Indexing & Abstracting
Full Text
Case ReportDOI Number : 10.36811/ijho.2021.110026Article Views : 35Article Downloads : 14
Successful Adjuvant Intraoperative Electron Beam Radiotherapy for Keloid: First Case Report and Review of Literature
Abdullah Essam Kattan1, Aws Abdulrahman Alsuhaibani2, Abdullah Alsuhaibani3 and Tareq Salah Hassan4*
1King Saud University, College of Medicine, Department of Surgery, Division of Plastic Surgery, Riyadh, Saudi Arabia
2Alfaisal University
3King Saud University, King Khalid University Hospital, Oncology center, Radiation Oncology Unit, Riyadh, Saudi Arabia
4Clinical Oncology and Nuclear Medicine Department, Faculty of Medicine, Assiut University, Assiut, Egypt
*Corresponding Author: Tareq Salah Hassan, Clinical oncology and Nuclear Medicine Department, Faculty of Medicine, Assiut University, Assiut, Egypt, Phone: 002 088 27 40 140; Email: tareqsalah41@yahoo.com; drtareqsalah@aun.edu.eg; Thassan1@ksu.edu.sa
Article Information
Aritcle Type: Case Report
Citation: Abdullah Essam Kattan, Aws Abdulrahman Alsuhaibani, Abdullah Alsuhaibani, et al. 2021. Successful Adjuvant Intraoperative Electron Beam Radiotherapy for Keloid: First Case Report and Review of Literature. Int J Hematol Oncol. 4: 726-741.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2021; Abdullah Essam Kattan
Publication history:
Received date: 17 December, 2021Accepted date: 27 December, 2021
Published date: 29 December, 2021
Abstract
Treatment of keloids is usually challenging, requiring a multimodal approach with no universally accepted treatment modality among the wide range of alternative keloid treatments. Excision of keloid lesion usually eliminates symptoms and it is the main treatment with considerable recurrence rate. Recurrence rate ranges from 45-100% when surgical excision is performed as monotherapy. Furthermore, Recurrent Keloids have a higher recurrence rate after surgery. In this case we discuss a challenging case of young female presented with third recurrence in lobule of the ear with defect necessitated flap reconstruction with concern for possible damage by the flap if radiation was given as external beam postoperatively. Intraoperative electron beam therapy was utilized with high safety and efficacy. To our knowledge this is the first case in the Middle East to use this technique in treating Keloid.
Conclusion
Treatment of keloids is usually challenging, requiring a multimodal approach. Excision of keloid lesion usually eliminates symptoms and it is the main treatment with considerable recurrence rate .Recurrence rate ranges from 45-100% when surgical excision is performed as monotherapy. Furthermore, Recurrent Keloids have a higher recurrence rate after surgery. Radiation is a valid option for decreasing risk of recurrence in recurrent keloid with high safety and efficacy profile. In this case we discuss a challenging case of young female presented with third recurrence in lobule of the ear with defect necessitated flap reconstruction with concern for possible damage by the flap if radiation was given as external beam postoperatively. Intraoperative electron beam therapy was utilized with high safety and efficacy. To our knowledge this is the first case in the Middle East to use this technique in treating Keloid.
Keywords: Keloid; Radiation; Intraoperative Radiation; IOeRT
Clinical Case
Female patient, 19 years old, presented with recurrent Right ear keloid for the third time with history of trauma to right ear lobe 4 years back with subsequent keloid formation. Patient underwent 2 previous excisions with subsequent recurrence occurred twice. Local injection of steroids has been tried after excising second recurrence but unfortunately failed. From plastic surgery point of view excision with reconstruction with free skin graft from skin behind the affected right ear was necessary to fill the resultant gap of tissue loss after the excision and so postoperative irradiation was of a matter of concern not to affect the integrity ,viability and healing of skin graft. Patient was referred to radiation oncology clinic for discussion of the option of adjuvant / intraoperative radiation aiming at reducing risk of recurrence after excision and before graft reconstruction not to expose graft to radiation. Patient was discussed and counseled regarding the situation, procedure and possible long term complications including theoretical risk of carcinogenesis at irradiated area. Patient agreed on the procedure and informed consent was obtained.
Technique
At the time of surgery, patient underwent surgical excision followed by intraoperative electron beam irradiation (IOeRT) 13 Gy in one fraction using intraop mobetron® and then graft reconstruction. Patient tolerated treatment well with smooth postoperative course and healing. With no early complications. After 3 years of follow up there is smooth course with no signs of recurrence or late complications. To our knowledge this is the first time of using this technique of intraoperative electron beam radiotherapy in treatment of keloid in the Middle East and early use in this indication across the globe.
Review of Literature and Discussion
Defin¬ition of keloids: Keloids are benign fibrous dermal tumors due to excessive collagen formation during tissue repair after skin injury that can sometimes evolve in an unfavorable way with pathological scar formation [1].
Aetology: Skin injury, after surgery or piercing, is the leading cause of generating keloids. It may follows burn injury, tattooing and even simple acnes. However, the cause of keloid formation remained a mystery [1].
Time of development and course: The hypertrophic scars usually occur 4 to 8 weeks postoperatively or after the injury. The time of keloid formation is variable, from generally within 3 months to many years after the dermal injury. Unlike the hypertrophic scars, which often gradually regress after years, keloids persist for longer period of time.
Risk Factors
Race: Black people are more likely to develop keloids, while the Caucasians are least likely. In African populations, the incidence is 6-16%, which is 15 times higher than whites [2].
Gender: Affect males and females equally [3] But due to the more piercing among women might bring the confounding bias of female predominance [4].Incidence of ear-lobe keloids following ear piercing is about 2.5% according to a survey of 1000 nurses in UK [5].
