Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ijia.2020.110001Article Views : 16Article Downloads : 12
Evaluating the Potential of Indirect Enzyme-linked Immunosorbent assay in the quality control of Allergens in Food
Muhammed Y
Faculty of Science, Department of Biochemistry, Federal University Gusau, Nigeria
*Corresponding Author: Muhammed Y, Faculty of Science, Department of Biochemistry, Federal University Gusau, Nigeria, Tel: +2349039150752; Email: Yusufmsani1002@outlook.com
Article Information
Aritcle Type: Research Article
Citation: Muhammed Y. 2020. Evaluating the Potential of Indirect Enzyme-linked Immunosorbent assay in the quality control of Allergens in Food. Int J Immunol Allergy. 1: 01-08.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2020; Muhammed Y
Publication history:
Received date: 08 February, 2020Accepted date: 14 February, 2020
Published date: 17 February, 2020
Abstract
Allergen is any food substance capable of causing allergic reaction due to recognition by immune system. The disorders associated with allergens are increasingly prevalent in the developed world and together include allergic rhinitis which is also known as hay fever, eczema, allergic asthma, atopic dermatitis, and food allergies with symptoms ranging from shortness of breath, sneezing, redness of eyes and running of nose. Also, others develop a potentially deathly systemic allergic reaction known as anaphylaxis, within seconds or minutes of exposure to allergens. Therefore, extremely small amount of allergen/antigen can be quantitatively measured using ELISA by taking the advantage of plastic surface ability to absorb low but detectable amount of protein. This is to ensure food safety. That is why it is important to monitor the quality of food prior to packaging and distribution. The protocols were that the unknown sample and standard solutions were added to the wells followed by washing, addition of enzyme linked with secondary antibody, washing, addition of substrate and reaction terminator using stop solution. The OD was then determined at 450 nm using microplate reader. The concentration of unknown antigen was determined to be 0.60ppm using a standard calibration curve. Therefore, ELISA has the potential of detecting small amount of protein in biological fluid within a short period of time with high sensitivity and selectivity. The sensitivity of ELISA in allergen detection has been supported by many studies without emphasizing on its implementation in food industry for quality control prior to distribution and packaging. Therefore, ELISA can be considered as the novel technology for quantification of extremely small amount of protein/allergen in an unknown sample.
Keywords: Allergens; Pea nuts; Food Quality Control; ELISA
Introduction
Food allergies have become the most important food safety concerns around the world. The prevalence of food allergy is more in North America, where 6% of young children and 4% of adults suffer from food allergies [1]. It is reported that food allergies cause approximately 150 to 200 fatalities per year, based on data from a five-year study of anaphylaxis in Minnesota from the Mayo Clinic. Fatal food anaphylaxis is most often caused by peanuts (50-62%) and tree nuts [2]. Allergen is any food substance capable of causing allergic reaction due to recognition by immune system. The term ‘allergy’ was originated by Clemens von Pirquet in 1906 who used it as a means of calling to attention due to reactivity develop by individual, or hypersensitivity reactions when exposed to certain allergens. Although the statement above is concerning the cause of serum sickness and disorders associated with allergens which are also associated with the triggering of IgE production that are specific for allergens together with the expansion of allergen-specific T-cell populations [3]. The mechanism of IgE in the mediation of allergic inflammatory reaction is that upon exposure to allergen, it binds to high affinity Fc receptor on mast cells and sensitizes them to release mediators involve in inflammation such as histamine (Figure 1). Some of the food and ingredients that are associated with triggering of allergic reaction are gluten, eggs, milk, soy, peanuts, walnut and other kinds of stone fruit like hazelnut, almond, cashew, then shrimp, shellfish, celery, sesame, lupine, mustard, sulphur dioxide [4]. But most of the potent allergens are found in pea nuts and tree nuts. With the prevalence rates of 0.8-1.5% for peanut allergy in the UK and US population and about 0.6% for tree-nut allergy in the US