Indexing & Abstracting
Full Text
Review ArticleDOI Number : 10.36811/ijpsh.2020.110023Article Views : 16Article Downloads : 21
A Review on ethnobotanical uses, biological activities and phytochemical aspects of Acacia senegal (L.) Willd. and Acacia seyal Delile. (Fabaceae)
René D Magnini1,2,3, Hilou A3, Millogo-Koné H2, Compaore S2,4, Pagès J-M1 and Davin-Regli A1*
1U1261, INSERM, UMR-MD1 «Membranes et Cibles Thérapeutiques», IRBA, Aix-Marseille Université, Faculté de Pharmacie, 27 Bd Jean Moulin, 13385 Marseille, France
2Institut de Recherche en Sciences de la Santé (IRSS/CNRST), Département de Médecine et Pharmacopée Traditionnelle/ Pharmacie (MEPHATRA-PH), 03 BP 7047 Ouaga 03, Burkina Faso
3Laboratoire de Biochimie et de Chimie Appliquée (LABIOCA), UFR/SVT, Université Ouaga I Pr Joseph Ki-Zerbo, 03 BP 848 Ouagadougou 03, Burkina Faso
4Laboratoire de Biologie et Ecologie Végétales (Labev), UFR/SVT, Université Ouaga I Pr Joseph Ki-Zerbo, 03 BP 848 Ouagadougou 03, Burkina Faso
*Corresponding Author: Davin-Regli A, UMR_MD1, U-1261, Membranes et Cibles Thérapeutiques, Faculté de Pharmacie, 27 Bd Jean Moulin, 13385 Marseille cedex 05, France, Tel: 33 (0)491835695; Email: anne-veronique.regli@univ-amu.fr
Article Information
Aritcle Type: Review Article
Citation: René D Magnini, Hilou A, Millogo-Koné H, Compaore S, et al. 2020. A Review on ethnobotanical uses, biological activities and phytochemical aspects of Acacia senegal (L.) Willd. and Acacia seyal Delile. (Fabaceae). Int J Plant Sci Hor. 2: 32-55.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2020; René D Magnini
Publication history:
Received date: 25 February, 2020Accepted date: 07 March, 2020
Published date: 10 March, 2020
Abstract
The genus Acacia is a group of tropical plants species used in folk medicine due to virtue of its many therapeutic properties. In this document, we review the Ethnopharmacology, biological and phytochemical activities of the two major plant species used. Although, several researchers has been done, Acacia senegal (L.) Willd. and Acacia seyal Delile. are among the species of the genus for which phytochemical study is limited, few bioactive compounds and properties described. Based on these current traditional uses, it is necessary to carry out more biochemical and pharmaceutical assays in order to identify the precise ingredient that supports the recommendation in traditional medicine. The characterization of the active compound that plays a role for treating human diseases (infection, cancer, etc.) represents a key step in phytochemical research of new compounds. Moreover, this information about the active compound will help the clinician/pharmacist to define a rational and combined use with the synthetic molecules for which resistance mechanisms are currently reported in clinical cases.
Keywords: Acacia Senegal; Acacia seyal; Antimicrobial; Biological activity; Phytochemistry; Tradional medicine
Introduction
Traditional medical practices vary from country to country and region to region, and are influenced by several factors including culture, history, attitudes and personal philosophy [1]. The renewed interest over the centuries and the transmission of experience from generation to generation are proof of the safety and effectiveness of this medicine. The lack of health care centers in remote areas, often linked to the high cost of conventional medicines, means that 80% of people in African countries use traditional medicine for their primary health needs [2]. Nowadays, developing countries such as Burkina Faso are adopting policies to promote traditional recipes through collaboration between health practitioners and traditional healers. Today, infectious diseases are the leading cause of death in the world and antibiotic resistance has become a global concern [3]. The emergence and spreading of pathogens that present resistance to many if not for all clinically used antibiotics has led WHO to classify them as a human health priority [4-6]. Therefore, researchers are increasingly turning to medicinal plants in search of new approaches to develop new effective drugs against microbial infections. The screening of potential antimicrobial activity of active molecules from medicinal plants is of concern [7]. Some recent reviews point on the possible use of natural products to combat multidrug resistant bacteria (for an example see. Interestingly, Acacia senegal (L.) Willd. and Acacia seyal (Del.), of the Fabaceae-Mimosoideae family, are well known in traditional medicine and often used in combination with other plants to combat microbial infections [12-14]. The available knowledge on these plants was searched using the keywords Acacia senegal (L.) Willd. and Acacia seyal Del. in the databases 'Google scholar’, 'NCBI', 'Springer Link', Free Scientific Publications' and' Web of Science'. Their properties are of a major interest in the research and development of new active molecules targeting multidrug resistant pathogens or the identification of adjuvant that can restore the antibiotic activity in resistant bacteria. This review summarizes the current knowledge regarding these two plants and presents some perspectives for a future study and application about their antimicrobial properties to combat antibiotic resistance.
Taxonomy of Fabaceae
Leguminosae Fabaceae previously identified and described by Adanson and de Jussieu are subdivided into three sub-families including Caesalpinioideae, Mimosoideae and Papilionoideae [15-17]. With about 765 genera and more than 19500 species, Fabaceae, constitute the third most important plant family [18,19]. The species in this family are well distributed in all tropical and warm temperate regions of the world. Recent data indicated that the Legume Phylogeny Working Group has subdivided the Fabaceae into six sub-families instead of three, namely Cercidoideae (12 genera, 335 species), Detarioideae (84 genera, 335 species), Detarioideae (84 genera, 335 species) and Cercidoideae (84 genera, 760 species), Duparquetioideae (1 genus, 1 species), Dialioideae (17 genera, 85 species), Caesalpinioideae (148 genera, 4400 species; includes genera of the Mimosoideae) and Papilionoideae with 503 genera, and 14,000 species [20,21]. Acacia genus belongs to the subfamily of Mimosoideae and is the second most important genus in the Fabaceae family, with about 1350 species currently recognized. The highest concentrations of Acacia sp. are found in Australia (955 species), with high numbers also in America (about 185 species), Africa (144 species) and Asia (89 species) [22, 23]. This family represents an important source of molecules that are involved in the treatment of various diseases.
Botanical description
Acacia senegal (L.): Willd. Acacia senegal is commonly known as white gum tree, with Acacia verek Guill. & Perott and Mimosa senegal L. as synonymes and vernacular names, gon pèelega (Moore) and Gommier of Senegal (French). It’s a Sahelian and Sudano-Sahelian species, belonging to the Fabaceae-Mimosoideae family [24]. It’s distributed in Senegal to Cameroon and Sudan. A. senegal occurs naturally in arid, semi-arid and subtropical regions, and is drought-resistant [25]. It’s also presents in tropical, Southern Africa and India. It is a phanerophytes, a thorny shrub tree of 2-6 or even 12 meters high with very branched and ascending branches [26]. The trunk is about 30 cm in diameter and the bark is light grey with a red slice marbled with white [27]. The leaves are green-grey, alternating and bipinnate, measuring 3.5-8 cm long with grapes of cream color small flowers. Seeds are greenish brown [26]. Pubescent then hairless pods measuring about 7 cm long x 2 cm wide represent the fruits. In Africa, flowering takes place at the foliage before the first rains but also sometimes at the end of the rainy season, especially from July to September. A. senegal is one of the species used to create the great African green wall. A. senegal is used to fertilize soils, as firewood, local construction and fence posts and the gum Arabic produced is traded internationally [28-30].
Acacia seyal (Delile.): Acacia seyal also called Gon-ponsego (Mooré); Gommier, Mimosa épineux (French) is phanerophyte, a thorny tree 6 to 17 m high with smooth and green bark [24]. The twigs are greenish and the leaves are alternating and bipinnate, from 3 to 10 cm long with 3-7 pairs of pinnules. The fruits are represented by narrow pods and contain 6 to 10 seeds that are brown when they are ripened. Flowering and fruiting usually take place in the second half of the dry season, before foliage. It is a species that is Sahelo-Saharan and Sudano-Sahelian. It’s found in low slopes and low ground and generally near rivers. This species has spread from Senegal to Cameroon, Egypt and Somalia [31].
