Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ijpmh.2021.110010Article Views : 23Article Downloads : 35
Comparing the mRNA levels in the brain stem of stressed rats with Tianeptine and Sertraline administration
Mehnaz Gitay1,3*, Kausar Saboohi1,2, Bushra Chaudhary2 and Samina Bano1
1Clinical Biochemistry and Psychopharmacology Research Unit, Department of Biochemistry University of Karachi, Karachi, Pakistan
2Biological and Biomedical Sciences, The Aga Khan University, Karachi, Pakistan
3Department of Biochemistry, DIMC, Ojha Campus, Dow University of Health Sciences, Karachi, Pakistan
*Corresponding Author: Dr. Mehnaz Gitay, Clinical Biochemistry and Psychopharmacology Research Unit, Department of Biochemistry University of Karachi, Karachi-75270, Pakistan, Email: mehnaz.nuruddin@duhs.edu.pk; quick_gitay@hotmail.com
Article Information
Aritcle Type: Research Article
Citation: Mehnaz Gitay, Kausar Saboohi, Bushra Chaudhary, et al. 2021. Comparing the mRNA levels in the brain stem of stressed rats with Tianeptine and Sertraline administration. Int J Psychiatr Ment Health. 3: 01-09.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2021; Mehnaz Gitay
Publication history:
Received date: 14 December, 2020Accepted date: 31 December, 2020
Published date: 04 January, 2021
Abstract
Since the discovery that antidepressants work in part by potentiating the actions of 5-HT within the serotonergic system the effects these drugs elicit on the serotonin transporter (SERT) protein have been an area of active research. The aim of the present study is to understand the mechanism of action of tianeptine and sertraline in relation to its effects on the expression of SERT gene and SERT protein in the brain stem of stressed rats. Albino Wistar rats were divided into two groups (n=12) i.e. saline and drug. Each group was further divided into two equal groups, stressed (Forced Swim Test-FST) and unstressed. Tianeptine and sertraline were administered to rats orally for 4 weeks prior to subjecting them to forced swim test and decapitation. Tianeptine increased the expression of SERT gene though the protein is reduced in the brain stem in stress. On the contrary sertraline decreased the expression of SERT gene but increased the protein in the brain stem. The increase in swimming time in FST by both the drugs indicates stress alleviating effects. It can be concluded that Tianeptine prevents stress induced changes through its effect on the serotonergic system, including SERT mRNA and protein. Sertraline complies to the reuptake inhibition property by reducing SERT gene expression. Results are discussed specifically, how changes in SERT expression following chronic antidepressant treatment may contribute to the therapeutic benefits of antidepressants.
Keywords: Antidepressants; Serotonin transporters; Stress; Serotonergic system; SERT gene expression
Introduction
Antidepressants (SSRIs) are believed to relieve depressed mood and anxiety by inhibiting reuptake of serotonin so as to cause increase in extracellular serotonin. This homeostasis alteration is thought to undergo further adaptive processes that together constitute the cellular mechanism of current antidepressant therapy and are not clear yet. Tianeptine is an antidepressant drug with structural similarities to the tricyclic antidepressant agents but a novel neurochemical profile. The main difference between this and other antidepressant agents is its action on serotonin (5-hydroxytryptamine; 5-HT). Tianeptine increases serotonin uptake in the brain and platelets. The behavioral and physical (atrophy of neuronal dendrites) effects of stress on the hypothalamic-pituitary-adrenal axis are reduced by tianeptine, and levels of noradrenalin (norepinephrine) and dopamine are indirectly increased in several regions of the brain. The exact mechanism of action of Tianeptine is still not clear [1]. It is known that inhibition of serotonin transporter protein function increases synaptic serotonin [2]. With this point, drugs that block the transporters known as selective serotonin reuptake blockers (SSRIs) are considered to treat disorders that are caused by low serotonin levels, depression for instance. The therapeutic action of SSRIs is due to the activation of post synaptic receptors followed by intermediate modulations and feedback mechanisms [3]. Sertraline, an SSRI, acts via blocking the reuptake of synaptic serotonin back in the neuron, increasing the duration of serotonin in the synapse and thus, its function. Be Mansour and group, in 2002 observed a downregulation in SERT with a time course study, up to 21 days [4]. It was concluded that the decline in SERT binding sites was not a result of reduced gene expression, in fact, the transporters were recovered after