Familial tendency and darker skin races: The keloid is gradually considered as a genetic disease with genetic predisposition that shows an autosomal dominant or X-linked inheritance pattern [6].Keloid is not uncommon in patients genetically predisposed to multiple lesions, related to previous surgery or trauma [3].
Wound tension: In spite of absence of controlled trials to prove, wound tension has been implicated as a factor in the development of keloid and hypertrophic scar formation. Tension may result from attempts to close a wound with tissue loss, overlying bone, or mobility over a joint [7,8] leading to abnormal fibroblastic reaction [9].
Pathophysiological Basis of Keloids Formation
Persistent chronic inflammation: Both keloids and hypertrophic scars are considered results of persistent chronic inflammation. Histologically, continuous local inflammation was observed along with keloid progression [10]. Fibroblast continuous proliferation by persistent activation: The full pathophysiological mechanism is not yet fully known but may be due to a defect in the regulatory mechanisms of cell growth that don't recognize the healing, resulting in continued proliferation of fibroblasts for continuous activation of cell growth factors [11]. Abnormal Fibroblasts response to stimulation; Fibroblasts seen in keloids have different properties than those seen in normal skin and hypertrophic scars. They respond abnormally to stimulation, show a greater capacity to proliferate and produce high levels of collagen (predominantly type I), elastin, fibronectin, and proteoglycan [12,13].
Others: Inflammatory cells, increased numbers of fibroblasts, angiogenesis, and new collagen deposition were all observed. Besides, inflammatory cytokines or mediators including interleukin (IL)-1α, IL-1β, IL-6, and tumor necrosis factor (TNF)-α also overproduced in keloids or hypertrophic scar tissues. This theory is supported by the effectiveness of corticosteroids keloid treatment [12,13].
Clinical Picture
Sites of predilection: As injury has more predilection for the upper half of the body, head, neck, chest, shoulders and arms as a common location. However, any part can be affected. Recently, with the use of new habitual piercing, the most common location is the ear lobe. In the US, the incidence of keloids on ear lobes after piercing, based on a survey of 1000 women, is around 2.5% [11,14].
Symptoms: Esthetic Problem pain and itching are the most common symptoms. Pain and itching are the most common symptoms observed in 91% and 96% of cases respectively [15]. The final clinical result of keloid formation is the development of a Progressive aesthetically unpleasant scar, Painful and itchy lesion. Function disturbance if located in certain areas of the skin (popliteal fossa, neck, and forearm), may induce significant functional limitations. Keloids don’t regress spontaneously but they spread on the surrounding healthy skin surface[16].
Recurrence: Keloids also have a high recurrence rate after surgical resection. Keloid management has always been frustrating and challenging. In the treatment of keloids, the recurrence after surgical excision is relatively high [17].Earlobe is exceptional, which indicate lower recurrence rate under similar treatment. Therefore, this site-specific characteristic provides us the site-specific treatment algorithms, for example, to decide whether or not the lesion requires radiation therapy [6].
Distinguishing hypertrophic scars from keloids: Although usually difficult but Keloids can be defined by Cosman et al's clinical criteria [18] (Table 1 ).
Differentials: In the ear lobe, the differential diagnosis includes a hypertrophic scar, an embedded foreign body, a sarcoid granuloma and an epidermal cyst [19].
|
Table 1: Differences between Keloid and Hypertrophic scars. |
||
|
|
Keloids |
Hypertrophic scars |
|
Time to formation |
Variable, within 3 months to many years after the dermal injury. |
Usually occur 4 to 8 weeks postoperatively or after the injury. |
|
Morphology |
Firm nodules |
More linear, nodular, or papular with more regular borders. |
|
Color |
Cosman et al's clinical criteria: be skin colored, dyspigmented, or erythematous due to telangiectasias more likely to be erythematous and telangiectatic in Caucasians , hyperpigmentation is more popular in Blacks [20]. |
|
|
Border |
Lesion always extends beyond the border of demarcated primary lesion, and often with irregular shape, Just like how the word “keloid” originated from the Latin word “crab [6]. |
Always within the original wound borders [6]. |
|
Course |
Persist for longer period of time. |
Often gradually regress after years. Spontaneous regression is debatable [21,22]. |
|
Symptoms |
Although both lesions are pruritic, the keloids are more likely to cause significant pain and hyperesthesia [6]. |
|
|
Response to treatment |
often resistant to treatment and have a higher rate of recurrence [23]. |
More responsive to treatment |
Treatment of Keloids: Treatment is usually challenging, requiring a multimodal approach with no universally accepted treatment modality among the wide range of alternative keloid treatments. This is due to the variable quality of reporting of treatment series [22]. Prevention is the best treatment strategy Predisposed persons should avoid nonessential surgery, particularly in body locations at high risk for the development of keloids [22].
Surgery: Excision of keloid lesion usually eliminates symptoms.Recurrence rate ranges from 45-100% when surgical excision is performed as monotherapy [24]. Furthermore, Recurrent Keloids have a higher recurrence rate after surgery [25]. Keloids following burns had a poorer success rate than those developing after surgery or mechanical trauma (p<0.001) [26]. Keloids existing for more than 2 years before therapy had a recurrence-free response rate of 78.9% in comparison to 68.8% for keloids existing between only 1 and 6 months [26].
Radiation therapy: Keloid scars are the commonest type of benign disease treated by radiotherapy because keloid scars are particularly refractory to most other therapeutic modalities [27].
