Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ijme.2020.110003Article Views : 17Article Downloads : 19
Enhanced interfacial strength of plasma treated polyethylene and glass
Vojtech Novácek1, Petr Špatenka1, Tatana Vacková1,*, Zdenka Jeníková1, Syam Krishna2 and Bedrich Veselý3
1Department of Material Engineering, Faculty of Mechanical Engineering, Czech Technical University in Prague, Karlovo nám. 13, 121 35 Prague 2, Czech Republic
2Department of Chemistry, Sree Narayana College, Kollam - 691001, Kerala, India
3Department of Applied Physics and Technics, University of South Bohemia, Colleague of Education, Jeronýmova 10, 371 15 Ceské Budejovice, Czech Republic
*Corresponding Author: Tatana Vackova, Department of Material Engineering, Faculty of Mechanical Engineering, Czech Technical University in Prague, Karlovo nám. 13, 121 35 Prague 2, Czech Republic, Email: Tatana.Vackova@fs.cvut.cz
Article Information
Aritcle Type: Research Article
Citation: Vojtech Novácek, Petr Špatenka, Tatana Vacková, et al. 2020. Enhanced interfacial strength of plasma treated polyethylene and glass. I J Mech Eng. 2: 01-08.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2020; Vojtech Novácek
Publication history:
Received date: 11 February, 2020Accepted date: 21 February, 2020
Published date: 24 February, 2020
Abstract
Low-temperature plasma treatment represents an important technology to influence surface properties of polymers. Less financially demanding and environmentally friendly way leads to a significant enhancement of adhesion to another type of surfaces. The article describes the influence of plasma treatment time of polyethylene (PE) powder leading to the increase of interfacial strength to glass substrate. This strength was quantified by the tensile test and compared with the industrially used chemical products, maleic anhydride or silane. The maximum value of the interfacial strength was achieved by plasma treatment with an exposure time of 60 s and is at least by 150 % higher than in the case of the interphase created by silane. The results of the research, also proven by ESCA measurement, represent the potential of using plasma treatment of powder semi-products for the preparation of fibre-reinforced composites with a thermoplastic matrix processed only at atmospheric pressures.
Keywords: Plastics; Glass; Surface treatment; Interface/interphase
Introduction
Thermoplastic composites from polyolefins are frequently applied to various branches of the industry. Very often reinforcement by glass fibres is used for the improvement of mechanical properties, which strongly depends on the interfacial adhesion and is predominately determined by the transfer of mechanical load from the matrix to the fibres [1]. Various methods have been proposed for enhancement of the interfacial strength between glass fibres and a polymer matrix. Surface treatment of fibres by organosilanes has been established as a standard process. The reactive group of silanes forms a covalent bond with functional groups of the particular matrix. Non-polar thermoplastics have to be modified by non-saturated organosililanes grafted directly on the polymer chain, i. e. maleic anhydride (MAH) or silanes coupling agents containing vinyl groups used for polyolefin grafting [2-4].
Polyethylene (PE) is frequently used polyolefin in various branches of industry. Because of PE hydrophobicity, the adhesion to glass is very low. The addition of small amount of MAH is a standard modification resulting in incorporation of polar OH groups [2,4]. An environmentally friendly and less costly way of surfaces modification is the plasma grafting of functional groups [5-7]. Recently this treatment has become a commonly used for surface cleaning and activation before adhesive bonding technology [6,8,9]. Plasma technology works at a low temperature and low pressure, so there is no influence on bulk properties of materials [10]. By modifying the plasma discharge newly functional groups, e. g. hydroxide (-OH), carbonyl (C=O), carboxyl (COOH) or amino groups (NH2), are created on the molecular chain of the PE [11,12]. Incorporation of these groups significantly improve surface polarity [13,14], which is the premise of outstanding adhesion to other substrates. Additionally, the treated PE can be in powder form in a minimum particle size for maximizing the treated surface.
