H22O8 |
318 |
0.53 |
|
2 |
12.870 |
7,7A-dimethyl-3A,4,5,7A-tetrahydro-3H-benzofuran-2-one |
C10H14O2 |
166 |
1.91 |
|
3 |
15.667 |
Propionamide, N-(3-fluoro-2-methylphenyl)-3-(4-fluorophenyl) |
C16H15F2NO |
275 |
0.30 |
|
4 |
16.217 |
Tau-Muurolol |
C15H26O |
222 |
0.76 |
|
5 |
16.580 |
Patchoulane |
C15H26 |
206 |
1.06 |
|
6 |
18.722 |
Aniline, N-(2-(diethylamino)ethyl)-2,4-dinitro- |
C12H18N4O4 |
282 |
1.64 |
|
7 |
25.010 |
Morpholine, 4,4'-(phenylmethylene)bis |
C15H22N2O2 |
262 |
0.49 |
|
8 |
25.173 |
1,2-benzenedicarboxylic acid, bis(2-ethylhexyl) ester |
C24H38O4 |
390 |
8.00 |
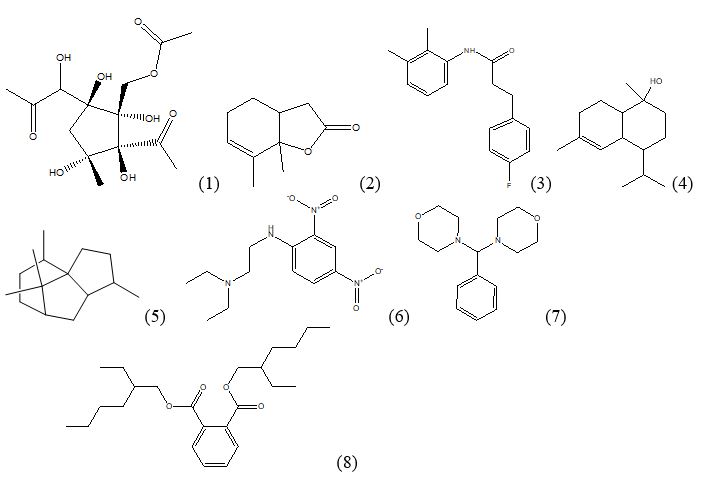
Figure 2: Compounds identified in the methanol extract of leaves of Euryops pectinatus ‘Viridae’ by GC-MS.
|
Table 4: Biological activity of the compounds identified in the methanol extract of leaves of Golden euryops. |
|
|
Name |
Activity* |
|
1,4-Diacetyl-3-acetoxymethyl-2,5-methylene-l-rhamnitol |
Anticancer (oral), Antitumor (Ovary), Inhibit Production of Tumor Necrosis Factor |
|
7,7A-dimethy-3A,4,5,7A-tetrahydro-3H-benzofuran-2-one |
Anti-HIV Integrase, Antidote (Hydrazine, Heavy Metals. Hypoglycin A) |
|
Propionamide, N-(3-fluoro-2-methylphenyl)-3-(4-fluorophenyl |
Antitumour, Anaphylactic, Increase NK cell activity, Narcotic, Nematicide, Neuroinhibitor, Neuroprotective, Neurostimulant, Neurosedative |
|
Tau-Muurolol |
Anticancer (skin, stomach), Antidiabetic, Antidote (snake venom, strychnine, Salicylate), Antioxidant synergist, Antitumor (Skin, stomach) |
|
Patchoulane |
Anti-HIV Integrase, Antidote (Hydrazine), Anaphylactic, Analgesic, Antiaging, Anti-IgE, Antiacne, Anticancer |
|
Aniline, N-(2-(diethylamino)ethyl)-2,4-dinitro- |
Nematicide, Anaphylactic, Antitumor , Increase NK cell activity, Narcotic |
|
Morpholine, 4,4'-(phenylmethylene)bis |
Increase SOD Activity, Disinfectant, Antidote (Diazepam, Digoxin) |
|
1,2-benzenedicarboxylic acid, bis(2-ethylhexyl) ester |
Arachidonic acid- Inhibitor |
|
*Source: Dr. Duke’s Phytochemical and Ethnobotanical Databases (online database). |
|
For the pharmacological as well as pathological discovery of novel drugs, the essential information regarding the chemical constituents are generally provided by the qualitative phytochemical screening of plant extract [12]. The spectrum for the phytocomponents of the methanolic leaf extract of Golden euryops was determined using GC-MS (Figure 1).