Surgical excision and adjuvant radiation therapy seems to be the best treatment modality , confirmed by many studies that the rate of side effects of this treatment modality is low and response rates are high It drops down the rate of recurrence down to 10-20% when radiation therapy adjuncts surgical excision [6,11,14,28-30].
Postoperative Mechanism of action of radiation: Tissue injury? Histamine release from mast cells?Fibroblasts ?TGF-1 beta release ?Fibroblast proliferation and angiogenesis? Collagen synthesis ( Figure 1).
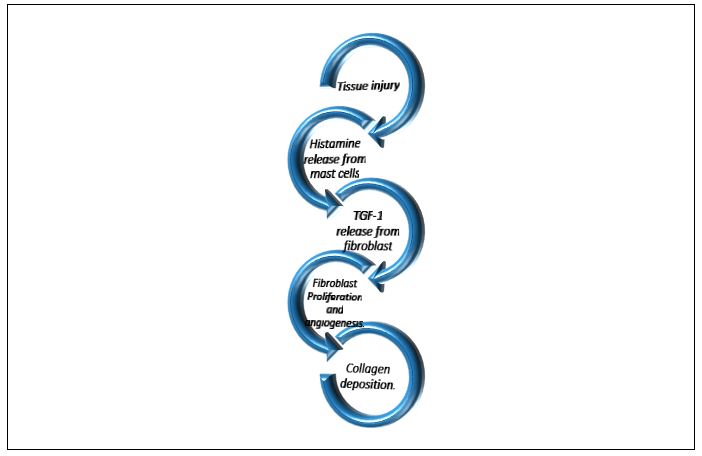
Figure 1: Simplified diagram of pathophysiology of keloid formation.
The proposed mechanism of action Radiation suppresses collagen synthesis in keloids or hypertrophic scars through inhibition of fibroblast proliferation and angiogenesis during the exaggerated wound-healing process [31]. This is mediated by the inhibition of TGF-beta 1 release from fibroblasts which is stimulated by histamine released from mast cells. Treatment is most effective during the first 24-72 hours after surgery which will be discussed later (Section 0).
History and evolution of treating keloid with radiation
First treated keloids: De Bearman and Gourgerot have first treated keloids using superficial X ray irradiation in 1906 [32]. Reports on using X ray as a treatment method for keloids surfaced around 1920 and 1933[33]. Originally, Kilovoltage therapy with energy of 100-400 keV were used; however, low energy X ray resulted with more acute skin side effects. Hyperpigmentation was the main complication in almost one third (30%) of cases [28]. Postoperative radiation for the prevention of keloids recurrence was recommended around the 1940s to 1950s as a proactive approach [30,34,35]. Then dose dependent approach was disclosed in the year 190 through a study conducted in Melbourne indicated potential dose dependent results [36]. Although radiation therapy has not been proven yet to be effective in the following years of the 1960s, and when treating keloids and hypertrophic scars both remained as a problem, preventive X ray irradiation has already been widely accepted and practiced.
Furthermore, low dose superficial X ray irradiation within 48 h postoperatively has proven to prevent keloid relapse [37]. Gradually, further well designed studies have supported the comparative effectiveness of combination therapy between surgical keloidectomy and postoperative X ray irradiation therapy [38,39]. Evidence confirming importance of postoperative radiotherapy; more long term retrospective studies on keloids detailed the importance of irradiation postoperatively. Kovalic and Perez, followed 75 patients with 113 keloids for a mean time of 9.75 years, demonstrated an overall control rate of 73% [25]. Some studies mentioned that half of the relapsed cases occurred within 6 months postoperatively and most cases of relapse occurred within 24 months [28,40] .
Technique:
X ray irradiation was gradually replaced by electron beam irradiation. Up to a dose of 15 Gy irradiation delivered postoperatively after surgical excision successfully prevented hypertrophic scars or keloids formation and recurrence and results was confirmed in 1990 [41]. Electron beam therapy had advantageous uniform dose distribution curve and steeper dose fall off is, with more condensed radiation deposition and less normal tissue damage [42]. Field set up: The electron field covers the normal skin area 0.5 or 1 cm from the margin of the operative suture lines. The surrounding normal tissue is shielded with lead (equivalent to 6 mm of Pb) [17].
Electron beam: This is usually applied with a 6-MeV energy with 0.5 or 1-cm thick silicone bolus as a tissue compensator is placed on the keloid surgical scar to ensure adequate radiation dose was applied to the surface of the skin. With the help of a bolus, reduced irradiation dose could achieve excellent clinical outcome [43]. There has not been any consensus formed in standardized treatment despite the International Clinical Recommendations on Scar Management contrived in 2002 [44]. Currently, Many modalities of radiation therapy can be used including radioactive isotope carrying α, β, γ, and neutrons particles, X rays at various energy level, electron accelerators, and heavy particle accelerators. Superficial and orthovoltage X rays (photons) therapy, brachytherapy (β rays using phosphorus 32 [30] strontium 90 [45]), γ rays using cobalt 60[46] with different efficacy and complications. Comparison between different radiation treatments modalities are summarized in (Table 2).