population have been reported (Grundy et al. 2002, Sicherer et al. 2003. Therefore, Peanut (Arachis hypogea) is a legume that belongs to the family of Leguminosae. It grows under the ground in peanut pods containing the peanut seed. On the other hand, Tree nuts are edible seeds that grow on trees. Some of the Tree nuts that are mostly associated with allergic reactions are hazelnut (Corylus avellana), almond (Prunus dulcis), pistachio (Pistachia vera), macadamia nuts (Macadamia integrifolia), cashew (Anacardium occidentale), walnut (Juglans regia), pine nut (Pinus pinea), pecans (Carya illinoinensis) and Brazil nut (Bertholetia excelssa) [5].The major allergens identified in peanuts are Ara h 1 which is chemically a glycoprotein, vicilin, with molecular weight of 63.5 kDa, Ara h 2 which is a glycoprotein, conglutin, with molecular weight of 17.5 kDa, Ara h 3 which is a legumin, with molecular weight of approximately 60 kDa, Ara h 4 which also a legumin, but with molecular weight of 37 kDa, Ara h 5 which is a profilin with molecular weight of 14-15 kDa, Ara h 6 which is a conglutin, with molecular weight of 14.5 kDa, Ara h 7 which is a conglutin, with molecular weight of 15.8kDa and Ara h 8 which is a pathogenesis-related protein, with molecular weight of 16.9 kDa. The major allergens recognised by the IgE in the sera of 90% allergic patients are Ara h 1 and Ara h 2, with are Ara h 3, Ara h 4, Ara h 5, Ara h 6, Ara h 7 and Ara h 8 less recognise by the patient immune system [5]. The disorders associated with allergens are increasingly prevalent in the developed world and together with allergic rhinitis which is also known as hay fever, eczema, allergic asthma and some food allergies. Also, others develop a potentially deathly systemic allergic reaction known as anaphylaxis, within seconds or minutes of exposure to allergens [6] Therefore, Food manufacturers are responsible for the safety of their products [4]. It is important that food company should detect trace number of allergens prior to distribution. Therefore, ELISA (Figure 2 and 3) can be used as rapid assay technique to check the purity of food in terms of allergens so as to prevent allergic reaction that can result to inflammation [7]. ELISA is a quantitative and qualitative analytical technique that detect the presence of antigen as a result of colour change through antigen-antibody interaction through the use of enzyme conjugated to secondary antibody, and can be employed in the detection of the presence of antigen in a biological fluid. ELISA also has potential application in the determination of food authenticity and quality such as dairy product, fish and poultry product [8]. ELISA history date back to 1960 when insulin concentration was determined by Yalow and subsequently pioneered by Engvall and Perlman in 1971 by replacing radioactive molecule with enzyme-conjugated antibody [9]. The principle of ELISA is that, wells are coated with known antibody to an antigen, when antigens are added to the well, they bind to the antibody and when secondary antibodies-enzyme conjugate are added they to bind to the antigen and when substrate are added the enzyme convert the substrate to coloured product (Figure 4). The enzyme can either be alkaline phosphatase that uses nitro phenyl phosphate as a substrate or horse reddish peroxidase that uses O-Phenyl-diamine-dihydrochloride as substrate [10]. Therefore, Vitamin, hormone, drugs, vitamin, protein, small peptides, show high level of specificity against the antibody or antigen raised for them, the reason is that the impossibility of antibodies to bind to other antigens not specific for them is very high [11]. It has been stated that there are several techniques for detection of hidden antigen in biological fluid such as RIA, immunoblotting, RIE, but these techniques suffered from limitations such as lack of sensitivity and selectivity [12] also reported that to protect consumers against the allergic reaction to peanut in food, it is essential to devise a sensitive and a specific method to detect hidden allergens as food allergies are common and can result in both acute and chronic disease, which might be increasing in prevalence, affect quality of life, and can be severe and potentially fatal [13]. However, the specific aim of this study is to evaluate the potential of ELISA as a rapid detection technique for quality control of peanut as potent allergen in food sample.
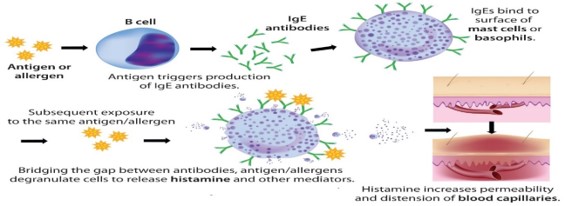
Figure 1: The mechanism of IgE in the mediation of inflammatory reaction upon exposure to allergen [14].