According to our knowledge, there is not enough published research focused on the enhancement of adhesion by a plasma treatment of PE powders to glass substrates. The recent worldwide research in enhancing the interfacial strength is focused on the treatment of fillers or on the combination of a plasma with chemical agents but not to plasma treatment of the basic matrix [15-22]. The aim of this work is investigation of adhesive properties of the plasma-treated PE powder onto glass sheet surfaces in comparison with generally used coupling agents (MAH, silane).
Materials and Methods
Materials
Plasma treated and untreated linear low-density polyethylene (PE; 0.935 g.cm-3, Tm 124 °C) powder with an average particle size of 280 μm was used. Standard soda-lime glass (60×20×4mm) with 75% of silicon dioxide with calcium oxide and nitric oxide was used. Two types of coupling agents were used: maleic anhydride (MAH; Merck, spol. s.r.o., Czech Republic) 5 wt.% was mixed with the untreated PE and industrially applied silane coating on the glass sheets (Johns Manville Slovakia, a.s., Slovakia).
Sample preparation
Plasma treatment of PE was performed in a laboratory device (Surface Treat, a.s.; LA400; Czech Republic). Neat PE powder was treated by oxygen plasma discharge (pulse microwave reactor 1kW, pulse duration 70 ?s, off time 130 ?s) with various treatment duration (10, 20, 30, 40, 50, 60, 120, 300 and 600s) under a pressure of 100Pa. Each batch for the plasma treatment contained 0.25kg of PE powder. Before the preparation, the PE powders and glass were dried in an oven at 90 °C for 120 min and consequently embedded in a dismountable mold (Figure 1) and put into the preheated oven (200°C, 25min). A fluoroplastic foil and liquid chemical separator (Frekote 700 NC) were used as separators. The samples were cooled to the room temperature on air.

Figure 1: Assembly of the PE/glass sheet/PE samples for tensile tests; width a = 20 mm and length b = 20 mm of the contact area. The whole length of each sample was 165 mm and the thickness 4 mm.
Methods
A tensile tester (Instron 5582; MA, USA) was used to determine the adhesion effects at a strain rate of 10 mm.min-1 and room temperature. 10 samples (Figure 1) from each system were tested. The interfacial strength or ultimate strength of bulk sample was calculated (R = Fmax/ab [MPa]) including the standard deviation. Electron spectroscopy for chemical analysis (ESCA) was used for the measurement of the surface chemical composition (10-9 mbar of residual gas in the analysis spectrometer chamber). Al Kα radiation was used for the excitation of photoelectrons. Firstly, the spectrum was measured in the field of binding energy 0-1000 eV, and then lines C 1s and O 1s were measured with a deviation ± 0.2eV in high-resolution mode. The surface stoichiometry was derived from the integral photoemitting lines after deducting the non-linear Shierley background. The transmission function of the hemispherical electron analyzer was corrected to the theoretical values of the partial photoionized cross-sections. The overlapping components of the spectra corresponding to the nonequivalent chemical states of carbon were fitted with Gauss-Lorentz functions (using software XPSPEAK 1.4) [23,24]. The melting and crystallization temperatures of PE powders were measured by DSC (STA 409PG LUXX; Netzsch, Germany; 300 °C, N atmosphere, rate 10°C.min-1, holding time 5min, cooling rate 5°C.min-1). The evaluation of resulting temperatures was carried out from three independent measurements. The fracture surfaces were observed by an optical light microscope (Neophot 32; Zeiss, Germany) and a scanning electron microscope using SE mode (Lyra, Tescan, Czech Republic).
Results and Discussion
Interfacial strength evaluation
The dependence of the interfacial strength between PE and glass sheets on the treatment time is shown in figure 2. Plasma treatment resulted in rapid increase of tensile strength (Rm). This corresponds to the behaviour of the powder hydrophility determined by the Washburn method [25]. The maximal value of Rm (15.27 ± 1.50) MPa was achieved in samples prepared from PE powder plasma-treated for 60 s. Samples prepared from powders treated for 120 s and longer, the strength had a decreasing character with increasing standard deviation. Lower ability of powder to melt together with roughening of the top layer of molten PE particles and subsequently the gradual colour changes of the solidified samples were observed. Similarly, as in Ref [5], this effect indicates polymer chain scission followed by higher crosslinking and subsequent decrease of the adhesion.