Ethnobotanical uses (parts, traditional uses, nutritional value) of A. Senegal and A. seyal.
Different parts of the plant species are used dry or in liquid form after maceration or decoction for general treatment of bacterial, viral, parasitic infections or used to treat symptoms in gastroenterology, dermatology, hematology, rheumatology and inflammation (Table 1 and 2). Locally applications can be performed for ophthalmological or dermatological problems.
Table 1: Different uses and methods of extract preparation of A. senegal in different African countries. |
||||||
|
Medical uses |
Plant parts |
Forms |
Plant association |
Medication administration
|
Country |
References |
|
Respiratory infections, Flue, sinusitis |
Bark
Gum |
Decoction
Powder |
|
Oral |
Burkina Faso |
[14,32] |
|
Toothaches |
Young leaves, Thorns |
Powder |
Diospyros mespiliformis Hochst. Ex A. DC. |
Inhalation gargles |
[33] |
|
|
Stomac ulcer Colic |
Bark Stems
Gum |
Powder
Decoction
|
|
Oral |
Senegal |
[34] |
|
Malaria fever |
Gum
|
water |
|
Oral |
[35] |
|
|
Malaria |
Bark Stem
|
Decoction |
|
Bath Oral |
Mali |
[36] |
|
Hemorrhoids STIs |
Roots |
decoction |
Guiera senegalensis J. F. Gmel
|
Oral |
[37] |
|
|
Liver deseases |
Roots |
Decoction |
Stereospermum kunthianum Cham.
Ficus thonningii Blume |
|
Niger |
[38] |
|
Laxative Cirrhosis Hepatitis |
Roots |
Powder |
|
Oral |
[39] |
|
|
wounds |
Bark |
Decoction |
|
Oral
|
[40] |
|
|
Malaria |
Stems Bark |
Decoction |
|
Oral |
Nigeria |
[41] |
|
Stomach aches Purgative STIs Diarrhea Stomach aches |
Roots
Bark |
Decoction |
|
Oral |
Kenya |
[42] |
|
Wounds |
Gum |
Paste |
|
Topical
|
[43] |
|
|
Bleedings |
Gum |
Paste |
Commiphora myrra (T. Nees) Engl
|
Oral |
||
|
Stomach aches |
Bark |
Macerate |
|
Oral |
[44] |
|
|
Laxatives |
Bark
Seeds |
Macerate |
|
Oral |
[45] |
|
|
Food suplement |
Leaves |
eaten by livestock |
|
Oral |
[46] |
|
|
Stomach aches
|
Bark |
Decoction |
|
Oral |
[46] |
|
|
Against Evil spirits |
Seeds |
Crushed |
|
Oral
|
Ethiopia |
[47] |
|
Eyes injuries Back pain Constipation Stomach aches |
Fresh gum |
Decoction |
|
Oral |
[48] |
|
|
Eyes injuries
|
Bark |
Drops |
|
Local |
[49] |
|
|
Mumps
|
Leaves |
Topic |
|
Oral |
||
|
Fertility
|
Roots |
Topic |
|
Oral |
||
|
Diarrhoea Mouth inflammation |
Roots |
|
|
Oral |
Angola |
[50] |
|
Abscesses and boils Cough |
Roots |
Decoction |
|
Local |
Tanzania |
[51,52] |
|
Haemorrhagic Diarrhea |
Barks and roots |
Decoction |
|
Oral |
[53] |
|
|
Headaches |
Roots |
Powder |
|
Smoked |
Uganda |
[54] |
|
Delivery pain in animals |
Bark |
Maceration |
|
Oral |
[55] |
|
|
Pospartum pain in animals |
Bark and roots
|
Maceration |
|
Oral |
||
|
Diarrhea Ulcers |
Gum |
Powder |
|
Oral |
Sudan |
[56,57] |
|
diabetes, Kidney failure |
Fruits |
Powder |
|
Oral |
||
|
Stomach ulcers and aches Abdominal pain |
Stem bark |
Decoction |
|
Oral |
Mauritania |
[58] |
|
Eyes drop |
Gum |
eyewash |
|
Local |
Morocco |
[59] |
|
Lung disases Stomach aches Liver diseases |
Powder |
|
Oral |
|||
|
Anti-inflammatory |
|
External use |
||||
|
Table 2: Different uses and methods of extract preparation of A. seyal in different African countries. |
||||||
|
Medical use |
Plant parts |
Forms |
Plant association |
Medication administration
|
Country |
Refs |
|
Dysentery Gastrointestinal pain |
Bark and roots |
Decoction |
|
Oral |
Burkina Faso |
[60] |
|
Leprosis |
Root bark |
Infusion |
|
Oral |
||
|
Nervous sensory Digestive disorders |
Bark gum |
Decoction
|
|
Crushing Instillation Oral bashing |
[61] |
|
|
Toothaches |
Bark and leaves |
Decoction
|
|
Oral |
[33] |
|
|
STIs
Bleeding |
Bark stems Trunks twigs |
Decoction
Powder |
Mytragyna inermis (Willd.) Kuntze.
Gossypium sp |
Oral |
[12] |
|
|
Keratitis Eyes aches |
Bark stems
|
chew |
Salt |
Instillation
|
[34] |
|
|
Dysentery |
Bark
|
Powder |
Honey |
Oral |
Senegal |
[62] |
|
Snake bites |
Bark stems |
Infusion |
|
Oral and local |
[63] |
|
|
Purgative Fortifying STIs |
bark, stem trunk, or twig |
Decoction |
|
Oral |
[13,14] |
|
|
Leprosy |
bark, stem trunk, or twig |
Decoction |
|
Oral |
||
|
Headaches
|
Liquid butter,
|
Local wash |
||||
|
Eye diseases |
Leaves |
|
Leptadenia hastata (Perr.) Decne Ziziphus mucronata Willd. |
|
||
|
Bilious fever and jaunice Urinary infections |
Roots |
Decoction |
Combretum glutinosum Perr. Ex DC. And milk |
Local wash
Oral |
||
|
Leprosy |
Red bark of trunk |
|
|
Oral |
[64] |
|
|
Wound injuries |
Leaves |
Decoction |
Milk |
local |
Niger |
[65] |
|
Malaria Spleen dilatation fever |
Bark |
Powder |
Milk Millet |
Oral |
[65] |
|
|
Asthenia Avitaminosis Sickle cell disease |
Roots |
Maceration |
Securidaca longipedunculata Fresen., Pergularia tomentosa L., Stereospermum kunthianum Cham., Feretia apodanthera Del., Annona senegalensis Pers., Securinega virosa (Roxb.ex willd.) Baill, Ziziphus mauritiana Lam., Boscia senegalensis (Pers.) Lam, Cassia sieberiana DC. |
Oral with millet milk
porridge |
[66] |
|
|
Arthritis Inflammation Liver desaese |
Bark |
Decoction |
|
Oral |
[67,68] |
|
|
Epilepsy |
Bark |
Maceration |
|
Oral |
Mauritanie |
[58] |
|
Pneumonia |
Bark Stem Trunk twig |
Decoction |
|
Oral |
Kenya |
[69] |
|
Malaria |
Roots |
Decoction |
|
Oral |
[70] |
|
|
Joint pain |
Bark stems leaves |
boiled |
Strychnos henningsii Pvetta crassipes ( K. Schum.) |
Oral |
[71] |
|
|
Intestinal parasites |
Roots |
|
|
Oral |
Ethiopia |
[49] |
|
Jaunice |
Leaves |
|
|
Oral |
||
|
Chest pain |
Roots |
crushed |
|
Oral |
[47]) |
|
|
Diarrhoea |
Roots |
Maceration |
|
Oral |
Uganda |
[55] |
|
Viral skin necrosis nodules |
Bark leaves |
Maceration |
|
Oral |
||
|
Bleeding and leaves |
Bark |
Decoction |
|
external |
Sudan |
[72] |
|
Leprosy |
||||||
|
Arthritis Rheumatisms Rheumatoid fever |
Wood |
|
|
smoked |
[73] |
|
|
Inflammation and stomach aches |
Leaves |
|
|
|
||
|
Laxative
|
Stem bark |
Decoction |
|
Oral |
Mauritania |
[58] |
|
Painful period |
Roots seeds |
Decoction |
Pennisetum americanum (L.) Leeke Capsicum annuum (L.)[Cult.] Zanthoxylum zanthoxyloides (Lam.) Zepem.&Timler |
Oral |
Togo |
[74] |
|
Appendicitis |
Roots |
Decoction |
|
Oral |
Benin |
[75] |
|
Conjonctivitis trachoma |
Gum |
Maceration |
|
Oral |
Mali |
[76] |
|
Conjonctivitis trachoma |
Leafed stem Bark of trunk |
Decoction |
|
Oral |
||
|
Purgative Syphilis Leprosy Headaches Chest pain |
Bark of trunk and leafed |
Decoction |
|
Oral |
||
|
fistula |
Leaves |
Powder |
Honey |
local |
Rwanda |
[77] |
|
dysentery |
Bark and roots |
crushed |
Water |
Oral |
Djibouti |
[78] |
|
Post-abortion care Stmach aches |
Bark |
Maceration |
|
Oral |
||
|
Infected wounds |
Seed |
Powder |
|
Local |
Algeria, Egypt, Morocco |
[79] |
|
Fever Dysmenorrhea Eye infections |
Seed |
Decoction |
|
Oral local |
[79] |
|
|
Stomach ulcers Rheumatisms |
Leaves bark |
Decoction |
|
Oral local |
[79] |
|
|
Rheumatisms Infecions post delivery |
Wood |
Fumigation |
|
Oral |
Algeria, Egypt, Morocco |
[59,80] |
|
Rheumatisms Respiratory tract infection |
Gum |
|
|
Oral |
||
|
Gastric ulcer |
Leaves bark |
|
|
Oral |
||
|
Livestock |
Pod |
|
|
Oral |
||
Phytochemistry, pharmacology and toxicological studies on the plants extracts
The Fabaceae family is an important source of biologically active molecules. However, few species have been examined specifically for these substances; in fact, the secondary metabolites of only a small proportion of Acacia species have been examined in detail [81]. Acacia senegal and Acacia seyal are among the few that have been studied.
