Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ijpsh.2020.110024Article Views : 31Article Downloads : 11
Vegetative growth of Cannabis sativa L. cultivars in Jamaica using 18/6 photoperiod
Machel A. EMANUEL*, Valrick V. HENRY and Dwight E. ROBINSON
Department of Life Sciences, Faculty of Science and Technology, The University of the West Indies, Mona Campus, Kingston 7, Jamaica
*Corresponding Author: Machel A. Emanuel, Life Science Cannabis Research Group, Department of Life Sciences, Faculty of Science and Technology, The University of the West Indies, Mona campus, Kingston 7, Jamaica, Email: machel.emanuel02@uwimona.edu.jm
Article Information
Aritcle Type: Research Article
Citation: Machel A. EMANUEL, Valrick V. HENRY, Dwight E. ROBINSON. 2020. Vegetative growth of Cannabis sativa L. cultivars in Jamaica using 18/6 photoperiod. Int J Plant Sci Hor. 2: 56-64.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2020; Machel A. EMANUEL
Publication history:
Received date: 02 April, 2020Accepted date: 16 April, 2020
Published date: 18 April, 2020
Abstract
Geographic isolation coupled with human selection of Cannabis sativa L. has given rise to the subspecies Cannabis sativa L. subspecies sativa and indica. Plant breeders have cross-pollinated the two subspecies sativa and indica which are considered to be Cannabis hybrids. The therapeutic and medical values of these subspecies are distinct in their applications for a cannabis consumer. The photo sensitive nature of cannabis allows for the manipulation of extending the vegetative cycle for cannabis cultivars that are not native to an equatorial climate. With the use of supplemental lighting to achieve a photo-period of 18/6 in Jamaica, the objective of the experiment is to determine which of the subspecies produces more biomass production vegetating under the same growing conditions in Jamaica. The data shows that despite the landrace sativa subspecies having the lowest leaf area index of 83.3 mm2, the photosynthetic capability produced an average height of 168.7 cm, 13.7 branches and129 leaves compared to a height of 143 cm,8.9 branches and 64 leaves for indica and a height of 132.2 cm, 6.6 branches and 66 leaves for the hybrid cultivar resulting in significant differences (p < 0.05) being observed among cultivars. Therefore, cultivating sativa type cultivars in Jamaica and tropical regions may require less energy input in a shorter time frame to achieve greater biomass output during the vegetative cycle, which may give rise to the possibility of harvesting greater yields with less inputs for the Tropical Ganja Farmer.
Keywords: Cannabis sativa; Indica; Hybrid; landrace; Jamaica
Introduction
There is still a level of ambiguity surrounding the classification of Cannabissativa L. from its medical applications, classical botanical taxonomy, and chemotaxonomy, DNA profiling and legal forms [1]. After the formal description of Cannabis sativa Linnaeus in 1753, Lamarck in 1785 published the description of a different species, Cannabis indica Lam. that exhibited different morphological characteristics. According to [2,3] who described Cannabis sativa L. as tall branched plants, used mainly for fiber, seeds and as an intoxicant, while Cannabis indica Lam. is a short, densely branched plant with firm stem, broad leaflets and high psychoactive capabilities. The recognition of Cannabis sativa L. and Cannabis indica Lam. as separate subspecies is primarily based on allozyme allele frequencies, morphological differences, different geographic ranges, and wild populations have been found within the indigenous range of Cannabis, central Asia, the northern Himalayas and western China [4]. Cannabis is now distributed worldwide from the equator to about 60° North latitude, and throughout much of the southern hemisphere [5]. The diversification of Cannabis occurred centuries ago where landrace variants became isolated in geographic regions and selected for certain traits by human intervention. The northern landraces are usually smaller in stature and earlier maturing than the landraces from more equatorial environments that tend