treatment cessation due to increased gene expression. Later, a study with 21 days of subcutaneous sertraline (7.5 mg/ kg / day) administration found to decrease serotonin transporter density and function [5]. Since the discovery that antidepressants work in part by potentiating the actions of 5-HT within the serotonergic system the effects these drugs elicit on the serotonin transporter (SERT) protein have been an area of active research. Specifically, antidepressants are thought to initially function by acting as SERT antagonists resulting in an immediate increase in the synaptic 5-HT concentration. SERT proteins are found in various regions of the brain as transporters, including axonal varicosities and terminal boutons [6]. Changes to the expression level of SERT in response to drugs could contribute to neuroadaptive changes associated with chronic antidepressant administration. Surprisingly, the effects of antidepressants on regulation of SERT expression remain largely unevaluated. Glucocorticoids are known to be released in physiological states directed by the circadian rhythm whereas in stress an enhanced release is observed, that might lead to a raised expression of SERT ultimately causing depression [7]. Ex-vivo studies performed on cortical and hippocampal synaptosomes of rats treated acutely and chronically with tianeptine [8,9] suggested the drug to enhance the uptake of serotonin from the synapse into the neuron. Broqua and group explored the influence of tianeptine (10mg/kg) on the neurochemical, neuroendocrinological and behavioral effects of stress on restraint stressed rats and did not associate the antidepressant properties of tianeptine with any such change [10]. Bano and colleagues investigated the acute effects of 10 mg /kg tianeptine on stress related parameters and concluded that tianeptine increased circulating corticosterone, though the pharmacological effect of the drug is attributed to the availability of tryptophan from the periphery to the brain [11]. Liu and coworkers found that prophylactic tianeptine treatment reversed the structural changes in the brain [12], while Kuroda and group concluded that 10 mg/kg tianeptine, twice a day for 14 days decreased both serotonin transporter mRNA and binding sites in rat brain [13]. Most of the research to date has concentrated on the SERT located downstream of the projection pathways with focus on the post-synaptic effects of the SERT inhibition or activity [14]. Following some authors , instead of studying individual raphe nuclei for transporter studies [15,16], collectively the brain stem shall represent the nuclei. SERT in the brain stem gives an account of the autoregulatory status of the serotonergic neurons. Elucidation of the antidepressive mechanism of tianeptine action is one of the most difficult problems. The analysis of available data has shown the lack of any information that could explain neurochemical mechanism of action of this antidepressant. It is well known that tianeptine activates serotonin reuptake in the presynaptic ending, thus promoting more withdrawal of serotonin from the synaptic cleft. In spite of all existing theories tianeptine on the clinical level reveals antidepressive, anxiolytic effects, an action similar to SSRIs which is rather puzzling, according to Ansseau (1993) [17]. On the contrary, sertraline is a well-known serotonin re-uptake inhibitor and its effect on SERT in alleviating depression may be well endorsed through previous data, though the present study was carried out with the therapeutic dose(30mg/kg) for which no data is still available. The aim of the present study is to elucidate the mechanism of action of tianeptine and sertraline in relation to SERT gene expression.
Materials and Methods
Animals and Treatment
All animal procedures described below were conducted in strict accordance with the national research council for the care and use of laboratory animals (1996). Ethical approval was obtained from institutional animal ethics committee. Locally bred Adult Albino Wistar rats (150-200 g) were housed 6 per cage under standard light and dark cycle at 250C ± 20 C room temperature and were maintained on lab chow and water ad libitum. All experiments were carried out in the light phase of the cycle and were carried out with minimum suffering to the animals. Rats were checked for open field locomotor activity. Tianeptine (20 mg/kg/ml) dissolved in saline was orally administered to the test group for 4 weeks. An equal volume of saline was given to the controls. Rats were decapitated on the 29th day. The rats of the FST group were made to swim before decapitation. Brains were collected brain stems were separated snap frozen in liquid nitrogen and stored at -70o C respectively, until analysis.