Acacia senegal
The data contained in Table 3 summarize the biological activities and molecules or groups of molecules that have been informed by the different authors about Acacia senegal (L) Willd. and their supposed involvement in biological activities. The dichloromethane extract from the root wood of A. senegal showed good activity against two bacterial species, E. coli and S. aureus while the ethanolic extract, dichloromethane and ethyl acetate showed significant antifungal activity against C. albicans. From the wood of the root, ten molecules were isolated, including eicosanyl 3-Oferuloyl-quinate, isolated from nature for the first time. The molecules of 3α-hydroxyeuph-25-ene and α-amyrin were isolated for the first time from this species [82]. The α-amyrin and its derivatives have presented various biological activities e.g. anti-HIV and anti-acyl coenzyme A: cholesterol acyltransferase (ACAT) activities [83]. Other authors have reported the antifungal activity of β-sitosterol isolated from the methanolic fraction of M. azedarach leaves against Ascochyta rabiei [84]. A recent study demonstrated by bio-autographic analysis that extracts of A. senegal leaves (Acetone, chloroform, ethanol and petroleum ether) possesses antioxidant derivatives (DPPH) and an antibacterial activity against Pseudomonas aeruginosa. Analysis revealed antibacterial activity of four fractions of acetone extract, four fractions of chloroform extract, two fractions of ethanolic extracts and four fractions of petroleum-ether extracts. The phytochemical compounds present in the extracts are glycosides, alkaloids and flavonoids. In addition, ethanolic extract was the richest in secondary metabolites and the antibacterial and oxidative activity observed is believed to be related to the presence of its compound groups [85]. However, to date, no molecules have been isolated and identified from the various fractions and certified to be responsible for the activity. Furthermore, methanol and ethanol extracts from the trunk bark of A. senegal showed antibacterial activity against K. pneumoniae, Proteus vulgaris, Salmonella typhi, Salmonella dysenteriae and E. coli. According to the authors, the tannins and saponins contained in the extracts are responsible for the observed activity. In addition, toxicity studies of ethanolic extract from stem bark revealed any significant toxicity against Artemia salina [86]. According to some authors, the hexanic fraction of A. senegal stem bark is active against respiratory pathogenic bacteria including Klebsiella pneumonia and Streptococcus pneumoniae [87]. Two flavonoids, namely Vicenin [Apigenin-6,8-bis-C-bis-C-b-D-glucopyranoside] and Quercetin-3-O-rutinoside (Rutin) are most commonly found in the genus Acacia [81]. Vicenin et al. isolated these flavonoids from Ocimum sanctum and showed an antibacterial effect against Escherichia coli and Proteus with inhibition zone diameters of 18.84 and 17.16 mm respectively [88]. Several authors have reported the antibacterial effect of rutin against Escherichia coli, Proteus vulgaris, Shigella sonnei, Klebsiella sp., Pseudomonas aeruginosa and Bacillus subtilis [89-91]. In addition, the combination of rutin with other flavonoids has shown strong antibacterial activity against Bacillus cereus and Salmonella enteritidis [92]. Ethanolic extract from the leaves of A. senegal has decreased the activity of the sucrose enzyme and appears to facilitate the control of carbohydrate hydrolysis and therefore reduces postprandial increases in blood glucose levels in diabetics [93]. Ethyl acetate extract from the bark of the stem of A. senegal significantly reduced blood glucose, serum TC, serum TTG, serum LDL, serum urea and creatinine levels, and increased serum HDL levels in alloxane-induced diabetic albino rats [94]. Neutral sugar gums (rhamnose, arabinose and galactose), acids (glucuronic acid and 4-methoxyglucuronic acid), calcium, magnesium, potassium and sodium have been identified [26].
|
Table 3: Summary of known molecules from Acacia senegal (L) Willd. |
||||||
|
Organs |
Extraction Solvent (s) |
Biological |
Familly/Molecules |
Active molecules |
Refs |
|
|
Activity |
isolated |
|||||
|
Leaves |
Ethanol |
Diabète (reduce the increase in blood sugar levels) |
|
|
[97] |
|
|
80% ethanol |
Antioxidant (DPPH) |
Phenolic compounds |
|
[93] |
||
|
Good cytotoxic activity against Hep G2 Cell line |
||||||
|
Acetone |
Antioxydant/ Antibacterial (Pseudomonas aeruginosa) |
Carbohydrates, phenol, glycosides, Quinones /anthraquinones, alkaloids, anthocyanins and leuco anthocyanins, volatile oils |
|
[85] |
||
|
Chloroform |
Antioxidant/ Antibacterial (Pseudomonas aeruginosa) |
Glycosides, saponins/glycosides, alkaloids, flavonoids |
||||
|
Ethanol |
Antioxidant/ Antibacterial (Pseudomonas aeruginosa) |
Carbohydrates, Amino acid and protein, phenols, sterols and steroids, alkaloids, flavonoids, anthocyanins and leucoanthocyanins, volatile oils |
||||
|
Petroleum ether |
Antioxidant/ Antibacterial (Pseudomonas aeruginosa) |
Leucoanthocyanin, Glycoside |
||||
|
Stem Root (heart Wood ) |
Ethanol, DCM and Ethyl acetate |
Antibacterial |
Steroids, triterpenoids, |
Ceryl cerotate, Eicosanoic acid, Tetracosanol, Docosanoic acid, 3α-Hydroxyeuph-25-ene, α-Amyrin, Stigmasterol, ß-Sitosterol, Betulin-3-O-stearate, Eicosanyl 3-O-feruloyl-quinate, ß-Sitosterol-ß-D-glucoside, D-Pinitol |
[82] |
|
|
(E. coli and S. aureus). |
quinic acid diester, cyclohexitol |
|||||
|
Antifongical |
|
|||||
|
C. albicans |
|
|||||
|
Stem Bark |
Ethanol, Methanol |
No significant toxicity against Artemia salina. |
Saponin, tannin and Sterols |
|
[86] |
|
|
Antibacterial (K. pneumoniae; P. vulgaris, S. typhi, S. dysenteriae, E. coli) |
||||||
|
Ethyl acetate |
Diabète |
Flavonoids |
|
[94] |
||
|
Methanolic |
Anthelminthic activity |
|
|
[95] |
||
|
(Fasciola gigantica) |
||||||
|
Pods |
Ethanol |
No Antioxidant activity and Enzymatic inhibition |
|
|
[56] |
|
|
Aqueous |
||||||
|
Ethanol |
All extract exhibit high toxicity on Brine shrimp |
|||||
|
Aqueous |
||||||
|
70 % ethanol |
Neurotoxicity |
|
|
[96] |
||
|
Hepatotoxicity |
||||||
|
Seeds |
70% ethanol |
antiatherosclerotic cardioprotective |
|
|
[98] |
|
|
70 % ethanol |
Neurotoxicity |
|
|
[96]) |
||
|
Hepatotoxicity |
||||||
However, the study did not pay any attention to the relationship between activity and the chemical compounds produced by the gum. Methanolic extract from the bark of the stem showed 100% mortality against adult Fasciola gigantica worms in vitro at concentrations of 1000, 500 and 250 ppm after 6, 12 and 24 hours respectively [95]. A recent study evaluated the efficiency of Acacia senegal extracts against in improving DEHP-induced liver and brain toxicity. Sprague Dawley rats in which acute hepatotoxicity and neurotoxicity was induced by Di-2- Ethylhexyl phthalate (DEHP), received as oral treatment ethanolic extract at 70% of A. senegal pods for 28 days under several conditions. The results showed that the extract of A. senegal has an ameliorative effect by restoring the activities of antioxidant enzymes to normal by reducing the level of LPO in both tissues. Also, the extract improved the levels of cerebral amino acids, monoamines and their metabolites [96].