Timing of radiation: As discussed previously ,treatment is most effective during the first 24-72 hours after surgery as Postoperative radiotherapy impacts wound healing in patients with keloids at a time when the connective tissue is more radiosensitive by decreasing fibroblast proliferation and causing a rapid degranulation of mast cells the main secretors of histamine [48]. This is during the first 24-72 hours after surgery. Increasing postoperative interval between surgery and radiation therapy negatively correlates with clinical outcome. German studies [26,49] indicated that shorter postoperative interval managed lower rate of relapses. Irradiation within 24 hours after surgery was widely employed. Although, based on studies conducted before the 1990s (before the shift of radiation technique from superficial to iridium brachytherapy that might cause significant bias). Postoperative radiotherapy used a single applied field, with the first fraction delivered within 24 hours of excision to maximize effectiveness. decreases recurrence rates to less than 10 % [50]. A short-time (<7 hours) interval between scar excision and irradiation results in a lower recurrence rate compared with longtime intervals (>24 hours). Doubling the duration of interval from 25.9 h to 43.5 h might produce significant diminution of apoptotic cells and reduced keloid fibroblasts number decrease [51].
|
Table 2: Types of modalities used [47]. |
||||
|
TYPE |
Mechanism |
Depth of penetration |
Advantages |
Disadvantages |
|
Electron beam
|
electrons delivered via linear accelerato |
X-Ray (photon) beams • inexpensive and easy to use
|
Radioactive source emitting gamma rays delivered via catheter |
Radioactive source emitting gamma rays delivered via catheter |
|
Superficial/ Orthovoltage
|
2-6 CM |
5mm/2cm |
Inserted into target tissue |
Fixed onto skin |
|
High-dose-rate (HDR) interstitial brachytherapy |
• Treats superficially without damage to underlying structures • Can treat broad area of skin without radiation dose drop-off |
• treats superficially without damage to underlying structures |
Involves less normal tissue |
Good for long keloids and uneven surfaces |
|
HDR superficial (surface conforming) Brachytherapy |
• Cannot use on curved surfaces • Need linear accelerator |
dose of radiation drops off at periphery so have uneven dose delivery |
Total dose must be delivered in short time frame |
Total dose must be delivered in short time frame |
Number of fractions: Single-fraction irradiation showed superior results in terms of recurrence rate and patient convenience [52]. Importance of anatomical site of keloid from radiotherapy point of view due to fewer tensions, keloids on ear lobes, head, and neck were generally considered easier to treat compared to other sites such as trunk (especially chest) and limbs. A dose response analysis verified the fact that earlobe keloids have significantly lower risk of recurrence after similar radiation therapy [15]. Earlobe controlling dose was suggested to be reduced from 15 Gy 3 fractions down to 10 Gy 2 fractions. The diminution of radiation dose might also explain the reason why no prominent adverse effects were observed in following up patients [30].
Dose and fractionation: Most of the current literature recommendations were based on retrospective studies other than prospective studies. Besides, the difficulty in clinical distinction between keloids and hypertrophic scars and the lack of histopathological confirmation decrease the validity of these studies.Therefore, due to the limitations, there are no preferred treatments of keloids in specific sites [53]. Before the year 2000, 15 Gy in total was considered as optimal dose with minimal adverse effect [54]. A dose of at least 15 Gy, delivered in fractions within 10 days of surgery, was recommended in that time by some investigators [22,55]. However, Theodore and colleagues ,through retrospective study reviewing treatment results of 354 sites in 199 patients with a single fraction and skin dose ranging from 2 to 20 Gy and median follow up period of 35 months, found that Postoperative electron beam irradiation with a single dose of 9 Gy or greater is highly effective in the prevention recurrence of hypertrophic scars or keloids [41] .A total dose >10 Gy can accordingly reduce the recurrence rate, while a total dose >30 Gy will limit the recurrence rate down to <10%. Kal and Veen considered an optimal schedule should at least include a total dose over 30 Gy less than 3 fractions within 48 hours postoperatively [56]. Less than 19.2 postoperative electron beam irradiation could achieve 90-95% favorable control in earlobe sites according to Xu and colleagues [30].
In the retrospective review done by Sakamoto et al. [15], 194 lesions in 119 patients From 1979 to 1994 received postoperative radiotherapy with total dose ranging from 16 Gy/8 fractions to 40 Gy/8 fr (mean: biologically effective dose (BED) 33.5 Gy). Kilo-voltage X-rays (55 or 100 kVp) or electron beams (4 or 6 MeV) with median follow up period of 36 months (12-164 months).A dose response relationship with cutoff point of 20 Gy for both efficacy and complications and relapse rates was observed .Relapse rate was 11% at 20 Gy in five fractions or higher dose, while 43% at less than 20 Gy. On the other hand, the incidence of adverse effects was significantly higher for patients receiving more than 20 Gy in five fractions.
In the retrospective review done by Sakamoto et al. [15], 194 lesions in 119 patients From 1979 to 1994 received postoperative radiotherapy with total dose ranging from 16 Gy/8 fractions to 40 Gy/8 fr (mean: biologically effective dose (BED) 33.5 Gy). Kilo-voltage X-rays (55 or 100 kVp) or electron beams (4 or 6 MeV) with median follow up period of 36 months (12-164 months).A dose response relationship with cutoff point of 20 Gy for both efficacy and complications and relapse rates was observed .Relapse rate was 11% at 20 Gy in five fractions or higher dose, while 43% at less than 20 Gy. On the other hand, the incidence of adverse effects was significantly higher for patients receiving more than 20 Gy in five fractions.
Single fraction replaced multiple fractions concept: Conventionally, 3 fractions were considered more reliable than single high dose scheme, becoming the standard scheme widely[57]. However, hypofractionated radiation therapy control rate was gradually reported significantly superior results to the traditional fraction [56]. Single fraction electron beam irradiation of a total dose of 10 Gy was used in UK, US, and Asia on patients who were refractory to other treatment modalities except for radiotherapy, demonstrating no recurrence and well tolerance [41,58,59]. In a relatively recent study by Song and colleagues [58] showed that a single dose of 10 Gy within the first 72 h after excision of keloids is both safe and effective with the advantage of being simpler, more convenient, and cheaper than multiple fractionation.