Forced Swim Test (FST)
Locally bred Albino Wistar rats were exposed to forced swim test as described in detail [18]. Behavior during test swimming session was scored using a time sampling method [9]. Every 5 seconds; one of the 3 behaviors was recorded. Immobility was scored when the animal was making movements sufficient just to stay afloat. Swimming was scored when the animal actively swam around the tank (46 cm high and 20cm inner diameter) filled with 30 cm water (temperature 210C ± 20C), making movement greater than those necessary to stay afloat. Climbing was scored when the animal made vigorous thrashing movements with its fore paws, usually directed against the sides of the tank. Behavioral activity was videotaped and the results are shown as the total time in seconds for each behavioral category.
Chemicals and drugs
Tianeptine Sodium salt for the present study was purchased from Sigma Aldrich. Sertraline HCl was a kind gift from a local pharmaceutical. All chemicals were of the highest analytical grade.
Quantification of SERT mRNA expression
To quantify SERT mRNA isolated from the brain stems, the tissues were weighed and accordingly homogenized in appropriate volumes of Trizol® reagent (Invitrogen) for isolation of total RNA. DNase 1 digestion was performed and the RNA was purified with chloroform followed by isopranol precipitation of RNA. The quantity and integrity of the RNA was checked with the nanodrop (ND 1000) and 1μg of RNA was used for reverse transcription. Reverse transcription was done with superscript III (Invitrogen), by adding the RNA, primers and dNTPs (10μl) to a nuclease free tube. Mixture was heated for 5 minutes at 650C and chilled on ice. 10μl of cDNA synthesis mix that comprises of 10x RT buffer, MgCl2, 0.1M DTT, RNase OUT and Superscript III is added to the mixture. The contents were mixed gently and incubated at 500C for 50 minutes, then at 850C for 5 minutes to terminate the reaction. RNase H, 1μl was then added and incubated at 370C for 20 minutes. Reported primers from Abumaria, 2006 were used [19]. Amplicons were amplified in the Bio-Rad c1000 thermocycler using Go Taq® qPCR Master Mix (Promega). The components of the reaction mixture are given below in the table. The real time cycler conditions are as follows: 950C for 2 minutes, cycling steps-950C for 15 seconds, 550C for 1 minute, back again to the initial step of the cycle, i.e. 950C for 15 seconds. The cycle continued 40 times, resulting in 60 meramplicons for SERT and 83 mer amplicons for GAPDH.
|
Name of Gene |
Forward Primer |
Reverse Primer |
|
SERT |
TCTGAAAAGCCCCACTGGACT |
TAGGACCGTGTCTTCATCAGGC |
|
GAPDH |
TGCCCCCATGTTTGTGATG |
TGGTGGTGCAGGATGCATT |
Western Blotting
Total protein extracts from the brain stem were obtained from the phenolic phase of the Trizol homogenates after RNA isolation according to manufacturer’s instructions. Samples from each group were pooled and proteins were precipitated with isopropyl alcohol and then washed with 0.3M guanidine hydrochloride in 95% ethanol. The pellet was dissolved in 1% SDS with incubation at 500C for 5 to 10 minutes. The proteins were quantitated with modified Lowry Method and 200 μg of proteins/well was used for SDS PAGE. Proteins were separated in a 10% SDS-polyacrylamide gel and transferred to Polyvinylidene difluoride membranes (PVDF-cat # Z310204-6EA, Sigma). The resulting blots were then probed with antibodies against SERT (Millipore: anti-Serotonin Transporter-cat # AB1594P) for 12 hours and bands were visualized after color development with NBT/BCIP. Two SDS PAGE gels were run simultaneously, in order to compare the location of the bands on the membrane (band size) on the protein marker of the simultaneous gel. JPEG images were viewed and compared with Adobe Photoshop according to the band intensities, and contrast adjustments were made [20].
Statistical Analysis
All results were expressed as mean ± standard error of mean (SEM). Data was analyzed using either 1- way ANOVA followed by Tukeys HSD or student’s t test where appropriate. P< 0.05 was considered statistically significant. The analysis was carried out using version 19 of SPSS software.