to be taller in stature and matures over a longer time span when compared to northern landraces [6]. These diverse habitats have conditioned a colorful and flavorful array of Cannabis varieties. In the 1970s and 1980s when prohibition peaked globally and Cannabis cultivation was driven further underground which led to the advent of hybridized cultivars. The cross-fertile nature is responsible for the inter-mixing of global indigenous cultivars that are responsible for the resin-coated inflorescences of the indica to the enjoyable uplifting cerebral effects of sativa cultivars. The cross-pollinating of indica and sativa type cultivars has given rise to hybrid cultivars where they are perceived to bring out the best of both worlds within a single cultivar. Cannabis breeders have developed a virtually limitless selection of cultivars to choose from [7]. Jamaica amended the Dangerous Drugs Act in 2015 which saw introduction of several changes to the law as it relates to cannabis. The Cannabis Licensing Authority (CLA) was established for the purpose of enabling a lawful, regulated cannabis industry for medical, therapeutic or scientific purposes [8]. The island of Jamaica, known globally for its ganja culture now possesses the opportunity to construct a legal cannabis industry by cultivating and processing the raw materials into value-added commodities. Choosing the cultivars best suited for Tropical cultivation in Jamaica could see sustainable agriculture production by optimizing the use of non-renewable energy and natural resources by integrating natural biological systems for the benefit of achieving greater economic returns [9]. The objective of the experiment was to determine which of the subspecies can produce greater biomass under the same growing conditions and vegetation period in Jamaica’s equatorial climate.
Materials and Methods
Source of planting material
Three cannabis cultivars were selected for germination (indica, sativa and hybrid). The indica, Big Bud seeds were acquired from seed company Sensi Seeds, Oudezijds Achterburgwal 150, 1012DV Amsterdam, The Netherlands [10]. The hybrid, Sugar Pine seeds were acquired from the seed company Flying Dutchman Gardens, Oudezijds Achterburgwal 131, 1012DT, Amsterdam, The Netherlands [11]. The landrace sativa is an heirloom cultivar collected in the Commonwealth of Dominica from a traditional ganja farmer located in the Jaco Flats region of Belles. After the successful germination of 5 seeds per cultivar, the seedlings were transplanted into 6-inch plastic pots with a commercial germination mix (Lambert LM-18 Organic Germination Mixcomprises of sphagnum peat moss, calcitic limestone, dolomitic limestone, organic wetting agent and organic slow release fertilizer). The seedlings were exposed to Hydrofarm FLT44 System Fluorescent Grow Light, with lumens of up to 10,000 lux for 18 hours per day. The seedlings were watered regularly and fertilized with (seaweed pellets 12-6-12) compost tea at pH 6.5, 1.5 ppm and allowed to grow in the vegetative state for a period of 10 weeks with pruning of the apical shoots every two weeks to increase lateral growth. Subsequently, the 15 plants, 5 each from the three cultivars were placed in a flowering room at 18 -27 °C and relative humidity of 40-50%where they were exposed to 12 hrs of Vivosun 1000-Watt High Pressure Sodium Grow Light Blub High fitted Vivosun 1000-Watt UL Listed Dimmable Electronic Digital Ballast for a period of 2 weeks to determine the sex of the plants.
Asexual propagation
Once the sex of the plants was determined the male plants and hermaphrodites were culled. One female plant was then selected from each cultivar and placed under hydro farm FLT44 System Fluorescent Grow Light, with lumens of up to 10,000 lux for 18 hours per day where 15 cuttings were removed from each plant for asexual propagation. A total of 45 cuttings were exposed to hydro farm FLT44 System Fluorescent Grow Light, with lumens of up to 10,000 lux for 18 hours per day, allowed to develop roots aeroponically for 10 to 14 days and harden for 14 days until 4 new apical nodes are wproduced.