Results of different radiation modalities
External beam radiation: Data are widely variable.The overall recurrence rate reported in literatures varied from 8% to 29.3%, due to different sites and chronological reasons, with a total dose of 15-20 Gy in 3 fractions [56,57].
Additionally, if the surgeons use improved tension decrease suturing methods, the relapse rate can reach as low as 2.2% in treating smaller (<3 cm) chest keloids [60]. In a large review comparing the effectiveness of different treatments found significantly greater control rates with megavoltage electrons or cobalt-60 X-rays than kilovoltage X-rays. The hypothesis for this difference was the improvement in dose homogeneity with deeper penetrating radiation gives lower recurrence rates [57].
High dose rate (HDR) brachytherapy: Later after external beam radiation, high dose rate (HDR) brachytherapy provided us with an alternative to external radiation therapy, particularly for patients who are resistant to adjuvant external beam radiation therapy or corticosteroids, which resulted in a recurrence rate ranging from 4.7% to 21% [61,62].
EBRT versus HDR brachytherapy: Largest single-institution case-control retrospective study (2004-2014) of keloid recurrence rates and complications between postoperative EBRT and HDR brachytherapy revealed that post excision radiotherapy showed significant reduction in keloid recurrence compared to surgery alone.
Recurrence rates were non-significant between external beam radiotherapy and brachytherapy.
Differences in relapse pattern: In cases developed recurrence, keloids treated with EBRT recurred significantly later than those treated by HDR brachytherapy by a mean of 2.5 years [63]. In a meta-analysis of thirty-three studies , High-dose-rate brachytherapy showed lower recurrence rates compared with low-dose-rate brachytherapy and external radiation [52]. In summary, the results from numerous studies demonstrated a significantly lower recurrence rate (ranging from 12% to 28%), proving the effectiveness of postoperative irradiation therapy [54].
Intraoperative Electron beam Radiotherapy: In a recent article by Yang et al. Fourteen patients with keloids underwent radiotherapy using the Intrabeam system from November 2016 to March 2018 compared to data from this cohort to earlier their own data from keloid patients who had previously been exposed to 6 MV electron beams using conventional accelerators. At 22.5 months median follow up there was zero recurrences in the intrabeam group with statistically significant difference compared to the control group (p= 0.016) and excellent cosmetic outcome in 90% of patients [64].
Safety of radiation therapy: The use of a potentially carcinogenic treatment for the treatment of a benign process such as keloid scaring is controversial. Although a large study found no increased risk of malignancy following radiation therapy for keloids, case reports have outlined the development of carcinoma (two breast, one thyroid) in three patients [27,65]. Moreover, reviewing the literature, only 5 out of more than 6500 cases has been described as potential and doubtful [27,59].
Conclusion: Despite the risk of radiation-induced malignancy being so low, this treatment in not recommended in very young patients unless previous alternative treatments have failed for several years and the keloids cause severe pain and disfiguration with extra caution when considering treatment of keloid or hypertrophic scars in young age and in radiosensitive areas such as the breast or thyroid.
Complications of radiation therapy: Complications or adverse effects of postoperative radiation therapy could be divided into two categories: acute skin reactions and late complications [30] (Table 3). As previously discussed and proved in the retrospective review done by Sakamoto et al. [15], There was a dose response relationship with cutoff point of 20 Gy for both efficacy and complications and relapse rates . The incidence of adverse effects was significantly higher for patients receiving more than 20 Gy in five fractions.
|
Effects |
RELATED TO |
COMPLICATION TYPE |
|
Acute (within 7-10 days after radiation |
Total dose of radiation given |
Erythema. Edema Desquamation. Ulceration. Necrosis |
|
Late (weeks to months after radiation) |
Dose of radiation per fraction (Fraction size) |
Pigmentary changes (hypo- or hyperpigmentation). Atrophy. Alopecia. Telangiectasias |
Final Conclusion
Surgery with adjuvant irradiation is safe and effective for treatment of keloid scars. Recent systematic review of 33 studies [52,63], although lacking of high-quality randomization , recommended the following: The use of high-dose-rate brachytherapy instead of low-dose rate brachytherapy or external radiation. (2) A short-time interval between operation and irradiation. Single fraction instead of multiple fractions irradiation. A minimum of 12- to 24-month follow-up post treatment.
Other modalities that might be of clinical use in keloid treatment
Intralesional Corticosteroids: Cornerstone for both prophylaxis and treatment of hypertrophic scars and keloids. Leads to softening, flattening, and improvement of symptoms after treatment [66, 67]. Mechanism of Action is through suppression of collagen synthesis by decreased gene expression within the keloid or hypertrophic scar. Combined with surgical excision, the recurrence rate falls below 50% within 5 years [68,69].
Compression therapy
First publication on compression therapy was in the 1960s [70]. Mechanism is not completely understood. But may be due to producing tissue ischemia, decreasing tissue metabolism and increasing collagenase activity [71-73]. Necessity of applying the pressure dressing 18 hours a day for at least 6 months, there is Poor response of Scars older than 6-12 months and Difficulty to reach the required amount of pressure (24-40mm Hg) in locations over a joint because of excessive skin movement. Despite its activity, it has many disadvantages and limitations.
Cryotherapy
May be used As monotherapy or in combination with other techniques [65]. It acts through induction of ischemic damage to the microcirculation leading to anoxia with eventual necrosis[74]. Achieves good response in majority of patients. It is more effective when combined with intralesional steroids [75]. It has a limited role in facial lesions or older scars more than 12 months which shows a poor response to treatment. Main side effect is permanent hypopigmentation resulting from the cold sensitivity of melanocytes .So, cryotherapy is not desirable in patients with darker skin phonotypes [76].