Acacia seyal
Table 4 also summarizes the molecules or groups of molecules identified from Acacia seyal (Del.). Ethanolic extracts (leaves, root bark and trunk) and dichloromethane from Acacia seyal showed interesting activity against Klebsiella pneumoniae [99]. Previous work on other species of the same genus (Acacia nilotica (L.) Willd ex Del., Acacia sieberiana DC.) has shown good antibacterial activity against Escherichia coli and Klebsiella pneumoniae [99]. Many authors have reported of acacia genus, many biologically active compounds e.g. ethyl gallate, octasanol, β-amyrin, α-betulin and flavonoids [100, 101]. Concerning A. seyal, we have few information on the phytochemical composition of the different parts. However, the authors attribute the activity found by the species to the presence of similar compounds. The methanolic extract from the bark showed good antibacterial activity. Four compounds were isolated (epicatechin, catechin, digallic catechin and β-sitosterol) and tested for their activities. The author indicated that the activity of the isolated compounds was less interesting compared to totum [102]. This shows a synergy of activity between the compounds. In addition, different teams have reported the activity of β-sitosterol on inhibiting the growth of S. aureus and E. coli [103,104]. Methanolic extract from the leaves of A. seyal reduced the incidence of green mold (Penicillium digitatum) by 56.1% on fruits inoculated per injury. The extract of A. seyal revealed a high content of gallic acid, salicylic acid, p- coumaric acid, caffeic acid, 3,4 dihydroxy benzoic acid, ferulic acid [105]. Isolated p- coumaric acid from Nauclea pobeguinii (Pobeg.) Merr. did not activate against bacteria tested (E. coli, E. aerogenes, K. pneumoniae, P. aeruginosa, P. stuartii) at a concentration of 256 μg/mL [106]. In other hand, researchers have reported that caffeic and p-coumaric acid cause membrane damage of 44% and 59%, respectively, in Gram-positive bacteria, Oenococcus oeni [107]. Also, p-cumaric and ferulic acids have shown synergistic activity with amikacin against E. coli, E. aerogenes and S. aureus [108]. Ethyl gallate has shown antibacterial activity and synergistically when combined with tetracycline and fusidic acid against specific resistant and methicillin-sensitive strains of Staphylococcus aureus [109]. Ethanolic extracts (leaves, bark) and dichloromethane extract from the bark of Acacia seyal showed an activity higher than 85% with respect to the enzyme acetylcholinesterase. Alkaloids are known to have many pharmacological properties, including inhibition of acetylcholinesterase enzyme activity and the author associate the activity with alkaloids [99]. A recent study showed that methanolic extract from the bark of A. seyal showed 100% mortality against Biomphalaria Pfeifferi at different doses used [110]. The root extract of A. seyal has demonstrated antimicrobial activity against fungal and bacterial pathogens [111]. The cytotoxic study of the hydroethanolic extract of the stem bark of A. seyal to reduce the protein content of Bcl-xL and Bcl-2 which in turn promotes the intrinsic induction of apoptosis. In addition, the phytochemical analysis of this extract shows that it is rich in pro-apoptotic components such as flavonoids [112]. The structure of the gum of A. senegal (L.) and A. seyal has recently been revised by methylation analysis and nuclear magnetic resonance (NMR) spectroscopy. It has been found that A. seyal gum is more strongly branched than A. senegal and is composed of galactopyranosyl bound to 1,3. Galacturonic acid was recently identified for the first time in A. seyal [113] (Figure 1-5).
|
Tableau 4: Summary of known bioactive molecules from Acacia seyal (Del.). |
|||||
|
Extraction Solvent (s) |
Biological |
Familly/Molecules |
Active molecules |
References |
|
|
Activity |
isolated |
||||
|
Leaves |
Ethanol Dichlorométhane |
Inhibition of acetylcholinesterase |
nd |
|
[99,114] |
|
Ethyl acetate |
Anti-inflammatory |
||||
|
|
Antibacterial |
||||
|
Methanol, acetone, water |
Antifungal (Penicillium digitatum) |
Phenolic compounds |
gallic acid, salicylic acid, p- coumaric acid, caffeic acid, 3,4 dihydroxy benzoic acid and ferulic acid |
[105,111] |
|
|
Leaves |
E. carotovora, |
|
|
||
|
Roots |
P. syringae pv, Syringae, R. solanacearum, |
||||
|
|
S. epidermidis, |
||||
|
|
X. campestris pv. Mangiferae indicae |
||||
|
Stem Root |
Dichloromethane |
Antiinflammatory (Inhibition of prostaglandin synthesis) |
|
|
[99,114] |
|
Ethyl acetate |
Antibacterial |
||||
|
Stem Bark |
méthanol, chloroform water |
anti-trichomonal activity |
|
|
[115] |
|
Ethanol, Dichloromethane |
Inhibition of acetylcholinesterase, |
|
|
[99,116] |
|
|
Ethyl acetate |
Antimycobacterial |
||||
|
|
(M. aurum A +) |
||||
|
70% Ethanol |
Anti-cancer |
|
|
[102] |
|
|
Ethanol |
Antimicrobial Staphylococcus aureus and Candida albicans |
Flavonoids, saponins, terpenoids, steroids, alkaloids, phenols and tannins. |
|
[117] |
|
|
Antioxydant (DPPH) |
|||||
|
(Wood) Aqueous, ethyl acetate, chloroform |
Antibacterial Staphylococcus aureus, Escherichia coli and Salmonella |
|
[118] |
||
|
Gum Arabic |
|
Complex of polysaccharides containing calcium, |
|
[113] |
|
|
magnesium, potassium salts, protein, gallic, ellagic and chlorogenic acids |
|||||
|
n-hexane |
Anticonvulsant |
Flavonoids, saponins, terpenoids, steroids, alkaloids, coumarin and tannins. |
|
[119] |
|
|
Ethanol |
|||||
|
Methanol |
Molluscicidal Activity (Biomphalaria pfeifferi) |
|
|
[110] |
|
|
Fruits |
methanol, chloroform water |
anti-trichomonal activity |
|
|
[115] |

Figure 1: Phylogeny and Classification of Fabaceae [20].
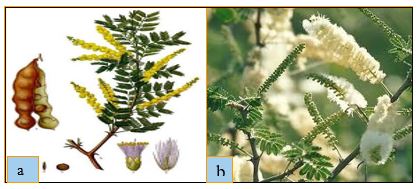
Figure 2: (a) fruit, inflorescence, (b) leaves of Acacia senegal (L.) Willd. [Marco Schmidt, (CC BY-NC-SA)].

Figure 3: (a) leaves, (b) inflorescence of Acacia seyal (Delile.) [P. Poilecot].