Vegetative growth
Upon successful root development, the 45 asexually propagated plants from 3 cultivars were transplanted into 6-inch plastic pots and placed in green house for 2 weeks exposed to hydro farm FLT44 System Fluorescent Grow Light, with lumens of up to 10,000 lux for 18 hours per day until four new were produced. Subsequently, the 45 asexually propagated plants were transferred to 14-inch plastic pots with (Lambert AFM-3 All Purpose Mix comprises of sphagnum peat moss, calcitic limestone, dolomitic limestone, organic wetting agent and organic slow release fertilizer), placed in the greenhouse (temperature 25-36 °C and 60-75 % relative humidity) where they were exposed to 18 hours of light per day (11 hours of natural light and 8 hours of Vivosun 600-Watt Metal Halide Grow Light Blub High fitted Vivosun 600-Watt UL Listed Dimmable Electronic Digital Ballast, natural light 6am to 4pm and Metal Halide supplemental lighting from 4pm to 12pm) for a period of 6 weeks. The plants were all fertilized with 2000 ml of (seaweed pellets 12-6-12) compost tea at pH 6.5 and 2.5ppm twice by-weekly and watered 1000 ml of water daily. No pesticides or fungicides were applied during this phase of the life cycle.
Data collection
Every week the height of each plant was measured from the base of the soil to the apical node, the number of leaves and number of branches of the main stem. After 6 weeks of vegetative growth 10 photosynthetic palmately compound leaves (fan leaves) were removed and the average leaf area index was determined for each cultivar.
Statistical analysis
The initial height (Figure 1) of 32.65, 25.15 and 18.75 cm respectively for sativa, indica and hybrid upon transplanting into 14-inch pots did not vary significantly. Within the first couple weeks of vegetative growth a similar trend was observed for the three cultivars; the sativa cultivar had a height of 68.8 cm, while the indica and hybrid cultivars had heights of 51.1 and 44.2 cm respectively. A significant difference in plant height was recorded for the sativa cultivar where an increase of 35.1 cm growth was achieved from week 2 to week 3 was observed. The difference in height observed for indica and hybrid cultivars between week 2 and 3 was 17.8 and 21.3 cm respectively. The sativa cultivar continued to increase significantly to heights of 124.6 and 145.7 and 168.7 cm for week 4, 5 and 6 respectively (Figure 2). The hybrid cultivar increased to heights of 87.2, 101.7 and 143 cm and the indica cultivar to heights of 79.6, 91.9 and 132.2 cm for weeks 4, 5, and 6 respectively (Figure 2). There was no significant difference in the number of leaves (Figure 3) recorded for the first couple weeks of 40,22 and 24 respectively for sativa, indica and hybrid cultivars. The sativa cultivar increased significantly for weeks 3, 4, 5 and 6 achieving a total number of 129 leaves. The indica and hybrid cultivar did not vary significantly for weeks 3, 4, 5 and 6, total number of leaves recorded were 64 and 66 respectively for indica and hybrid. There was no significant difference in the number of branches (Figure 4) observed for the first 3 weeks of vegetative growth. After three weeks the number of branches for the sativa, indica and hybrid were 13.7, 8.9 and 6.6 respectively. There was a significant difference for the sativa cultivar during weeks 4 and 5, with an average number of 16.9 and 19.4 branches respectively when compared to the indica and hybrid cultivars with average numbers of 9.8 and 12.4, 9.4 and 12 branches respectively. After 6 weeks vegetative growth the sativa cultivar displayed 22.5 branches, while the indica and hybrid had 17.4 and 16.5 branches respectively. There was a significant difference observed for the leaf area index (Figure 5) of the indica cultivar of 128 mm2 when compared to sativa and hybrid cultivars of 83.3 and 90.83 mm2 respectively (Figure 6). The vegetative stage of the cannabis life cycle is directly related to the quantity of inflorescence clusters produced upon harvest [12]. The cannabis spp. is a quantitative short-day plant, the flowering cycle can be induced by alternating the photo-period from a long day (18/6) to a short day (12/12) [13-15]. The Caribbean being situated in the Tropical