Topical silicone gel sheeting or cushions
By application for at least 12 hours daily for 2-4 months. It shows significant scar softening and decreased pruritus. Although many of the studies performed is lacking adequate controls [65] .The relatively simple nature of this treatment makes it a popular treatment option[77].
Interferon
Through decreasing types I and III collagen production from fibroblasts [78-81].Although archives improvements of up to 50% , It has questionable activity [82].
Fluorouracil
Only Two published studies have demonstrated improvement with intralesional use of fluorouracil [83] . No prospective, well-controlled clinical studies are available[76].
Laser therapy
One of the most advantageous modalities. Initial studies showed unacceptably high rates of scar recurrence and other adverse effects including pain, atrophy, and dyspigmentation [84-87]. The first encouraging results was by Alster and colleagues by using pulsed dye laser (PDL laser) in treatment of hypertrophic scars and follow up duration of 10 months [88]. It demonstrated marked improvements in scar erythema, texture, height, and pliability [89, 90].
Other agents
Bleomycin, tamoxifen, tretinoin, tacrolimus, pentoxifylline, colchicine, calcium antagonists, tranilast, zinc. Studies are often limited by poor design, a paucity of subjects, and short-term follow-up [76].
|
Table 4: Keloids of the ear: the range of reported outcomes for treatment options [22]. |
||
|
Treatment |
Worst reported recurrence rate (%) |
Best reported recurrence rate (%) |
|
Steroids alone |
50% at 5 years 7 |
11.2% at 4 years |
|
Surgery alone |
100% at 5 years |
50% at 5 years |
|
Silicone-gel sheet alone |
- |
21% at 6 months |
|
Laser surgery |
95% at 1 year |
30% at 1 year |
|
Surgery and steroids |
12 85% at 5 years |
5% at 5 years |
|
Surgery, steroids and pressure |
- |
100% at 18 months |
|
Surgery, intralesional verapamil and pressure |
- |
48% at 28 months
|
|
Surgery and radiotherapy |
12.5% at 12 months |
2.8% at 5.6 years |
|
Surgery and local flap a |
|
0% at 26 months |
|
aOnly one paper with long-term results available |
||
Conclusion
Treatment of keloids is usually challenging, requiring a multimodal approach. Excision of keloid lesion usually eliminates symptoms and it is the main treatment with considerable recurrence rate .Recurrence rate ranges from 45-100% when surgical excision is performed as monotherapy. Furthermore, Recurrent Keloids have a higher recurrence rate after surgery. Radiation is a valid option for decreasing risk of recurrence in recurrent keloid with high safety and efficacy profile. In this case we discuss a challenging case of young female presented with third recurrence in lobule of the ear with defect necessitated flap reconstruction with concern for possible damage by the flap if radiation was given as external beam postoperatively. Intraoperative electron beam therapy was utilized with high safety and efficacy. To our knowledge this is the first case in the Middle East to use this technique in treating Keloid.
References
1 Pontoriero A. 2015. Post-operative radiotherapy of keloids. A 10-years’ experience of kilo voltage irradiation. 13: 201.
2 Ha JM. 2014. Comparison of the effectiveness of nonablative fractional laser versus pulsed-dye laser in thyroidectomy scar prevention. 26: 615-620. Ref.: https://pubmed.ncbi.nlm.nih.gov/25324655/ DOI: https://doi.org/10.5021/ad.2014.26.5.615
3 Davies DJ. 1985. Plastic and reconstructive surgery. Scars hypertrophic, scars, and keloids. 290: 1056. Ref.: https://pubmed.ncbi.nlm.nih.gov/3921108/ DOI: https://doi.org/10.1136/bmj.290.6474.1056
4 Rabello FB. 2014. Update on hypertrophic scar treatment. 69: 565-573. Ref.: https://pubmed.ncbi.nlm.nih.gov/25141117/ DOI: https://doi.org/10.6061/clinics/2014(08)11
5 Simplot TC. 1998. Comparison between cartilage and soft tissue ear piercing complications. 19: 305-310. Ref.: https://pubmed.ncbi.nlm.nih.gov/9758178/ DOI: https://doi.org/10.1016/s0196-0709(98)90003-5
6 Xu J. 2017. The radiation therapy in keloids treatment: a comprehensive review of pathomechanism, damage mechanisms and cellular response.