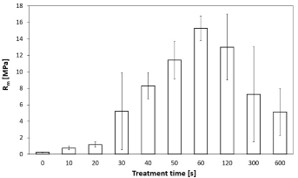
Figure 2: Dependence of adhesion on plasma treatment time of PE.
The quantified maximum interfacial strength of plasma-treated PE/glass sheet samples were compared with coupling agents (MAH, silane; Figure 3). Extremely low adhesion was observed between the untreated PE and the glass sheet (0.24 ± 0.03 MPa). After addition of the MAH the interfacial strength increased to (3.72 ± 3.69 MPa). In the case of the samples with silane, the increase was even higher (5.92 ± 4.21 MPa). The interphase prepared by combining plasma treatment and silane applied to glass showed another increase (13.04 ± 3.57 MPa).
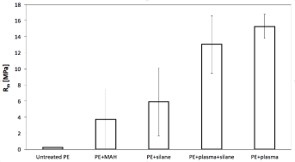
Figure 3: Comparison of the adhesion of PE onto glass sheets with different interphase. From the left a) untreated PE/without any modification; b) MAH/modified powder; c) silane/modified glass; d) combination of plasma/ treated powder and silane/modified glass; e) plasma/ treated PE powder at 60s.
Moreover a few days later after the tensile test, cohesive glass failure occurred in some PE/glass samples on the interphase in the middle of the glass sheet area, as shown in the figure 4 (half of the tested sample). The reason is the equilibrium shrinking as a result of crystallization processes. In the case of samples with MAH, the cohesive glass failure did not appear. In the samples, both with the silane modification and combination of silane/plasma-treated PE, the cohesive glass failure appeared only in rare cases. But the cohesive failure occurred in the glass sheets in all the samples prepared by plasma-treated PE. This effect is further proof of the excellent adhesion of all prepared interphases.

Figure 4: Cohesive glass failure in the interphase PE/glass due to shrinkage as a result of equilibrium crystallization processes in the semi-crystalline thermoplastic PE. The picture depicts only halves of the broken samples.
Very low adhesion of untreated PE is due to the nonpolar character of the PE chains [8]. The adhesion caused by grafted MAH groups on the PE chains is due to mostly hydrogen bonding with hydroxide groups bonded to glass [2], which are absorbed water from the air and form silanol groups [27,28]. However, the resulting strength of PE samples with MAH as coupling agent seems to be reduced. This could probably be connected with the limited ability of the MAH groups to react with the hydroxyls of the glass sheet without providing mechanical anchoring between surfaces during the sample processing.
The increase in the strength, in the case of silane, can be attributed to the improved interfacial adhesion between glass and PE due to the coupling and is comparable with results elsewhere [26]. The silane chemical is bonded to glass by covalent and hydrogen bonding due to the presence of silanol groups on the glass [4,27]. Surprisingly, the adhesion of plasma-treated PE to the clear glass was higher than to the glass treated by the silane-based coupling agent. This is the proof that grafting of reactive radical functional groups by plasma treatment extremely increases the polarity of the nonpolar thermoplastic. Primarily hydroxide groups created on the PE surface by plasma treatment [5] give the premise of a major chemical bond with the glass directly to form silanols and hydrogen bonds [27]. The reason for higher adhesion and especially more frequent cohesive violations of the glass sheets could be attributed to the newly formed polymer-glass interphase after plasma [30]. Another reason could be the higher concentration and uniform distribution of the functional reactive groups in comparison to silane modification of the glass surface, where some uncovered areas may appear [3,26]. According to our knowledge, most of the papers about composites deals with plasma treatment of fibres rather than matrices [17,22,29]. Plasma-treated PE with glass filler appears to be a unique composite system. Therefore, the resulting properties cannot be compared but it can be concluded that the resulting strength is equivalent or higher [22,29].