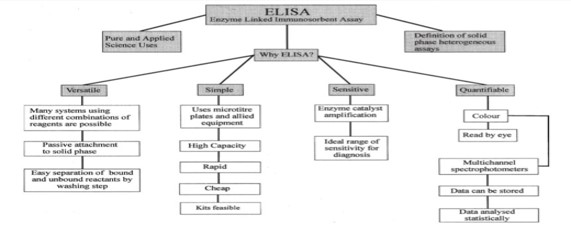
Figure 2: Flow chart which illustrates the suitability of ELISA as a novel technique [15].
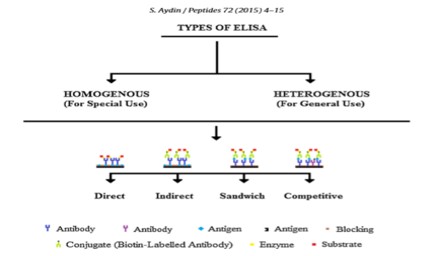
Figure 3: Types of ELISA based on the immobilization of antibodies on a plastic surface [11].
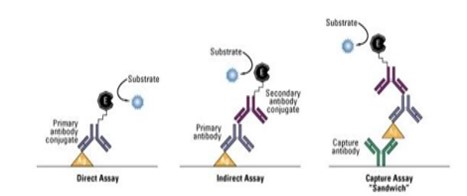
Figure 4: Antigen interaction with antibody, and interaction of secondary antibody with the same antigen in the presence of enzyme and substrate (Thermofisher Scientific, Overview of ELISA).
Materials and Methods
Unknown Sample, Standard Solution, Reagents and Chemicals
Wells were purchased coated with monoclonal antibodies against peanut protein and blocked according to manufacturer’s design, standard solutions (0 ppm, 0.5 ppm, 0.25 ppm, 1 ppm and 2.5 ppm) blank solution (Control variation and contribution of plastic in optical density.), Unknown peanut solutions, washing buffer (remove unbound antibodies, antigens and unbound enzyme-antibody conjugate from the wells), Enzyme Conjugate solution (detect the antigen based on colour change as a result of an enzymatic reaction to yield a coloured product) Substrate solution (transformed to coloured product), Stop solutions (stop colour formation). Microplate reader (450nm) (determines optical density of coloured solution) and Stopwatch were used in this experiment.
Reaction between Enzyme labelled Antibody and Peanut Antigen
ELISA Immuno plate containing 12 wells was purchased coated with monoclonal antibody against peanut and also the wells were blocked to avoid any other antibody/protein binding. The wells strip was placed on a sample holder, followed by the addition of standard solutions of known concentration, that is 0 ppm, 0.25 ppm, 0.5 ppm, 1 ppm, 2.5 ppm to well 1, 2, 3, 4 and 5, to well 6, blank solution was added and well 7 and 8 contains the unknown antigen solution, followed by gentle shaking of wells on a flat surface and the well strip was incubated for 10 minutes at room temperature, after incubation the liquid in the wells was discarded and the wells were washed 5 times with a washing buffer, followed by addition of 100 μl of conjugate solution to each well and mixing by shaking on a flat surface for 10 seconds, after mixing, the well strip was incubated at room temperature for 10 minutes, after which the liquid was discarded again and the well strip was tapped on a tissue paper to remove residual liquid from the wells. 100 μl of substrate solution was added again to each well followed by shaking on a flat surface for about 10 seconds to mix the solution, the well strip was incubated at room temperature, no washing was done in this step, then 100 μl of stop solution was added to each well, then shook for 10 seconds on a flat surface.
Peanut Detection through ELISA Reader
The OD density of each well was obtained after 30 minutes using Bio-Rad microplate reader at 450, followed by processing of data with Microplate manager.
Estimation of the Peanut Concentration using Calibration Curve
A calibration curve was plotted on Microsoft excel using the OD and the concentration of the Standard solution.