7 Ketchum LD. 1974. Hypertrophic scars and keloids a collective review. 53: 140-154. Ref.: https://pubmed.ncbi.nlm.nih.gov/4590747/ DOI: https://doi.org/10.1097/00006534-197402000-00004
8 Stucker FJ. 1992. An approach to management of keloids. 118: 63-67. Ref.: https://pubmed.ncbi.nlm.nih.gov/1728280/ DOI: https://doi.org/10.1001/archotol.1992.01880010067018
9 Urioste SS. 1999. Keloids and hypertrophic scars: review and treatment strategies. In Seminars in cutaneous medicine and surgery. Ref.: https://pubmed.ncbi.nlm.nih.gov/10385284/ DOI: https://doi.org/10.1016/s1085-5629(99)80040-6
10 Ogawa R. 2017. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. 18: 606. Ref.: https://pubmed.ncbi.nlm.nih.gov/28287424/ DOI: https://doi.org/10.3390/ijms18030606
11 Klumpar DI. 1994. Keloids treated with excision followed by radiation therapy. 31: 225-231. Ref.: https://pubmed.ncbi.nlm.nih.gov/8040405/ DOI: https://doi.org/10.1016/s0190-9622(94)70152-0
12 McCoy BJ. 1981. Effects of various sera on growth kinetics and collagen synthesis by keloid and normal dermal fibroblasts. 67: 505-510. Ref.: https://pubmed.ncbi.nlm.nih.gov/7208694/ DOI: https://doi.org/10.1097/00006534-198104000-00014
13 Russell SB. 1995. Glucocorticoid regulation of elastin synthesis in human fibroblasts: down-regulation in fibroblasts from normal dermis but not from keloids. 104: 241-245. Ref.: https://pubmed.ncbi.nlm.nih.gov/7829880/ DOI: https://doi.org/10.1111/1523-1747.ep12612788
14 Sclafani AP. 1996. Prevention of Earlobe Keloid Recurrence with Postoperative Corticosteroid Injections versus Radiation Therapy A Randomized, Prospective Study and Review of the Literature. 22: 569-574. Ref.: https://pubmed.ncbi.nlm.nih.gov/8646474/ DOI: https://doi.org/10.1111/j.1524-4725.1996.tb00376.x
15 Sakamoto T. 2009. Dose–response relationship and dose optimization in radiotherapy of postoperative keloids. 91: 271-276. Ref.: https://pubmed.ncbi.nlm.nih.gov/19201502/ DOI: https://doi.org/10.1016/j.radonc.2008.12.018
16 Szulgit G. 2002. Alterations in fibroblast α1β1 integrin collagen receptor expression in keloids and hypertrophic scars. 118: 409-415. Ref.: https://pubmed.ncbi.nlm.nih.gov/11874478/ DOI: https://doi.org/10.1046/j.0022-202x.2001.01680.x
17 Lee SY. 2015. Postoperative electron beam radiotherapy for keloids: treatment outcome and factors associated with occurrence and recurrence. 27: 53-58. Ref.: https://pubmed.ncbi.nlm.nih.gov/25673932/ DOI: https://doi.org/10.5021/ad.2015.27.1.53
18 Cosman B. 1961. The surgical treatment of keloids. 27: 335-358.
19 Mann R. 1983. Sarcoidal tissue reaction—another complication of ear piercing. 8: 199-200. Ref.: https://pubmed.ncbi.nlm.nih.gov/6851241/ DOI: https://doi.org/10.1111/j.1365-2230.1983.tb01766.x
20 Supp DM. 2014. Inhibition of hyaluronan synthase 2 reduces the abnormal migration rate of keloid keratinocytes. 35: 84-92. Ref.: https://pubmed.ncbi.nlm.nih.gov/24043232/ DOI: https://doi.org/10.1097/bcr.0b013e3182a2a9dd
21 Moustafa MF. 1975. Presumptive evidence of the effect of pregnancy estrogens on keloid growth. 56: 450-453. Ref.: https://pubmed.ncbi.nlm.nih.gov/1099591/ DOI: https://doi.org/10.1097/00006534-197510000-00019
22 Ragoowansi R. 2001. Ear-lobe keloids: treatment by a protocol of surgical excision and immediate postoperative adjuvant radiotherapy. 54: 504-508. Ref.: https://pubmed.ncbi.nlm.nih.gov/11513512/ DOI: https://doi.org/10.1054/bjps.2001.3656
23 Murray J. 1994. Keloids and hypertrophic scars. 12: 27-37. Ref.: https://pubmed.ncbi.nlm.nih.gov/8180942/ DOI: https://doi.org/10.1016/0738-081x(94)90254-2
24 Ramakrishnan KM. 1974. Study of 1,000 patients with keloids in South India. 53: 276-280. Ref.: https://pubmed.ncbi.nlm.nih.gov/4813760/ DOI: https://doi.org/10.1097/00006534-197403000-00004
25 Kovalic JJ. 1989. Radiation therapy following keloidectomy: A 20-year experience jeffrey. 17: 77-80. Ref.: https://pubmed.ncbi.nlm.nih.gov/2745211/ DOI: https://doi.org/10.1016/0360-3016(89)90373-8
26 Wagner MA. 2000. Results of prophylactic irradiation in patients with resected keloids: a retrospective analysis. 39: 217-220. Ref.: https://pubmed.ncbi.nlm.nih.gov/10859014/ DOI: https://doi.org/10.1080/028418600430806
27 Botwood NC. 1999. The risks of treating keloids with radiotherapy. 72: 1222-1224. Ref.: https://pubmed.ncbi.nlm.nih.gov/10703484/ DOI: https://doi.org/10.1259/bjr.72.864.10703484
28 Borok TL. 1988. Role of ionizing irradiation for 393 keloids. 15: 865-870. Ref.: https://pubmed.ncbi.nlm.nih.gov/3182326/ DOI: https://doi.org/10.1016/0360-3016(88)90119-8
29 Chaudhry M. 1994. Ear lobe keloids, surgical excision followed by radiation therapy: a 10-year experience. 73: 779-781. Ref.: https://pubmed.ncbi.nlm.nih.gov/7805600/
30 Xu J. 2017. Radiation therapy in keloids treatment: history, strategy, effectiveness, and complication. 130: 1715. Ref.: https://pubmed.ncbi.nlm.nih.gov/28685723/ DOI: https://doi.org/10.4103/0366-6999.209896
31 Ship AG. 1993. Sternal keloids: successful treatment employing surgery and adjunctive radiation. 31: 481-487. Ref.: https://pubmed.ncbi.nlm.nih.gov/8297076/