Results
Figure 1: Shows the behavior of rats during forced swim test (FST) in terms of swimming, climbing and floating expressed in seconds. Data analyzed by students t-test indicates that swimming time is significantly increased by tianeptine (P=0.0005) as well as by sertraline (P=0.010) but floating time is significantly decreased by both the drugs (Tian-P=0.0005, Sert-P=0.042). Sertraline decreased climbing time significantly (P=0.04).
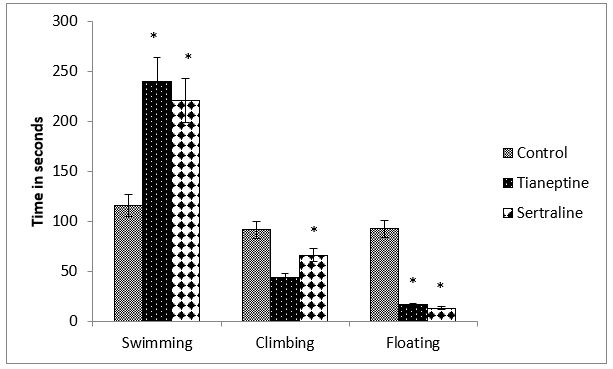
Figure 1: Effect of chronic tianeptine and sertraline administration on FST behaviour. Values are means± SEM for each group of 6 rats. Statistical analysis was performed using student’s t-test .The significance of the differences is indicated by *P<0.05.
Figure 2: Shows SERT gene expression after 4 weeks of tianeptine and sertraline administration. 1-way ANOVA indicates a significant difference among the groups (F=204.405, P=0.0001). Post hoc analysis indicates an increase in SERT mRNA (P= 0.0001) by FST. SERT mRNA is significantly increased (P=0.0001) in the tianeptine treated FST group too. Sertraline decreased SERT mRNA expression significantly (P=0.0001) as compared to the control group. In the sertraline treated group SERT mRNA is significantly decreased (P= 0.0001) by FST in comparison to the control FST group but increased significantly (0.007) when compared to the sertraline administered group.
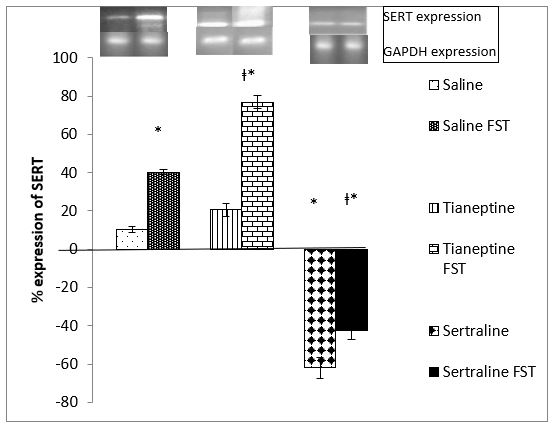
Figure 2: Effect of 4 weeks Tianeptine and Sertraline administration on SERT gene expression. Values are means± SEM for each group of 6 rats. The significance of the differences is indicated by *P<0.05 when stressed group is compared with respective control and †P<0.05 when drug treated group is compared with similarly treated saline control.
Table 1: Shows SERT protein in the brain stem after chronic tianeptine administration. According to the band intensity SERT protein is 1.98 times more expressed due to FST. It is 1.92 times more expressed in the brain stem of tianeptine administered group than in the saline group, while in the FST group tianeptine reduces SERT proteins 0.75 times as compared to the unstressed group. It also indicates that Sertraline does not alter SERT protein in the brain stem when the FST group is compared with drug + FST, but increases it in the sertraline treated unstressed group.