region of the world where a stable photoperiod of (12/12) is observed throughout the year, would allow for the induction of the flowering cycle of cannabis cultivars prematurely. It is generally believed that cannabis is indigenous to the temperate parts of Asia, after the seeds germinate during the spring season and the day length begins to increase throughout the summer then the plants are allowed to vegetate [2,16]. Once the summer solstice has been achieved and the day length begins to decrease cannabis would begin to enter and complete the flowering stages before the frost arrives during winter. Therefore, cultivating cannabis in a tropical region, cultivators would have difficulty maintaining the vegetative stage and often time results in the cannabis plants entering the flowering stage prematurely compromising the yield per plant. By extending the photo period with supplemental lighting the cultivator can extend the vegetative cycle to achieve more biomass production which is directly related to the potential yield per plant. In a Tropical climate flowering cannabis can occur consistently and simultaneously throughout the year once the plants are exposed to an uninterrupted 12 hr dark cycle daily. The results have demonstrated the cultivation of the three cultivars investigated in Jamaica’s Tropical climate with supplemental lighting having a direct influence on the rate biomass production observed during the vegetative cycle. The Cannabis sativa L. species is considered to be a polymorphic subspecies which differ in their phenotypic characteristics, chemical profile and developmental rates [17-20]. The plasticity of cannabis has been long recognized, but not completely understood as it is known cannabis cultivars grown under different latitudes, environmental conditions and habitat types will all influence the morphological and phenotypic characteristics [21,22]. The natural evolution of selective biotic pressures has resulted in various physiological requirements for biomass accumulation during the vegetative cycle [23]. Landrace cannabis cultivars possess genetic integrity achieved over long generations of breeding due to natural selection and human intervention that will allow the cultivar to be best suited and adapted for their environmental conditions with distinct physiological and morphological characteristics [24]. According to [25], the subspecies sativa is native to South Asia such as Myanmar and Thailand, making it better suited to be exploited for production in the tropical regions of the world. Whereas, the subspecies indica originated from Afghanistan, Pakistan and Kazakhstan making it adapted for temperate cultivation [6,27,26]. Study on photosynthetic rates of plants from tropical and temperate origins observed a significant difference in the photosynthetic rates of the two populations except for temperate plants grown under temperate conditions. Despite the landrace sativa cultivar possessing the smallest leaf area index it was able to produce greater biomass when compared to the indica and hybrid cultivars examined. The physiological and morphological adaptations of the sativa cultivar to accumulate greater biomass production over the same period of time under the same conditions may allow for greater yields harvested from sativa type cultivars with less inputs when compared to indica and hybrid cultivars. The photoperiod and temperature are two environmental stimuli which are dependent on climatic conditions which directly influence the photosynthetic rates and ability for biomass production [28] reported a Mexican variety of cannabis to have a relatively high photosynthetic rates at temperatures up to 30C. The rate of photosynthesis is one of the physiological responses that can be influenced by temperature, the selection of suitable cultivars for various environmental conditions will provide for better productivity and economic returns of cultivating cannabis as an horticultural commodity [29-31].


Figure 2: Photograph showing the indica, hybrid and the landrace sativa after six weeks of vegetative growth.
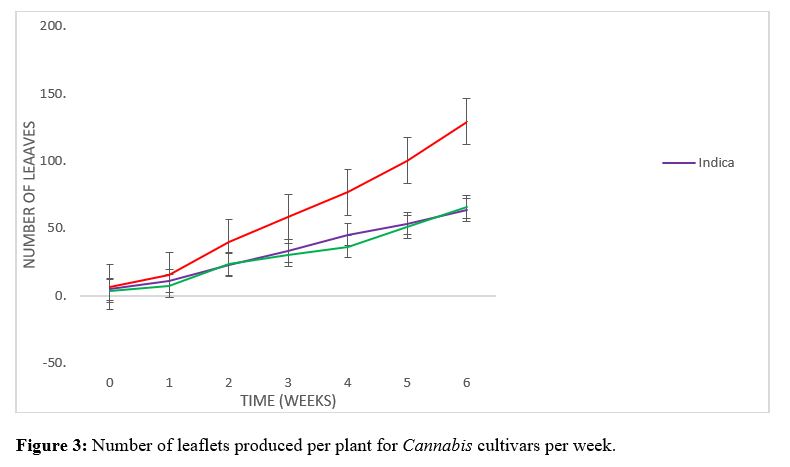


Figure 5: Photographs showing the fan leaves of the three cultivars indica, hybrid and the landrace sativa.