Figure 4: Some molecular structure from Acacia senegal (L.) Willd.

Figure 5: Some molecular structure from Acacia seyal (Del.).
Conclusion
This literature review provides an opportunity to learn about the therapeutic potentialities of Acacia senegal (L.) Willd. and Acacia seyal (Delile.). Although phytochemical knowledge of both species is limited, it appears to be a rich source of various active compounds with a wide range of pharmacological and therapeutic properties. For traditional use, it has become more common for several plants to be used in combination to treat a disease. This shows that the synergy of activity is well known to traditional healers. Among the diseases traditionally managed by A. senegal and A. seyal, infectious diseases occupy a prominent place. The pharmacological activity is objectively based on empirical experience and with the recent development of tools/methods based on Omics technologies (e.g. genomic, proteomic, transcriptomic, membranomic, etc.), it is important to measure the effects of these natural compounds on the physiology and metabolism of selected targeted cells (cancer cells, parasites, bacteria). Interestingly, this panel of research will be used to characterize the antimicrobial potential of Acacia species found in Burkina Faso. With the rise of resistant infections, natural extracts could be assayed in combination with usual antibiotics on multiresistant bacterial strains (MDR) to formulate future combined therapeutic strategies. To this aim, different approaches could be envisaged in this way. For instance, today a main resistance mechanism is associated with the lack of internal concentration of active antibiotics close to its target [120]. It will be interesting to test the capability of Acacia extracts to permeabilize the bacterial membrane and improve the activity of antibiotics in resistant bacterial strains as previously reported for some other natural products [106, 121,122]. Alternatively, it will be interesting to use the purified extracts in order to impair the activity of efflux pumps present in multidrug resistant bacteria that expel the antibiotic before it blocks the target [123,124]. This mode of action has been reported for different natural compounds that block or inhibits the antibiotic flux across the pump channel [125-127]. These different perspectives are especially attractive taking into account the methods recently reported that allow measuring the drug transport across bacterial membrane [120]. Another approach can be to research some compound having new activity against bacterial physiology [128, 129]. To conclude, the Acacia represents an attractive source for future development of antimicrobial compounds that could be identified and characterized using the new tools available in biochemical, physicochemical and biological domains.
Acknowledgements
We thank J-M Brunel for and J. Vergalli for their helpful advices and fruitful discussions.
Authors’ contributions
RDM, HMK and AH had collected all data reported. RDM wrote the paper. AH and ADR supervised the study. All authors read and approved the final manuscript.
Availability of data and materials
Data can be requested from the corresponding author.
Ethics approval and consent to participate
All participants were asked for their free prior informed consent.
References
1. Sofowora A. 2010. Plantes médicinales et médecine traditionnelle d'Afrique: Academie suisse des sciences naturelles, Karthala Ed. 375. Ref.: https://bit.ly/3cxqhA9
2. World Health Organization. Programme on Traditional Medicine. Principes me?thodologiques ge?ne?raux pour la recherche et l' e?valuation relatives a? la me?decine traditionnelle. Gene?ve: Organisation mondiale de la Sante?. 2000. Ref.: https://bit.ly/2Tuv8Lc
3. Westh H, Zinn CS, Rosdahl VT et al. 2004. An international multicenter study of antimicrobial consumption and resistance in Staphylococcus aureus isolates from 15 hospitals in 14 countries. Microbiol Drug Res. 10: 169-178. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/15256032
4. Boucher HW, Talbot GH, Bradley JS, et al. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 48: 1-12. Ref. : https://bit.ly/38py0gj
5. Tacconelli E, Carrara E, Savoldi A, et al. 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 18: 318-327. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/29276051
6. WHO reports, World Health Organization, Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline, Including Tuberculosis WHO. 2017.
7. Sathishkumar T, Baskar R, Rajeshkumar M. 2012. In vitro antibacterial and antifungal activities of Tabernaemontana heyneana Wall. leaves. J Appl Pharm Sci. 2: 17. Ref.: https://bit.ly/38tHx6j
8. Thawabteh A, Juma S, Bader M, et al. 2019. The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens. Toxins (Basel). 11. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/31717922
9. Liu M, El-Hossary EM, Oelschlaeger TA, et al. 2019. Potential of marine natural products against drug-resistant bacterial infections.Lancet Infect Dis. 19: 237-245. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/31031171
10. Mulat M, Pandita A, Khan F. 2019. Medicinal Plant Compounds for Combating the Multi-drug Resistant Pathogenic Bacteria: A Review. Curr Pharm Biotechnol. 20: 183-196. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/30854956
11. Zhao Y, Li H, Wei S, et al. 2019. Antimicrobial Effects of Chemical Compounds Isolated from Traditional Chinese Herbal Medicine (TCHM) Against Drug-Resistant Bacteria: A Review Paper. Mini Rev Med Chem. 19: 125-137. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/30332952
12. Fernandez De La Pradilla C. (1981 and 1985) Des plantes qui nous ont gueris: Jeunesse d'Afrique, Ouagadougou, Burkina Faso Tome 1 and Tome 2.
13. Kerharo J, Adam JG. 1964. Plantes médicinales et toxiques des Peul et des Toucouleur du Sénégal. Journal d'agriculture traditionnelle et de botanique appliquée. 11: 384-444. Ref.: https://bit.ly/2PR6WAl
14. Kerharo J, Adam J-G. 1974. La pharmacopée sénégalaise traditionnelle: plantes médicinales et toxiques. In: Journal d'agriculture tropicale et de botanique appliquée. 21: 76-77. Ref.: https://bit.ly/2vGKAKQ
15. Adanson, M. Families des plantes, 2 vols. Vincent, Paris. 1763 ; 325: 27.
16. de Jussieu AL (1789) Genera plantarum secundum ordines naturales disposita juxta methodum in horto regio parisiensi exaratam, anno 1774, numerisé 8 nov. 2012: veuve Herissant. 499.
17. Group AP. 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc. 161: 105-121. Ref.: https://bit.ly/2PTgeM9
18. LPWG, Legume Phylogeny Working Group. 2013. Legume phylogeny and classification in the 21st century: Progress, prospects and lessons for other species-rich clades. Taxon. 62: 217-248. Ref.: https://bit.ly/2xfpbsM
19. Lewis G, Schrire B, Mackinder B, Rico L, et al. 2013. Linear sequence of legume genera set in a phylogenetic context-a tool for collections management and taxon sampling. S Afr J Bot. 89: 76-84. Ref.: https://bit.ly/3cDoSIj
20. Miller JT, Seigler D, Mishler BD. 2014. A phylogenetic solution to the Acacia problem. Taxon. 63: 653-658. Ref.: https://bit.ly/3aumGB6
21. Azani N, Babineau M, Bailey CD. et al. 2017. The LPWG. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon. 66: 44-77. Ref.: https://bit.ly/3cDp22n
22. Brockwell J, Searle SD, Jeavons AC, et al. 2005. Nitrogen fixation in acacias: an untapped resource for sustainable plantations, farm forestry and land reclamation. Australian Centre for International Agricultural Research (ACIAR). 132. Ref.: https://bit.ly/2PPG0kz
23. Maslin B, Stirton C. 1997. Generic and infrageneric classification in Acacia (Leguminosae: Mimosoideae): a list of critical species on which to build a comparative data set. Bull Intern Group Study of Mimosoideae. 20: 22-44. Ref.: https://bit.ly/3aumZfe
24. Thiombiano A, Schmidt M, Dressler S, et al. 2012. Catalogue des plantes vasculaires du Burkina Faso. Boissiera: mémoires des Conservatoire et Jardin botaniques de la Ville de Genève. 65: 1-391. Ref.: https://bit.ly/2IseAwI
25. Omondi SF, Kireger E, Dangasuk OG, et al. 2010. Genetic diversity and population structure of Acacia senegal (L) Willd. in Kenya. Trop Plant Biol. 3: 59-70. Ref.: https://bit.ly/2PSey5t
26. Duke JA. 2012. Handbook of legumes of world economic importance: Springer Science & Business Media. 346. Ref.: https://bit.ly/2PLYn9Z
27. Kew RBG. The state of the world’s plants report-2016. Royal Botanic Gardens, Kew.
28. Fagg C, Allison G. 2004. Acacia Senegal and the gum arabic trade: monograph and annotated bibliography: Oxford Forestry Institute, University of Oxford,Tropical forestry papers. 42. Ref.: http://www.ox.ac.uk/
29. Odee DW, Telford A, Wilson J, et al. 2012. Plio-Pleistocene history and phylogeography of Acacia senegal in dry woodlands and savannahs of sub-Saharan tropical Africa: evidence of early colonisation and recent range expansion. Heredity. 109: 372. Ref.: https://go.nature.com/2PUoZWq
30. Odee DW, Wilson J, Omondi S, et al. 2015. Rangewide ploidy variation and evolution in Acacia senegal: a north–south divide? AoB Plants. Ref.: https://bit.ly/3cwvfNP
31. Arbonnier M. 2009. Arbres, arbustes et lianes des zones sèches d'Afrique de l'Ouest. Versailles Ed Quae, MHNH. 574.
32. Nacoulma O, Millogo-Rasolodimby J. 1985. Les produits de la ruche et leurs utilisations au Burkina Faso. Rev Med Pharm Afr. 9: 759-767.