|
Table 1: The amount of SERT protein in the brain is estimated as the ratio of intensity with photoshop. |
|
|
Group |
Intensity as compared to saline control |
|
FST |
+1.98 |
|
Tianeptine |
+1.92 |
|
Tianeptine FST |
-0.75 |
|
Sertraline |
+1.02 |
|
Sertraline FST |
No change |
Discussion
The two antidepressants are compared in stress models of Forced Swim Test (FST). FST induces the stress response observed as decreased swimming time and increased corticosterone. The rise in corticosterone leads to tryptophan degradation in the liver, rendering it unavailable to the brain for serotonin synthesis causing depression. On the other hand, the rise in brain tryptophan due to stress is the physiological response of the body to stress through a rise in the free fraction of tryptophan and increased uptake by the brain [11]. Depression is primarily caused when the rate of hepatic tryptophan depredatory mechanism exceeds the physiological adaptive process to combat the effects of stress. Furthermore, depression is also the result of increased serotonin turnover in the brain. Behavioral analysis shows significant increase in swimming time in chronically tianeptine treated rates (figure 1). Such an increase indicates the effect of tianeptine on the serotonergic system. Since tianeptine is a reuptake enhancer, the serotonin deficit in the synapse apparently does not support the antidepressant action of the drug. Previously, scientists have revealed the antidepressant activity of tianeptine with the help of stress paradigms (e.g. Psychosocial stress) used as models of depression in animal studies. The antidepressant activity of tianeptine can be attributed to the ability of tianeptine to oppose the effects of chronic stress on brain structure and plasticity [21]. In a study, it was observed that tianeptine reversed the shrinkage of dendrites, even in the presence of glucocorticoids [22]. Abumaria and coworkers reported an increase in SERT expression by chronic social stress concurrent to which increased SERT gene expression is observed in FST subjected rats too, indicating an up regulation of transporters, enhancing synaptic serotonin reuptake Abumaria [19]. An activated HPA axis is known to enhance serotonin reuptake, confirming the possible increase in reuptake by FST [23]. Serotonin reuptake from the synapse is necessary to normalize the membrane potential of the neuron, preparing it for neurotransmitter firing again. Since the two main sources of serotonin available for release are expected to be newly synthesized serotonin and serotonin recycled after reuptake by SERT [24], SERT plays a key role in regulating serotonin signaling by clearing serotonin from the extracellular space and recycling it to the intracellular space where it can be repackaged into vesicles [25]. Since tianeptine is an effective antidepressant and enhances serotonin re-uptake, this property challenges the monoamine theory of depression. In light of Uzbay’s explanation, the mechanism of action of tianeptine can be well explained by the neuroplasticity theory of depression [26]. Changes in neuroplasticity due to stress leads to the development of psychiatric disorders and drugs that effect neuroplasticity have significant effect on depression treatment. Tianeptine enhances the SERT gene expression most probably to increase the number of transporters in order to elevate the serotonin reuptake from the synapse. This increased reuptake prepares the neuron for neurotransmitter firing, thus increasing the firing rate. It was reported by Zhou and coworkers that fluoxetine and tianeptine increased the density of serotonin and SERT immunoreactive axons in certain areas of the brain, though no significant change in the expression of tryptophan hydroxylase or SERT gene was reported [27]. The decrease in the brain stem transporters in FST with prior Tianeptine administration may activate the 5HT1A auto receptors to regulate a controlled serotonin synthesis, positing an effective recycling of the serotonin taken back into the neuron. Thus, the 5HT1A receptor activation counteracts the stress induced neuronal hyperactivity. In the absence of stress, the brain stem SERT protein is comparatively higher than in the presence of stress provoking enhanced auto receptors activation by Tianeptine in stress. The increased SERT gene expression, but decreased brain stem protein suggests a possible trafficking of SERT protein from the somatodendritic region to the terminals in the stressed group. Treatment with Sertraline for 4 weeks, shows the property of the drug to down regulate SERT gene expression irrespective of the presence or absence of stress. Reduced SERT synthesis facilitates a decrease in the reuptake increasing synaptic serotonin availability and function. Furthermore, the increase in the somatodendritic transporters (brain stem), suggests the importance of serotonin reuptake to prevent the activation of the 5HT 1A receptors, due to which the regulation of serotonin synthesis and firing takes place. Contrary to our results, previous study by Kim and coworkers in 1999 observed an increase in SERT mRNA hybridization by insitu technique, with Sertraline (10 mg / kg, i.p.) for 14 days. The result was justified as a compensatory mechanism to reduce synaptic levels of serotonin which were increased by long term sertraline treatment [28]. There are reports that some antidepressants, including SRIs reduce the expression of SERT [4,29,30] resulting in enhanced serotonergic neurotransmission. Zhao and group in 2009 researched the effect of Sertraline (7.5 mg /kg) treatment for 14 days on SERT mRNA. The difference in the result may probably be due to the difference in dosage and duration. A marked decrease in SERT mRNA with 30mg/kg in present study is the first finding in our knowledge with respect to the dosage, duration and result [5].