Conclusion
The use of supplemental lighting for extending photoperiod in an equatorial climate is a practical solution for preventing the premature induction of the flowering cycle for Cannabis sativa L. The wide variety of cannabis seeds available on the international market has allowed breeders and growers to constantly create, develop and cultivate cannabis cultivars with varying euphoric and psychoactive capabilities for medical, recreational and sacramental purpose. The plasticity of cannabis as a horticultural commodity, colonizing the globe by forming an intrinsic relationship with humans has demonstrated physiological and morphological adaptations for growing in various climatic and environmental conditions. The cultivation of sativa cultivars in an equatorial climate appears to have an advantage to the rate of biomass production during the vegetative cycle compared to indica and hybrid cultivars. Therefore, a Tropical farmer equipped with sativa type cultivars may have the possibility of achieving greater yields consistently with less input cost compared to indica type cultivars. Further investigations into the processes associated with the physiological development of cannabis plants and the potential yield production associated with various cultivars from different geographic origins will provide for greater clarity for cultivar specific cultivation.
Acknowledgements
The authors thank, Mr. Dwayne Johnson for his assistance in the maintenance of the cannabis plants throughout the experimental protocols.
References
1. Small E. 2007. Cannabis as a source of medicinals, nutraceuticals, and functional foods. In: Acharya SN, Thomas JE (Eds) Advances in Medicinal Plant Research. Research Signpost, Trivandrum, Kerala, India. 1-39.
2. Schultes RE, Klein WM, Plowman T, et al. 1974. Cannabis: an example of taxonomic neglect. Harvard University Botanical Museum Leaflets. 23: 337-367. Ref.: https://bit.ly/2yl8nRu
3. Anderson LC. 1980. Leaf variation among Cannabis species from a controlled garden. Harvard University Botanical Museum Leaflets. 28: 61-69. Ref.: https://bit.ly/34CRGxd
4. Hillig KW. 2005. Genetic evidence for speciation in Cannabis (Cannabaceae). American Journal of Botany. 91: 966-975. Ref.: https://bit.ly/2Kbj2AV
5. Hillig KW. 2004. A chemotaxonomic analysis of terpenoid variation in Cannabis. Biochemical Systematics. 32: 875-891. Ref.: https://bit.ly/34EjIIF
6. Clarke RC, Merlin MD. 2013. Cannabis evolution and ethnobotany. University of California Press Berkeley. Ref.: https://bit.ly/2XE5wOh
7. Seedfinder 2020. Seedfinder online database.
8. Emanuel MA, Haughton AY, K’nife K. 2018. Policy analysis and implications of establishing the Caribbean Cannabis Economy (CCE): lessons from Jamaica. Drugs and Alcohol Today 18: 99-107. Ref.: https://bit.ly/2Vbr2rV
9. Ahmed AIS. 2019. Overview: Integrated Management of Plant Diseases towards Sustainable Development. International Journal of Plant Science and Horticulture. 1:173-179. Ref.: https://bit.ly/2yfn7Sb