Result and Discussion
This study is initiated to investigate the use of ELISA in quality control of allergens in food industry prior to packaging and distribution. This graph was plotted on Microsoft excel, the concentration of the pea nut was extrapolated from the equation from the graph after taking the absorbance of the known and unknown sample solution using microplate reader. The results shows the suitability of using ELISA to detect antigen in part per million in an unknow food sample using standard solution.
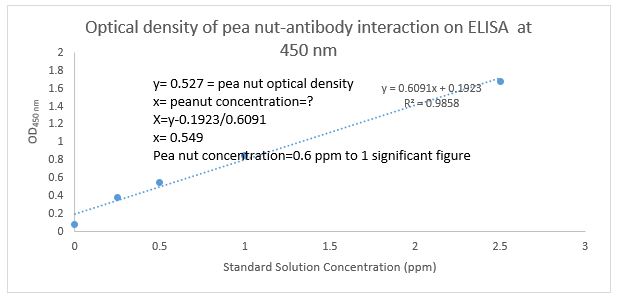
Figure 5: Peanuts antigen concentration (ppm) at 450nm run in the presence of monoclonal antibody against the peanut on ELISA. The result shows that the concentration of the unknown antigen is 0.6ppm, this concentration was extrapolated from the calibration curve of standard solution.
Therefore, ELISA provides a means of quantitative measurement of extremely small amount of protein and serves as a tool for protein purification. The technique takes the advantage of observation that plastic surface based on their design can adsorb low but detectable amount of protein. Therefore, specific antibodies against an antigen are made to adsorb on the surface of plastic wells and the wells do not allow any other protein binding because they are blocked with blocking protein such as BSA [16]. The specific peanut antibody interacts with the peanut antigen and results in colour development, the amount of the colour development is proportional to the concentration of antigen present in the unknown solution [17-23]. Findings of this experiment showed that the amount of peanut was determined to be 0.6ppm (Figure 5), this is supported by [7]. in their studies where they used monoclonal antibodies against peanut and rabbit secondary antibody, that the ELISA could be used as assay technique for detection of peanut contamination in food products, and also the useful range for detection of peanut in ice cream is 40 μg of peanut/ml to 2.0mg/ml. Several studies compare ELISA with other methods of protein detection such as Bradford, radioallergosorbent assay (RAS), radioimmunossay (RIA), and it was found that ELISA is a rapid, sensitive and specific technique for both quantitative and qualitative detection of trace amount of allergens in the food system compared to others as mentioned. However, our studies do not compare ELISA with any other technique. In accordance with studies carried out by [12]. they reported that gel precipitation immuno techniques cannot be automated in routine analysis and to date only ELISA for detection of level of 1 ppm of antigen is available commercially. this is due to the fact that other techniques involved the use dangerous radioactive molecules as label [7,12]. The improvement in the development of specific monoclonal antibodies, polyclonal antibodies and rapid enzymatic detection system will continue to revolutionise food quality control assay techniques. Therefore, ELISA technology can be exploited in all sort of food allergen quality control. The test procedure provided in this experiment is to encourage food industries to implement rapid quantification of allergens in food prior to packaging and distribution so as to ensure safety.
Conclusion
The study contributes to our understanding of sensitive and selective immunological technique for rapid quantification of Peanut antigen in an unknown sample. Therefore, ELISA can be considered as the most reliable technique to exploit in the quality control of allergen in food substances to ensure their safety prior to packaging and distribution.
Future Recommendation
Design of rapid ELISA test strip for different allergens found in food is highly recommended as it will play an important role in the quality control of allergens and protection consumers from inflammation reaction.
Acknowledgement
My acknowledgement goes to the school of biosciences University of Nottingham for the provision of the research facilities and the sample.