33. Tapsoba H, Deschamps JP. 2006. Use of medicinal plants for the treatment of oral diseases in Burkina Faso. J ethnopharmacol. 104: 68-78. Ref.: https://bit.ly/2TsN1tS
34. Aké-Assi YA. 1992. Contribution au recensement des espèces végétales utilisées traditionnellement sur le plan zootechnique et vétérinaire en Afrique de l'Ouest. Lyon, Université Claude Bernard. 220.
35. Thoen D, Thiam A. 1990. Utilisations des plantes ligneuses et sub-ligneuses par les populations de la region Sahelienne du lac de Guiers (Senegal). Bull Med TradPharm. 4: 169-178.
36. Diallo D, Diakite C, Mounkoro P, et al. 2007. Knowledge of traditional healers on malaria in Kendi (Bandiagara) and Finkolo (Sikasso) in Mali. Le Mali medical. 22: 1-8. Ref.: https://bit.ly/2TsQSa6
37. Maiga A, Diallo D, Fane S, et al. 2005. A survey of toxic plants on the market in the district of Bamako, Mali: traditional knowledge compared with a literature search of modern pharmacology and toxicology. J Ethnopharmacol. 96: 183-193. Ref.: https://bit.ly/2TKqcjV
38. Adjanohoun EJ. 1980. Médecine traditionnelle et pharmacopie: contribution aux études ethnobotaniques et floristiques au Niger: Paris, France, Agence de coopération culturelle et technique. 250.
39. Astor G, Von Massow F, Rauwald H. 1992. Pharmacopée nationale des plantes traditionnelles. Niger. Deutsche Gesellschaft für Technische Zusammenarrbeit (GTZ) GmbH, Eschborn.
40. Moussa A. 1998. Quels remèdes pour les principales pathologies du dromadaire chez les Touareg de la région de Tchin-Tabaraden (Niger). Pharm Méd Trad Afr. 10: 114-127. Ref.: https://bit.ly/2TIoYFZ
41. Iyamah P, Idu M. 2015. Ethnomedicinal survey of plants used in the treatment of malaria in Southern Nigeria. J Ethnopharmacol. 173: 287-302. Ref.: https://bit.ly/2Ts2FFL
42. Kokwaro JO. 2009. Medicinal plants of east Africa. University of Nairobi Press. 478.
43. Bekalo I, Keengwe M, Mathias E, et al. 1996. Ethnoveterinary medicine in Kenya: a field manual of traditional animal health care practice. Nairobi, Kenya: Intermediate Technology Development Group and International Institute of Rural Reconstruction. 226.
44. Ichikawa M, Kimura D, Terashima H. 2001. AFlora: A database of traditional plant use in tropical Africa. Syst Geograph Plants. 71: 759-764. Ref.: https://bit.ly/32WiNCv
45. Heine B, Heine I, Ko?nig C, et al. 1988. Plant concepts and plant use: An ethnobotanical survey of the semi-arid and arid lands of East Africa. Part IV : plants of the Borana (Ethiopia and Kenya). Saarbruken, Germany: Verlag Breitenbach publisher.
46. Bussmann RW. 2006. Ethnobotany of the Samburu of Mt. Nyiru, South Turkana, Kenya. J Ethnobiol Ethnomedicine. 2: 35. Ref.: https://bit.ly/2Tt9zdV
47. Lulekal E, Kelbessa E, Bekele T, et al. 2008. An ethnobotanical study of medicinal plants in Mana Angetu District, southeastern Ethiopia. J Ethnobiol Ethnomed. 4: 1-10. Ref.: https://bit.ly/2wu7chP
48. Belayneh A, Asfaw Z, Demissew S, et al. 2012. Medicinal plants potential and use by pastoral and agro-pastoral communities in Erer Valley of Babile Wereda, Eastern Ethiopia. J Ethnobiol Ethnomedicine. 8: 42. Ref.: https://bit.ly/32VPUGH
49. Teklehaymanot T. 2017. An ethnobotanical survey of medicinal and edible plants of Yalo Woreda in Afar regional state, Ethiopia. J Ethnobiol Ethnomed. 13: 40. Ref.: https://bit.ly/2v3ApzW
50. Urso V, Signorini MA, Tonini M, 2016. Wild medicinal and food plants used by communities living in Mopane woodlands of southern Angola: Results of an ethnobotanical field investigation. J Ethnopharmacol. 177: 126-139. Ref.: https://bit.ly/2TLawx5
51. Johns T, Mhoro E, Sanaya P, et al. 1994. Herbal remedies of the Batemi of Ngorongoro District, Tanzania: a quantitative appraisal. Econ Bot. 48: 90-95. Ref.: https://bit.ly/3czHWHB
52. Ruffo C. 1991. A survey of medicinal plants in Tabora region. Tanzania in Traditional Medicinal Plants, Dar Es Salaam University Press-Ministry of Health, Tanzania. 391. Ref.: https://bit.ly/3awLWGP
53. Minja M. 1999. The Maasai wonder plants. People and Plants’ Training Workshop held at the Tropical Pesticides Research Institute-Arusha Tanzania.
54. Tabuti JR, Lye KA, Dhillion S. 2003. Traditional herbal drugs of Bulamogi, Uganda: plants, use and administration. J Ethnopharmacol. 88: 19-44. Ref.: https://bit.ly/32UN7xe
55. Gradé JT, Tabuti JR, Van Damme P. 2009. Ethnoveterinary knowledge in pastoral Karamoja, Uganda. J Ethnopharmacol. 122: 273-293. Ref.: https://bit.ly/2Ir0Xhn
56. Hilmi YAM, Abdalgadir H, Khalid A, et al. 2014. A study of antioxidant activity, enzymatic inhibition and in vitro toxicity of selected traditional sudanese plants with anti-diabetic potential. BMC Complement Altern Med. 14: 149. Ref.: https://bit.ly/39r57C0
57. Musa MS, Abdelrasool FE, Elsheikh EA. et al. 2011. Ethnobotanical study of medicinal plants in the Blue Nile State, South-eastern Sudan. J Med Plants Res. 5: 4287-4297. Ref.: https://bit.ly/2xatLIB
58. Niang A. 1987. Contribution a? l’e?tude de la Pharmacopée traditionnelle mauritanienne. Ecole Nationale de Médecine vétérinaire,Sidi Thabet, Tunisie. 156.
59. Bellakhdar J. 1997. La pharmacopée marocaine traditionnelle, Medecine arabe ancienne et savoirs populaires. Ibis Press, Paris: Editions le Fennec, Casablanca. 764.
60. Sérémé A, Millogo Rasolodimby J, et al. 2008. Concentration en tanins des organes de plantes tannifères du Burkina Faso. J Soc Ouest-Afr Chim. 25: 55-61. Ref.: https://bit.ly/2TCAMJW
61. Zerbo P, Rasolodimby JM, Ouedraogo ON, et al. 2011. Plantes médicinales et pratiques médicales au Burkina Faso: cas des Sanan. Bois et forêts des tropiques. 307: 41-53. Ref.: https://bit.ly/38o3m73
62. Chevalier A. 1905. Les végétaux utiles de l'Afrique tropicale française: études scientifiques et agronomiques Dépot des publications. 152.