10. Leafly. 2020a. Big Bud. https://www.leafly.com/strains/big-bud.
11. Leafly. 2020b. Sugar Pine. https://www.leafly.com/strains/sugar-pine.
12. Meijer WJM, van der Werf HMG, Mathijssen EWJM, 1995. van den Brink PWM.. Constraints to dry matter production in fibre hemp (Cannabis sativa L.). The European Journal of Agronomy. 4: 109-117. Ref.: https://bit.ly/3cgAujw
13. Van der Werf HMG, Mathissen EWJM, Haverkort AJ. 1996. The potential of hemp (Cannabis sativa L.) for sustainable fibre production: a crop physiological appraisal. Annual Applied Biology. 129: 109-123. Ref.: https://bit.ly/2KayugQ
14. Struik PC, Amaducci S, Bullard MJ, et al. 2000. Agronomy of fibre hemp (Cannabis sativa L.) in Europe. Industrial Crops and Products. 11: 107-118. Ref.: https://bit.ly/3emIJg1
15. Kottek M, Grieser J, Beck C, et al. 2006. World map of Ko?ppen-Geiger cli- mate classification updated. Meteorologische Zeitscrift. 15: 259-263. Ref.: https://bit.ly/2RET3WK
16. Kudo Y, Kobayashi M, Momohara A, et al. 2009. Radiocarbon dating of fossil hemp fruits in the earliest Jomon period. Shokuseishi kenkyu? (Japanese Journal of Historical Botany) 17: 27-32.
17. Small E, Cronquist A. 1976. A practical and natural Taxonomy for Cannabis. Taxon. 25: 405-435. Ref.: https://bit.ly/2XGB9Hc
18. Small E. 2015. Evolution and classification of Cannabis sativa (Marijuana, Hemp) in relation to human utilization. The Botanical Review. 81: 189-294. Ref.: https://bit.ly/2RKgpui
19. Zang Q, Chen X, Guo H, et al. 2018. Latitudinal adaptation and genetic insights into the origins of Cannabis sativa L. Frontiers in Plant Science. 9: 1876. Ref.: https://bit.ly/2K9w9Tf
20. Mc Partland J. 2018. Cannabis sativa and cannabis indica versus “Sativa” and “Indica”. Cannabis sativa L. -Botany and Biotechnology. Springer International Publishing. 101- 121. Ref.: https://bit.ly/2V9U0Zg
21. Haney AW, Bazzaz FA. 1970. Botany and Chemistry of Cannabis. Churchill London. 39-48.
22. Hey J, Pinho C. 2012. Population genetics and objectivity in species diagnosis. Evolution 66: 1413-1429. Ref.: https://bit.ly/2Vf1338
23. Cosentino SL, Testa G, Scordia D, et al. 2012. Sowing time and prediction of flowering of different hemp (Cannabis sativa L.) genotypes in southern Europe. Industrial Crops and Products 37: 20-33. Ref.: https://bit.ly/2XBrUYF
24. Simmonds NW. 1993. Introgression and incorporation. Strategies for the use of crop resources. Biological Review. 68: 539-562. Ref.: https://bit.ly/3cnHHi0
25. Cherniak L. 1982. The great books of Cannabis, Vol. I: Book II. Cherniak/Dame Oakland CA. 207- 213.
26. Small E. 2017. Cannabis: a complete guide. CRC Press, Boca Raton, FL.
27. Bazzaz FA, Dusek D, Seigler DS, et al. 1975. Photosynthesis and cannabinoid content of temperate and tropical populations of Cannabis sativa. Biochemical Systematics and Ecology. 3: 15-18. Ref.: https://bit.ly/34Hl6Kk
28. Chandra S, Lata H, Khan IA, et al. 2011. Temperature response of photosynthesis in different drug and fiber varieties of Cannabis sativa L. Physiology and Molecular Biology of Plants 17: 297. Ref.: https://bit.ly/2xxRTpf
29. Berry J, Bijorkman O. 1980. Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology. 31: 491-543. Ref.: https://bit.ly/2xxVVhm
30. Larcher W. 1994. Photosynthesis as a tool for indicating temperature stress events. In Schulze ED, Caldwell MM (eds) Ecophysiology of photosynthesis. Ecological Studies, Springer Verlag, Berlin. 261-277. Ref.: https://bit.ly/3adOh9c
31. Joshi SC, Maikhuri RK. 1996. Seasonal changes in photochemical efficiency and leaf area of nitrogen and non-nitrogen fixing tree species grown in degraded land. International Journal of Sustainable Future for Human Security. 3: 1-11. Ref.: https://bit.ly/2VqC3o8




