Abbreviations
ELISA: Enzyme linked immunosorbent assay
RIA: Radio immunoassay
OD: Optical density
RIE: Rocket immunoelectrophoretic
BSA: Bovine serum albumin
References
1. Waserman S, Watson W. 2011. Food allergy. Allergy, Asthma & Clinical Immunology. 7: S7. Ref.: https://bit.ly/31Udhjh
2. Lanser BJ, Wright BL, Orgel KA, et al. 2015. Current options for the treatment of food allergy. Pediatric Clinics. 62: 1531-1549. Ref.: https://bit.ly/38tBzD9
3. Galli SJ, Tsai M, Piliponsky AM. 2008. The development of allergic inflammation. Nature. 454: 445-454. Ref.: https://go.nature.com/39yRhNm
4. Gojkovic V, Marjanovic-Balaban Z, Vukic M, et al. 2015. Allergens management system in the food production. J Hyg Eng Des. 12: 76-84. Ref.: https://bit.ly/2SGTdNe
5. Boye JI, Danquah AO, Lam Thang C, et al. 2012. Food allergens. Food biochemistry and food processing. 798-819.
6. Sicherer SH, Sampson HA. 2010. Food Allergens, American Academy of Allergy, Asthma & Immunology. 125: 116-125. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/20042231
7. Hefle SL, Bush RK, Yunginger JW, et al. 1994. A sandwich enzyme-linked immunosorbent assay (ELISA) for the quantitation of selected peanut proteins in foods. Journal of Food Protection, 57: 419-423. Ref.: https://bit.ly/3buVAeq
8. Asensio L, González I, García T, et al. 2008. Determination of food authenticity by enzyme-linked immunosorbent assay (ELISA). Food control, 19: 1-8. Ref.: https://bit.ly/2tUVFXS
9. Engvall E, Perlmann P. 1971. Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G. Immunochemistry. 8: 871-874. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/5135623
10. Lequin RM. 2005. Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clinical chemistry. 51: 2415-2418. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/16179424
11. Aydin S. 2015. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides. 72 : 4-15. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/25908411
12. Holzhauser T, Vieths S. 1999. Indirect competitive ELISA for determination of traces of peanut (Arachis hypogaea L.) protein in complex food matrices. Journal of agricultural and food chemistry, 47: 603-611. Ref.: https://bit.ly/2vsZ7JU
13. Sicherer SH, Sampson HA. 2014. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. Journal of Allergy and Clinical Immunology. 133: 291-307. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/24388012
14. Maggio E. 2017. Polysorbate, Biotherapeutics, and Anaphylaxis: A review. Ref.: https://bit.ly/37p4Ihz
15. Crowther JR. 2000. The ELISA guidebook (Vol. 149). Springer Science & Business Media.
16. Crommelin DJ, Sindelar RD, Meibohm B. 2013. Pharmaceutical biotechnology: fundamentals and applications. Springer Science & Business Media. Ref.: https://bit.ly/2OTg9aZ
17. Swinehart DF. 1962. The beer-lambert law. Journal of chemical education. 39: 333. Ref.: https://bit.ly/2SMSrOw
18. Clark MF, Lister RM, Bar-Joseph M. 1986. ELISA techniques. In Methods in enzymology (Vol. 118, pp. 742-766). Academic Press. Ref.: https://bit.ly/37sdYBv
19. Liu FT, Bohn JW, Ferry EL, et al. 1980. Monoclonal dinitrophenyl-specific murine Ig E antibody: preparation, isolation, and characterization. Journal of Immunology, 124: 2728-2737. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/7373045
20. Ohashi T, Mawatari K, Sato K, et al. 2009. A micro-ELISA system for the rapid and sensitive measurement of total and specific immunoglobulin E and clinical application to allergy diagnosis. Lab on a Chip. 9: 991-995. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/19294312
21. Sun DW. 2008. Modern techniques for food authentication. Academic Press.
22. Thacker JD, Casale ES, Tucker CM. 1996. Immunoassays (ELISA) for rapid, quantitative analysis in the food-processing industry. Journal of agricultural and food chemistry. 44: 2680-2685. Ref.: https://bit.ly/2u1NgCc
23. Xiao Y, Isaacs SN. 2012. Enzyme-linked immunosorbent assay (ELISA) and blocking with bovine serum albumin (BSA)-not all BSAs are alike. Journal of immunological methods. 384: 148-151. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/22732194




