Electron spectroscopy for chemical analysis (ESCA) and DSC
The ESCA analyses were performed on selected samples. Oxygen content was measured both on the glass substrate and PE sheet. The oxygen concentration (Figure 5) on the non-treated PE powder was 1.02 % and increased up to 7.17 % after plasma treatment whereas the oxygen content on the clear glass sheet was 55.4 % in accordance to the chemical composition of the glass sheet. After the tensile strength test, the oxygen concentration measured on the surface of the PE plate after its avulsion from the glass plate decreases to 2.60 %. This may be caused by removing bonds that remained on the opposite glass sheet - substantial decrease of the oxygen concentration on the corresponding surface of the glass to 13.44 %. This explanation is also supported by the concentration of carbon atoms, which was 71.90 % and 97.09 % in the opposite PE plate.

Figure 5: ESCA results of polar functional group rich in oxygen content. From the left a) untreated PE powder; b) plasma treated PE powder; c) plasma treated PE part after removing the glass sheet; d) glass sheet after removing the plasma treated PE part; e) glass sheet in the basic state.
The values correspond to the results of papers [5] and [11]. The measured inequality of groups rich in oxygen from both sides of the glass and the significant decrease of the groups on the PE part is the proof of the presence of the bonds from the PE adhered to the glass of the sample. This is due to the high adhesion between plasma-treated PE and the glass. This fact is confirmed in the macroscale plastic deformation of PE residues which adhered to the surface of the glass sheet after tensile test. Moreover, the dramatic increase of carbon concentration was observed on the glass sheet under the PE part when compared with the basic state the glass sheet. The resulting spectra of the opposite surfaces are depicted in figure 6.
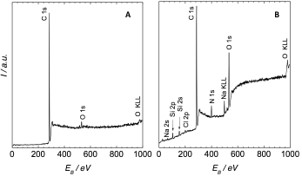
Figure 6: ESCA spectra measured in the field of binding energy 0-1000 eV: a) surface of PE sheet after tensile test, a) surface of glass sheet after tensile test.
The advantage of the plasma treatment is that only upper thin surface layer of the material is modified whereas the bulk of material reminds unchanged thus the plasma treatment does not affect the mechanical properties of the original material. To check whether the plasma treatment influenced the powder thermal properties the DSC analyses was performed. The resulting thermograms (Figure 7) did not show any significant differences between used powders. Both types of PE show the same crystallization temperature 113 ± 1 °C and approximately the same Tm; 128 ± 1 °C untreated PE, 127 ± 1 °C plasma-treated PE. A more significant deviation at plasma treated PE after 250 °C found as a certain slight crosslinking occurs in the polymer [5].
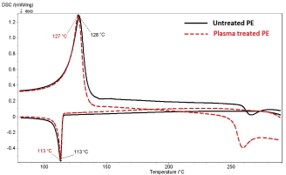
The character of the DSC curves shows no changes in the bulk material after plasma treatment. This confirms results of mechanical properties from tensile tests of pure matrices untreated PE (Rm = 21.1 ± 0.4 MPa) and plasma-treated PE (Rm = 20.8 ± 0.9 MPa), respectively. The perimeter of the treatment is only a surface issue, where the new functional reactive groups are grafted, furthermore examined in [11].
The results of the research represent the excellent potential for the preparation of glass reinforced thermoplastic materials. The required functional groups on the surface of the matrix can be prepared due to the possibility of varying composition by selecting different plasma gases and process parameters [5,31]. The figure 8. depicts preliminary results of plasma treated PE with excellent adhesion to the glass fibre in the PE composite.
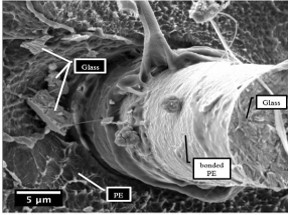
Conclusions
In this work, the effects of plasma treatment of PE powder to increase the interfacial strength to the glass substrate using the tensile tests were evaluated. Furthermore, the interfacial strength of samples prepared with commonly used industrial chemicals such as MAH, silane, even silane in combination with plasma was quantified and compared:
• The highest interfacial strength between PE and glass was achieved in samples with plasma treated PE powder.