63. Oumar F. 1962. Traitement des morsures de serpents avec des plantes du Djolof (Sénégal) Notes africaines, 93, 13-14, à partir de: Adam, J., Les plantes utiles en Afrique occidentale. (N. africaine Ed. Vol. 93).
64. Kalis S. 1997. Médecine traditionnelle, religion et divination chez les Seereer Siin du Sénégal: la connaissance de la nuit: L'Harmattan Edition. 335.
65. Dupire M. 1959. Pharmacopée peule du Niger et du Cameroun. Aequatoria. 22: 147-148.
66. Adam JG, Echard N, Lescot. 1972. Plantes médicinales Hausa de l'Ader (République du Niger). J Agriculture traditionnelle et botanique appliquée. 19: 259-399. Ref. : https://bit.ly/2Tqg8xQ
67. Puffet H. 1985. Pharmacopée vétérinaire traditionnelle des éleveurs du Sud-Niger. Tropicultura. 3: 14-15.
68. Saadou M. 1993. Les plantes medicinales du Niger: premier supplement a? l’enquête ethnobotanique de 1979. Rev Med Pharmacop Afr. 7:11-24.
69. Lindsay R, Hepper F. 1978. Medicinal Plants of Marakwet, Kenya: Royal Botanic Gardens (Kew). Herbarium, Kew, Richmond, United Kingdom. 49.
70. Nguta J, Mbaria J, Gakuya D, et al. 2010. Traditional antimalarial phytotherapy remedies used by the South Coast community, Kenya. J Ethnopharmacol. 131: 256-267. Ref.: https://bit.ly/3aBvSnr
71. Wambugu SN, Mathiu PM, Gakuya DW, et al. 2011. Medicinal plants used in the management of chronic joint pains in Machakos and Makueni counties, Kenya. Journal Ethnopharmacol. 137: 945-955. Ref.: https://bit.ly/32W9Mt8
72. Doka I, Yagi S. 2009. Ethnobotanical survey of medicinal plants in West Kordofan (Western Sudan). Ethnobot Leaflets. 11: 8. Ref.: https://bit.ly/39oRQd0
73. El-Ghazali G, El Tohami M, El Egami A, et al. 1997. Medicinal plants of the Sudan: Part 4. Medicinal plants of Northern Kordofan. Medicinal and Aromatic Plants Research Institute. National Center for Research, Khartoum.
74. Adjanohoun E, Ahyi M, Aké Assi L, et al. 1986. Contributions aux Études Ethnobotaniques et Floristiques au Togo, Médecine Traditionelle et Pharmacopée: Agence de Coopération Culturelle et Technique, Paris, France. 671.
75. Adjanohoun EJ. 1989. Contribution aux études ethnobotaniques et floristiques en République Populaire du Bénin: Paris, France, Agence de coopération culturelle et technique. 895.
76. Malgras D. 1992. Arbres et arbustes guérisseurs des savanes maliennes. Ed Karthala and ACCT, Paris. 478.
77. Durand JM. 1960. Les plantes bienfaisantes du Rwanda et de l'Urundi. Astrida, Groupe scolaire. 89.
78. Hassan-Abdallah A, Merito A, Hassan S, et al. 2013. Medicinal plants and their uses by the people in the Region of Randa, Djibouti. J Ethnopharmacol. 148: 701-713. Ref.: https://bit.ly/2VQuUzA
79. Hammiche V, Maiza K. 2006. Traditional medicine in Central Sahara: pharmacopoeia of Tassili N’ajjer. J Ethnopharmacol. 105: 358-367. Ref.: https://bit.ly/38o6sYJ
80. Boulos L. 1983. Medicinal Plants of North Africa. Reference Publications, Inc. Medicinal Plants of the World. 1: 286. Ref.: https://bit.ly/2uXIOVo
81. Seigler DS. 2003. Phytochemistry of Acacia-sensu lato. Biochem Syst Ecol. 31: 845-873.
82. Jain R, Sharma P, Bhagchandani T, et al. 2012. Phytochemical investigation and antimicrobial activity of Acacia senegal root heartwood. J Pharm Res. 5: 4934-4938. Ref.: https://bit.ly/39uWQwD
83. Morita M, Shibuya M, Kushiro T, et al. 2000. Molecular cloning and functional expression of triterpene synthases from pea (Pisum sativum) New αamyrin producing enzyme is a multifunctional triterpene synthase. Eur J Biochem. 267: 3453-3460. Ref.: https://bit.ly/38nH3ys
84. Jabeen K, Javaid A, Ahmad E, et al. 2011. Antifungal compounds from Melia azedarach leaves for management of Ascochyta rabiei, the cause of chickpea blight. Nat Prod Res. 25: 264-276. Ref.: https://bit.ly/2VNemZa
85. Samrot AV, Sahiti K, Raji P, et al. 2016. TLC bio-autography guided identification of antioxidant and antibacterial activity of Acacia senegal Der Pharm Lett. 8: 41-47. Ref.: https://bit.ly/2wAL8SA
86. Okoro S, Kawo A, Arzai A. 2012. Phytochemical screening, antibacterial and toxicological activities of Acacia senegal extracts. BAJOPAS. 5: 163-170. Ref.: https://bit.ly/2PPWI3h
87. Mudi S, Salisu A. 2009. Studies on brine shrimp lethality and activity of stem bark extract of Acacia senegal L. on respiratory tract pathogenic bacteria. Int J Biomed Health Sci. 5: 139-143. Ref.: https://bit.ly/2TrGvTV
88. Ali H, Dixit S. 2012. In vitro antimicrobial activity of flavanoids of Ocimum sanctum with synergistic effect of their combined form. Asian Pac J Trop Dis. 2: 396-398. Ref.: https://bit.ly/39FaHR2
89. Araruna MK, Brito SA, Morais-Braga MF, et al. 2012. Evaluation of antibiotic & antibiotic modifying activity of pilocarpine & rutin. Indian J Med Res. 135: 252-254. Ref.: https://bit.ly/3cGedwP
90. de Queiroz Pimentel RB, da Costa CA, Albuquerque PM, et al. 2013. Antimicrobial activity and rutin identification of honey produced by the stingless bee Melipona compressipes manaosensis and commercial honey. BMC Complement Altern Med. 13: 151. Ref.: https://bit.ly/3awhWed
91. Dubey S, Ganeshpurkar A, Bansal D, et al. 2013. Experimental studies on bioactive potential of rutin. Chronicles of young scientists. 4: 153. Ref.: https://bit.ly/2wALkBi
92. Arima H, Ashida H, Danno GI. 2002. Rutin-enhanced antibacterial activities of flavonoids against Bacillus cereus and Salmonella enteritidis. Biosci Biotechnology Biochem. 66: 1009-1014. Ref.: https://bit.ly/2Ir54dj
93. Abdelhady MIK, Kamal A, Youns M. 2012. Biological activity and total phenolic contents of ethanolic extracts of three species of Acacia leaves. J Pharm Res. 5: 691-695.
94. Batra S, Batra N, Nagori BP. 2013. Preliminary phytochemical studies and evaluation of antidiabetic activity of stem bark of Acacia senegal (L.) Willd. in alloxan induced diabetic albino rats. Int J Med Res Rev. 1: 611-616. Ref.: https://bit.ly/32WdFOK
95. Alsadeg AM, Koko WS, Osman E. 2015. In Vitro anthelminthic activity of the methanol stem bark extract of Acacia senegal against Fasciola gigantica. Int Inv J Biochem Bioinform. 3: 18-22. Ref.: https://bit.ly/2Ipnv2k
96. Seif MM, Ahmed-Farid O, AboulthanaWM. 2017. Evaluation of the protective effect of Acacia senegal extract against di-(2-ethylhexyl phthalate) induced hepato-and neurotoxicity in rats. Annu Res Rev Biol.19: 1-17. Ref.: https://bit.ly/2TrGP55
97. Abdelhady MIS, Youns M. 2014. In-vitro evaluation of the anti-diabetic activity of alcoholic extracts of certain plants belonging to families Meliaceae and Fabaceae. Nat Prod Indian J. 10: 99-101.