Acknowledgement
We thank the Higher Education Commission (HEC), Pakistan for financial support to Mehnaz Gitay (HEC, Indigenous Scholar).
References
1. Petermann M, Kronenberg G, Mosienko V, et al. 2020. Alterations in BDNF protein concentrations in the hippocampus do not explain the pro-neurogenic effect of citalopram on adult neurogenesis. Pharmacopsychiatry. Ref.: https://pubmed.ncbi.nlm.nih.gov/33197939/
2. Fuller RW. 1994. Uptake inhibitors increase extracellular serotonin concentration measured by brain microdialysis. Life Sci. 55: 163-167. Ref.: https://pubmed.ncbi.nlm.nih.gov/8007758/
3. Sprouse J, Clarke T, Reynolds L, et al. 1996. Comparison of the effects of Sertraline and its metabolite Desmethylsertraline on blockade of central 5HT reuptake in vivo. Neuropsychopharmacology. 14: 225-231. Ref.: https://pubmed.ncbi.nlm.nih.gov/8924190/
4. Benmansour S, Owens WA, Cecchi M, et al. 2002. Serotonin clearance in vivo is altered to a greater extent by antidepressant-induced downregulation of the serotonin transporter than by acute blockade of this transporter. J Neurosci. 22: 6766-6772. Ref.: https://pubmed.ncbi.nlm.nih.gov/12151556/
5. Zhao Z, Han- Zhang T, Bootzin E, et al. 2009. Association of Changes in Norepinephrine and Serotonin Transporter Expression with the Long-Term Behavioral Effects of Antidepressant Drugs. Neuropsychopharmacology. 34: 1467-1481. Ref.: https://pubmed.ncbi.nlm.nih.gov/18923402/
6. Schloss P, Williams DC. 1998. The serotonin transporter: a primary target for antidepressant drugs. J Psychopharmacology. 12: 115-121. Ref.: https://pubmed.ncbi.nlm.nih.gov/9694022/
7. Glatz K, Mössner R, Heils A, et al. 2003. Glucocorticoid-regulated human serotonin transporter (5-HTT) expression is modulated by the 5-HTT gene-promotor-linked polymorphic region. J Neurochem. 86: 1072-1078. Ref.: https://pubmed.ncbi.nlm.nih.gov/12911615/
8. Mennini T, Mocaer E, Garattini S. 1988. Tianeptine and amitryptyline controlled double-blind trial in depressed alcoholic patients. Neuropsychobiology. 19: 79-85.
9. Fattaccini CM, Bolanos F-Jimenez, Gozlan H, et al. 1990. Tianeptine stimulates uptake of 5-hydroxytryptamine in vivo in the rat brain. Neuropharmacology. 29: 1-8. Ref.: https://pubmed.ncbi.nlm.nih.gov/1689469/
10. Broqua P, Baudrie V, Chaouloff F. 1992. Differential effects of the novel antidepressant tianeptine on L-5-hydroxytryptophan (5-HTP)-elicited corticosterone release and body weight loss. EurNeuropsychopharmacol. 2: 115-120. Ref.: https://pubmed.ncbi.nlm.nih.gov/1633432/
11. Bano S, Gitay M, Ara I, et al. 2010. Acute effects of serotonergic antidepressants on tryptophan metabolism and corticosterone levels in rats. Pak. J. Pharm. Sci. 23: 266-272. Ref.: https://pubmed.ncbi.nlm.nih.gov/20566438/
12. Liu W, Shu XJ, Chen FY, et al. 2011. Tianeptine reverses stress-induced asymmetrical hippocampal volume and N-acetylaspartate loss in rats: an in vivo study. Psychiatry Res. 194: 385-392. Ref.: https://pubmed.ncbi.nlm.nih.gov/22047727/
13. Kuroda Y, Watanabe Y, McEwen BS. 1994. Tianeptine decreases both serotonin transporter mRNA and binding sites in rat brain. Eur J Pharmacol. 268: 3-5. Ref.: https://pubmed.ncbi.nlm.nih.gov/7925606/
14. Mac Gillivray L. 2012. The Regulation of Brain Serotonergic and Dopaminergic Neurons: The Modulatory Effects of Selective Serotonin Reuptake Inhibitors, Atypical Neuroleptics and Environmental Enrichment. Thesis: McMaster University.