• The maximal increase of adhesion, in comparison with untreated PE, between polymer and glass was observed in samples with PE powder which was plasma treated for 60 s.
• The interfacial strength of the plasma-treated PE was at least 150% higher in comparison to the case of the interphase created by silane chemical preparation.
• Results from ESCA measurements of polar functional groups confirmed the achieved excellent adhesive properties of plasma treated polymer to the glass.
• Moreover, DSC measurements did not show any changes of properties in bulk PE after its plasma treatment.
Funding
This work was supported by the Ministry of Education, Youth and Sport of the Czech Republic, program NPU1, project No. LO1207 and project No. CZ.02.1.01./0.0./0.0./16_ 019/0000826.
References
1. Gibson RF. 2010. A review of recent research on mechanics of multifunctional composite materials and structures. Compos Struct. 92: 2793-2810. Ref.: https://bit.ly/2T3AhIp
2. Ehrenstein GW. 2009. Polymer composites materials, Scientica, Praha.
3. Brown HR. 2003. Adhesion between polymers and other substances - A review of bonding mechanisms, systems and testing. Mater Forum. 24: 49-58. Ref.: https://bit.ly/2Vg2bnz
4. Gelest Inc. Silane Coupling Agents: Connecting Across Boundaries. Morisville (USA). http://www.gelest.com, 2006, (accessed 20.06.17).
5. Horakova M, Spatenka P, Hladík J, et al. 2011. Investigation of adhesion between metal and plasma-modified polyethylene. Plasma Process Polym. 8: 983-988. Ref.: https://bit.ly/2HE2qAC
6. Wachinger G, Thum C, Llopart L, et al. New trends in CFRP treatment and surface monitoring for automated structural adhesive bonding. Proceedings of the international conference on composite materials ICCM17 2009; Edinburgh. Ref.: https://bit.ly/37NljvD
7. Prachár J, Novák I, Borsig E. 2014. The possibility of modifying the polypropylene surface using barrier discharge plasma and immobilization of the antibacterial polysaccharide. Chem Listy. 108: 579-585.
8. Quitzau M, Wolter M, Kersten H. 2009. Plasma treatment of polyethylene powder particles in a hollow cathode glow discharge. Plasma Process Polym. 6: 392-396. Ref.: https://bit.ly/2SJm2tA
9. Arenas JM, Ali?a C, Narbo?n JJ, et al. 2013. Considerations for the industrial application of structural adhesive joints in the aluminium–composite material boxing. Compos Part B. 44: 417-423. Ref.: https://bit.ly/2SLVecc
10. Pi?chal J, Hladi?k J, Špatenka P. 2009. Atmospheric-air plasma surface modification of polyethylene powder. Plasma Process Polym. 6: 148-153. Ref.: https://bit.ly/2V9oKKh
11. Píchal J, Aubrecht L, Hladík J, et al. 2006. DBD modification of polyethylene powder hydrophobicity. Czech J Phys. 56: 1290-1296. Ref.: https://bit.ly/32d4Gbz
12. Encinas N, Di?az-Benito B, Abenojar J, et al. 2010. Extreme durability of wettability changes on polyolefin surfaces by atmospheric pressure plasma torch. Surf Coat Tech. 205: 396-402. Ref.: https://bit.ly/2SJ1WzA
13. Navaneetha Pandiyaraj K, Selvarajan V, Deshmukh RR, et al. 2009. Adhesive properties of polypropylene (PP) and polyethyleneterephthalate (PET) film surfaces treated by DC glow discharge plasma Vacuum. 83: 332-339. Ref.: https://bit.ly/2VbRsu6
14. Pamreddy A, Skácelová D, Hani?inec M, et al. 2013. Plasma cleaning and activation of silicon surface in Dielectric Coplanar Surface Barrier Discharge. Surf Coat Tech. 236: 326-331. Ref.: https://bit.ly/32d4LvT