98. Ram H, Jatwa R, Purohit A. 2014. Antiatherosclerotic and cardioprotective potential of Acacia senegal seeds in diet-induced atherosclerosis in rabbits. Biochem Res Intern. 6. Ref.: https://bit.ly/2xd9MJn
99. Eldeen I, Van Staden J. 2008. Cyclooxygenase inhibition and antimycobacterial effects of extracts from Sudanese medicinal plants. S Afr J Bot. 74: 225-229. Ref.: https://bit.ly/2vH3aCA
100. Abd el Nabi OM, Reisinger E, Reinthaler FF, et al. 1992. Antimicrobial activity of Acacia nilotica (L.) Willd. ex Del. var. nilotica (Mimosaceae). J Ethnopharmacol. 37: 77. Ref.: https://bit.ly/2Q7nzYP
101. Neuwinger HD. 1996. African ethnobotany: poisons and drugs: chemistry, pharmacology, toxicology. CRC Press. 941. Ref.: https://bit.ly/2IoD5er
102. Zingue S, Njuh AN, Tueche AB, et al. 2018. In vitro cytotoxicity and in vivo antimammary tumor effects of the hydroethanolic extract of Acacia seyal (Mimosaceae) stem bark. BioMed Res Int. 13. Ref.: https://bit.ly/38tkjNA
103. Ododo MM, Choudhury MK, Dekebo AH. 2016. Structure elucidation of β-sitosterol with antibacterial activity from the root bark of Malva parviflora. SpringerPlus. 5: 1210. Ref.: https://bit.ly/32VVCs7
104. Sen A, Dhavan P, Shukla KK, et al. 2012. Analysis of IR, NMR and antimicrobial activity of β-sitosterol isolated from Momordica charantia. Sci Secure J Biotechnol. 1: 9-13. Ref.: https://bit.ly/2vH3WiY
105. Mekbib SB, Regnier TJ, Sivakumar D, et al. 2009. Evaluation of Ethiopian plant extracts, Acacia seyal and Withania somnifera, to control green mould and ensure quality maintenance of citrus (Citrus sinensis L.) Fruits. 64: 285-294. Ref.: https://bit.ly/3aABvlU
106. Seukep JA, Sandjo LP, Ngadjui BT, et al. 2016. Antibacterial and antibiotic-resistance modifying activity of the extracts and compounds from Nauclea pobeguinii against Gram-negative multi-drug resistant phenotypes. BMC Complement Altern Med. 16: 193. Ref.: https://bit.ly/38qc6tC
107. Campos FM, Couto JA, Figueiredo AR, et al. 2009. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int J Food Microbiol. 135: 144-151. Ref.: https://bit.ly/2InqWX7
108. Hemaiswarya S, Doble M. 2010. Synergistic interaction of phenylpropanoids with antibiotics against bacteria. J Med Microbiol. 59: 1469-1476. Ref.: https://bit.ly/39oWurs
109. Soe WM, Myint NL, Sing LC, et al. 2011. Ethyl gallate as a combination drug can overcome resistance in MRSA. Lett Drug Design Discover. 8: 65-68. Ref.: https://bit.ly/39raz7W
110. Ismail MA, Koko WS, Osman EE, et al. 2016. Molluscicidal Activity of Acacia seyal (Dell) Bark Methanolic Extract Against Biomphalaria pfeifferi Snails. Int Biol Biomed J. 2: 73-79. Ref.: https://bit.ly/2vLQv1c
111. Mekbib SB. 2016. In vitro antimicrobial assay of selected medicinal plants against medically important plant and food-borne pathogens. J Medicinal Plants Studies. 4: 163-169. Ref.: https://bit.ly/3cCyXW8
112. Zingue S, Michel T, Cisilotto J, et al. 2018. The hydro-ethanolic extract of Acacia seyal (Mimosaceae) stem barks induced death in an ER-negative breast cancer cell line by the intrinsic pathway of apoptosis and inhibited cell migration. J Ethnopharmacol. 15 : 41-50. Ref. : https://www.ncbi.nlm.nih.gov/pubmed/29783017
113. Nie S-P, Wang C, Cui SW, et al. 2013. A further amendment to the classical core structure of gum arabic (Acacia senegal). Food Hydrocolloids. 31: 42-48. Ref.: https://bit.ly/2uYtQ1v
114. Eldeen I, Van Staden J. 2007. In vitro pharmacological investigation of extracts from some trees used in Sudanese traditional medicine. S Afr J Bot. 73: 435-440. Ref.: https://bit.ly/39tqvGH
115. Osman E, Koko,W, Dahab M. 2011. In vitro anti-trichomonal activity of three
endogenous Sudanese forestry medicinal trees. Int J Nat Prod Pharm Sci. 2: 11-19. Ref.: https://bit.ly/3aye0cS
116. Eldeen I, Van Staden J. 2007. Antimycobacterial activity of some trees used in South African traditional medicine. S Afr J Bot. 73: 248-251. Ref.: https://bit.ly/39raYY0
117. Abdllha HB, Mohamed AI, Almoniem KA, et al. 2016. Evolution of antimicrobial, antioxidant potentials and phytochemical studies of three solvent extracts of five species from Acacia used in sudanese ethnomedicine. Adv Microbiol. 6: 691-698. Ref.: https://bit.ly/32XfkDv
118. Mariod AA, Fadle N, Hasan AA. 2014. Antimicrobial screening of wood extracts of Combretum hartmannianum, Acacia seyal and Terminalia brownie. European J Mol Biol Biochem. 1: 77-80. Ref.: https://bit.ly/2TLKMkh
119. Garba U, Sadiq FSD, Abdullahi Y. 2018. Anticonvulsant Screening of Ethanol and N-Hexane extracts of Acacia seyal Del. (Stem Bark) in rats. Niger J Pharm Biomed Res. 3: 17-21. Ref.: https://bit.ly/3axWYM0
120. Masi M, Réfregiers M, Pos KM, et al. 2017. Mechanisms of envelope permeability and antibiotic influx and efflux in Gram-negative bacteria. Nat Microbiol. 2: 17001. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/28224989
121. Bolla JM, Alibert-Franco S, Handzlik J, et al. 2011. Strategies for bypassing the membrane barrier in multidrug resistant Gram-negative bacteria. FEBS Lett. 585: 1682-1690. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/21549704
122. Fadli M, Chevalier J, Saad A, et al. 2011. Essential oils from Moroccan plants as potential chemosensitisers restoring antibiotic activity in resistant Gram-negative bacteria. Int J Antimicrob Agents. 38: 325-330. Ref. : https://bit.ly/2TuRn3E
123. Wang Y, Venter H, Ma S. 2016. Efflux Pump Inhibitors: A Novel Approach to Combat Efflux-Mediated Drug Resistance in Bacteria. Curr Drug Targets. 17: 702-719. Ref.: https://bit.ly/32SfuMH
124. Ohene-Agyei T, Mowla R, Rahman T, et al. 2014. Phytochemicals increase the antibacterial activity of antibiotics by acting on a drug efflux pump. Microbiology open. 3: 885-896. Ref.: https://bit.ly/2TuRqwm
125. Fankam AG, Kuete V, Voukeng IK, et al. 2011. Antibacterial activities of selected Cameroonian spices and their synergistic effects with antibiotics against multidrug-resistant phenotypes. BMC Complement Altern Med. 11: 104. Ref. : https://bit.ly/2TCGImc
126. Touani FK, Seukep AJ, Djeussi DE, et al. 2014. Antibiotic-potentiation activities of four Cameroonian dietary plants against multidrug-resistant Gram-negative bacteria expressing efflux pumps. BMC Complement Altern Med. 14: 258. Ref.: https://bit.ly/2TLLDkZ
127. Zaynab M, Fatima M, Abbas S, et al. 2018. Role of secondary metabolites in plant defense against pathogens. Microb Pathog. 124: 198-202. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/30145251
128. Chandra Mohana N, Yashavantha Rao HC, Rakshith D, et al. 2018. Omics based approach for biodiscovery of microbial natural products in antibiotic resistance era. J Genet Eng Biotechnol. 16: 1-8. Ref. : https://bit.ly/2VQAoua




