15. Lesch KP, Heils A, Riederer P. 1996. The role of neurotransporters in excitotoxicity, neuronal cell death, and other neurodegenerative processes. J Mol Med. 74: 365-378. Ref.: https://pubmed.ncbi.nlm.nih.gov/8841949/
16. Sur C, Betz H, Schloss P. 1996. Immunocytochemical detection of the serotonin transporter in rat brain. Neuroscience. 73: 217-231. Ref.: https://pubmed.ncbi.nlm.nih.gov/8783244/
17. Ansseau M. 1993. The paradox of tianeptine. European Psychiatry. 8: 89-93.
18. Bano S, Dawood S. 2008. Serotonergic mediation effects of St John's Wort in rats subjected to swim stress. Pak J Pharm Sci. 21: 63-69. Ref.: https://pubmed.ncbi.nlm.nih.gov/18166522/
19. Abumaria N, Rygula R, Hiemke C, et al. 2007. Effect of chronic citalopram on serotonin-related and stress-regulated genes in the dorsal raphe nucleus of the rat. Eur Neuropsychopharmacology. 17: 417-429. Ref.: https://pubmed.ncbi.nlm.nih.gov/17182223/
20. Guerzoni C, Bardini M, Mariani SA, et al. 2006. Inducible activation of CEBPB, a gene negatively regulated by BCR/ABL, inhibits proliferation and promotes differentiation of BCR/ABL-expressing cells. blood. 107: 4080-4089. Ref.: https://pubmed.ncbi.nlm.nih.gov/16418324/
21. Czéh B, Michaelis T, Watanabe T, et al. 2001. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with Tianeptine. Proc Natl Acad Sci USA. 98: 12796-12801. Ref.: https://pubmed.ncbi.nlm.nih.gov/11675510/
22. Magariños AM, Deslandes A, McEwen BS. 1999. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol. 371: 113-122. Ref.: https://pubmed.ncbi.nlm.nih.gov/10357248/
23. Tafet G, Toister- Achituv M, Shinitzky M. 2001. Enhancement of serotonin uptake by cortisol: a possible link between stress and depression. Cogn Affect Behav Neurosci. 1: 96-104. Ref.: https://pubmed.ncbi.nlm.nih.gov/12467107/
24. Borue X, Condron B, Venton BJ. 2010. Both synthesis and reuptake are critical for replenishing the releasable serotonin pool in Drosophila. J Neurochem. 113: 188-199. Ref.: https://pubmed.ncbi.nlm.nih.gov/20070864/
25. Murphy DL, Lerner A, Rudnick G, et al. 2004. Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Mol Interv. 4: 109-123. Ref.: https://pubmed.ncbi.nlm.nih.gov/15087484/
26. Uzbay TI. 2008. Tianeptine: Potential influences on neuroplasticity and novel pharmacological effects. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 32: 915-924.
27. Zhou L, Huang KX, Kecojevic A, et al. 2006. Evidence that serotonin reuptake modulators increase the density of serotonin innervation in the forebrain.J Neurochem. 96: 96-406. Ref.: https://pubmed.ncbi.nlm.nih.gov/16300628/
28. Kim CY, Kim SY, Hong JP, et al. 1999. Effect of Sertraline on the Regulation of Serotonin Transporter mRNA Levels in Rat Brain. J Korean Neuropsychiatr Assoc. 38: 1071-1076.
29. Benmansour S, Cecchi M, Morilak DA, et al. 1999. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci. 19: 10494-10501. Ref.: https://pubmed.ncbi.nlm.nih.gov/10575045/
30. Gould GG, Pardon MC, Morilak DA, et al. 2003. Regulatory effects of reboxetine treatment alone, or following paroxetine treatment, on brain noradrenergic and serotonergic systems. Neuropsychopharmacology. 28: 1633-1641. Ref.: https://pubmed.ncbi.nlm.nih.gov/12825093/




