15. Xie J, Xin D, Cao H, et al. 2011. Improving carbon fiber adhesion to polyimide with atmospheric pressure plasma treatment. Surf Coat Tech. 206: 191-201. Ref.: https://bit.ly/2Pc0inX
16. Sever K, Erden S, Gu?lec HA, et al. 2011. Oxygen plasma treatments of jute fibers in improving the mechanical properties of jute/HDPE composites. Mater Chem Phys. 129: 275-280. Ref.: https://bit.ly/38L80wV
17. Wu GM, Shyng YT, Kung SF, et al. 2009. Oxygen plasma processing and improved interfacial adhesion in PBO fiber reinforced epoxy composites. Vacuum. 83: 271-274. Ref.: https://bit.ly/2SYoJpY
18. Su M, Gu A, Liang G, et al. 2011. The effect of oxygen-plasma treatment on Kevlar fibers and the properties of Kevlar fibers/bismaleimide composites. Appl Surf Sci. 257: 3158-3167. Ref.: https://bit.ly/2SIXkth
19. Lee HS, Kim S, Noh YJ, et al. 2014. Design of microwave plasma and enhanced mechanical properties of thermoplastic composites reinforced with microwave plasma-treated carbon fiber fabric. Compos Part B. 60: 621-626. Ref.: https://bit.ly/2wuFgu5
20. Bozaci E, Sever K, Sarikanat M, et al. 2013. Effects of the atmospheric plasma treatments on surface and mechanical properties of flax fiber and adhesion between fiber–matrix for composite materials. Compos Part B. 45: 565-572. Ref.: https://bit.ly/39PtGIu
21. Li Y, Zhang J, Chenga P, et al. 2014. Helium plasma treatment voltage effect on adhesion of ramie fibers to polybutylene succinate. Ind Crop Prod. 61: 16-22. Ref.: https://bit.ly/38MB7zZ
22. Cech V, Knob A, Hosein H-A, et al. 2014. Enhanced interfacial adhesion of glass fibers by tetravinylsilane plasma modification. Compos Part A. 58: 84-89. Ref.: https://bit.ly/2HJStBE
23. Shirley DA. 1972. High-resolution x-ray photoemission spectrum of the valence bands of gold. Phys Rev B. 5: 4709-4714. Ref.: https://bit.ly/2T0rs2k
24. Scofield JH. 1971. J Electron Spectrosc Relat Phenom. 8: 128.
25. Špatenka P, Jeníková Z, Vacková T, et al. Plasma and Particles. SAMPE Conference Proceedings. Long Beach, CA, May 23-26, 2016. Society for the advancement of material and process engineering.
26. Moon CK, Lee JO, Cho HH, et al. 1992. Effect of diameter and surface treatment of fiber on interfacial shear strength in glass fiber/epoxy and HDPE. J Appl Polym Sci. 45: 443-450. Ref.: https://bit.ly/2HNEomF
27. Takeda S, Fukawa M. 2005. Role of surface OH groups in surface chemical properties of metal oxide films. Mater Sci Eng B. 119: 265-267. Ref.: https://bit.ly/2uVbmPw
28. Navarra G, Vella E, Grandi S, et al. 2009. Temperature effects on the IR absorption bands of hydroxyl and deuteroxyl groups in silica glass. J Non-Cryst Solids. 355: 1028-1033. Ref.: https://bit.ly/2vUMKGp
29. Balkova R, Jancar J, Cech V. 2009. Effect of RF-plasma deposition parameters on the composition and properties of organic layers deposited on glass fibers. Compos Sci Technol. 69: 2485-2490. Ref.: https://bit.ly/2T5fswa
30. Gao SL, Mäder E. 2002. Characterisation of interphase nanoscale property variations in glass fibre reinforced polypropylene and epoxy resin composites. Composites A. 33: 559-576. Ref.: https://bit.ly/2vTtl8Q
31. Hladik J, Spatenka P, Aubrecht L, et al. 2006. New method of microwave plasma treatment of HDPE powders. Czech J Phys. 56: 1120-1225. Ref.: https://bit.ly/2SKY69b




