Eight compounds are reported to have biological activity (Figure 2, Table 4). The activity prediction is based on Dr. Duke’s Phytochemical and Ethnobotanical databases and available literature. Compound 1,4-Diacetyl-3-acetoxymethyl-2,5-methylene-l-rhamnitol is also reported in the methanol extract of Aspergillus terreus that showed antibacterial and anti-fungal activity [13]. Similarly another compound a Patchoulane?type Sesquiterpene, 6?Acetoxy Cyperene isolated from Cyperus rotundus rhizomes is reported to induce caspase?dependent apoptosis in human ovarian cancer cells [14].
Conclusion
In the present study, methanolic extract of Euryops pectinatus ‘Viridae’ commonly known as Golden euryops, a weed was analysed using mass spectrometry. Through these results, we can conclude that E. pectinatus can be exploited to isolate and characterize important bioactive compounds for pharmaceutical and industrial applications. Several phytochemicals from plants have recently attracted a great deal of attention, mainly due to their role in anticancerous, antimicrobial, antioxidant properties etc. At present there are so many economically important plant species that are being utilized for their important secondary metabolites. Plants contain secondary metabolites meant for defense against various pathogens. Identification of local weeds belonging to Asteraceae family containing bioactive potential against microorganisms, cancer, etc would open new doors in the field of pharmacology, as use of plants are safe and cost-effective.
Acknowledgement
The author thanks the Kalinga University Authorities for providing necessary lab facilities for carrying out the work.
References
1. Chauhan BS. 2020. Grand challenges in weed management. Front Agro. 1: 3.
2. Pillay P, Maharaj VJ, Smith PJ. 2008. Investigating South African plants as a source of new antimalarial drugs. J Ethnopharm. 119: 438-454. Ref.: https://pubmed.ncbi.nlm.nih.gov/18687395/ DOI: https://doi.org/10.1016/j.jep.2008.07.003
3. Sasidharan S, Chen Y, Saravanan D. 2011. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr J Tradit Complement Altern Med. 8: 1-10. Ref.: https://pubmed.ncbi.nlm.nih.gov/22238476/
4. Lai H, Singh NP. 2006. Oral artemisinin prevents and delays the development of 7, 12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer in the rat. Cancer Lett. 231: 43-48. Ref.: https://pubmed.ncbi.nlm.nih.gov/16356830/ DOI: https://doi.org/10.1016/j.canlet.2005.01.019
5. Sun J, Chu YF, Wu XZ. 2002. Antioxidant and antiproliferative activities of fruits. J. Agri. Food Chem. 50: 7449-7454. Ref.: https://pubmed.ncbi.nlm.nih.gov/12452674/ DOI: https://doi.org/10.1021/jf0207530
6. Turner S. 2001. Euryops pectinatus. SANBI, Plantz Africa.
7. Shrivastava AK, Tikariha A, Patra S. 2014. Seasonal and Floristic Biodiversity of Weeds growing in Chunkatta and Bhilai area of Chhattisgarh, India. Int J Curr Microbio Appl Sci. 3: 318-326.
8. Tikariha A, Shrivastava AK, Patra S. 2016. Phytosociological analysis of weeds in Durg district of Chhattisgarh. IOSR J Env Sci Toxicol Food Tech. 10: 14-21.
9. De S, Dey YN, Ghosh AK. 2010. Phytochemical investigation and chromatographic evaluation of the different extracts of tuber of Amorphaphallus paeoniifolius (Araceae). Int J Pharma Biomed Res. 1: 150-157.
10. Evans WC, Trease. 2002. GE Trease and Evans pharmacognosy. WB Saunders, China. 193-407.
11. Kokate CK, Purohit AP, Gokhale SB. 2003. Pharmacognosy, 22nd ed. Pune, Nirali Prakashan. 109-257.
12. Mukherjee PK. 2002. Quality control of Herbal Drugs: An approach of evaluation of Botanicals. Business Horizons pharmaceuticals publishers: New Delhi.
13. Mohammed GJ, Kadhim MJ, Hussein HM. 2016. Characterization of bioactive chemical compounds from Aspergillus terreus and evaluation of antibacterial and antifungal activity. Int J Pharmacog Phytochem Res. 8: 889-905.
14. Ahn JH, Lee TW, Kim KH. 2015. 6?Acetoxy Cyperene, a Patchoulane?type sesquiterpene isolated from Cyperus rotundus rhizomes induces caspase?dependent apoptosis in human ovarian cancer cells. Phytother Res. 29: 1330-1338. Ref.: https://pubmed.ncbi.nlm.nih.gov/26062076/ DOI: https://doi.org/10.1002/ptr.5385




