32 De Bearman. 1906. Chelides des maqueuses. 7: 151.
33 Finzi. 1920. The treatment of tumours by radium and x rays. 8: 68-79.
34 Rockwell WB. 1989. Keloids and hypertrophic scars: a comprehensive review. 84: 827-837. Ref.: https://pubmed.ncbi.nlm.nih.gov/2682703/ DOI: https://doi.org/10.1097/00006534-198911000-00021
35 Kruger AJS. 1954. Keloids and their treatment with special reference to the radiotherapy. 93: 426-433. Ref.: https://pubmed.ncbi.nlm.nih.gov/13178898/
36 Van den Brenk H. 1960. Radiation in the management of keloids and hypertrophic scars. 47: 595-605. Ref.: https://pubmed.ncbi.nlm.nih.gov/13841012/ DOI: https://doi.org/10.1002/bjs.18004720603
37 Levy DS. 1976. Postoperative irradiation in the prevention of keloids. 127: 509-510. Ref.: https://pubmed.ncbi.nlm.nih.gov/183542/ DOI: https://doi.org/10.2214/ajr.127.3.509
38 Caronni EJC. 1967. Surgical removal and immediate radiotherapy (iridium 192) in treatment of keloids. Preliminary note. 19: 874-882. Ref.: https://pubmed.ncbi.nlm.nih.gov/5189379/
39 Carayon A. 1965. Resillot, SURGICAL TREATMENT OF KELOIDS. SURGERY-RADIOTHERAPY COMBINATION OR" SHAVING OFF". 10: 80-84. Ref.: https://pubmed.ncbi.nlm.nih.gov/14306079/
40 Arnault J. 2009. Keloids treated with postoperative Iridium 192* brachytherapy: a retrospective study. 23: 807-813. Ref.: https://pubmed.ncbi.nlm.nih.gov/19470053/ DOI: https://doi.org/10.1111/j.1468-3083.2009.03190.x
41 Lo TC. 1990. Single-dose electron beam irradiation in treatment and prevention of keloids and hypertrophic scars. Radiother Oncol. 19: 267-272. Ref.: https://pubmed.ncbi.nlm.nih.gov/2126387/ DOI: https://doi.org/10.1016/0167-8140(90)90153-n
42 Li M. 2016. A dosimetric comparison between conventional fractionated and hypofractionated image-guided radiation therapies for localized prostate cancer. 129: 1447. Ref.: https://pubmed.ncbi.nlm.nih.gov/25955230/ DOI: https://doi.org/10.1259/bjr.20150080
43 Seegenschmiedt MO. 2015. German Cooperative Group on Radiotherapy for Non-malignant Diseases (GCG-BD). Radiotherapy for non-malignant disorders: state of the art and update of the evidence-based practice guidelines. 2015. 88: 20150080. Ref.: https://pubmed.ncbi.nlm.nih.gov/25955230/ DOI: https://doi.org/10.1259/bjr.20150080
44 Mustoe TA. 2002. International clinical recommendations on scar management. 110: 560-571. Ref.: https://pubmed.ncbi.nlm.nih.gov/12142678/ DOI: https://doi.org/10.1097/00006534-200208000-00031
45 Guix B. 2001. Treatment of keloids by high-dose-rate brachytherapy: a seven-year study. 50: 167-172. Ref.: https://pubmed.ncbi.nlm.nih.gov/11316560/ DOI: https://doi.org/10.1016/s0360-3016(00)01563-7
46 Shen J. 2015. Hypofractionated electron-beam radiation therapy for keloids: retrospective study of 568 cases with 834 lesions. 56: 811-817. Ref.: https://pubmed.ncbi.nlm.nih.gov/26224888/ DOI: https://doi.org/10.1093/jrr/rrv031
47 Cheraghi N. 2017. RADIATION THERAPY for the Adjunctive Treatment of Surgically Excised Keloids: A Review. 10: 12. Ref.: https://pubmed.ncbi.nlm.nih.gov/28979658/
48 Caccialanza M. 2002. Postoperative radiotherapy of keloids: a twenty-year experience. 12: 58-62.
49 Seegenschmiedt MH. 2004. Radiation therapy for nonmalignant diseases in Germany. 180: 718-730. Ref.: https://pubmed.ncbi.nlm.nih.gov/15549190/ DOI: https://doi.org/10.1007/s00066-004-9197-9
50 Eaton DJ. 2012. Radiotherapy treatment of keloid scars with a kilovoltage X-ray parallel pair. 102: 421-423. Ref.: https://pubmed.ncbi.nlm.nih.gov/21889225/ DOI: https://doi.org/10.1016/j.radonc.2011.08.002
51 Kal HB. 2005. Biologically effective doses of postoperative radiotherapy in the prevention of keloids. 2005. 181: 717-723. Ref.: https://pubmed.ncbi.nlm.nih.gov/16254707/ DOI: https://doi.org/10.1007/s00066-005-1407-6
52 Hoang D. 2017. Surgical excision and adjuvant brachytherapy vs external beam radiation for the effective treatment of keloids: 10-year institutional retrospective analysis. 37: 212-225. Ref.: https://pubmed.ncbi.nlm.nih.gov/27553611/ DOI: https://doi.org/10.1093/asj/sjw124
53 Caviggioli F. 2010. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. 126: 1130-1131. Ref.: https://pubmed.ncbi.nlm.nih.gov/20811265/ DOI: https://doi.org/10.1097/prs.0b013e3181e3b804
54 Ogawa R. 2007. Postoperative radiation protocol for keloids and hypertrophic scars: statistical analysis of 370 sites followed for over 18 months. 59: 688-691. Ref.: https://pubmed.ncbi.nlm.nih.gov/18046154/ DOI: https://doi.org/10.1097/sap.0b013e3180423b32
55 Doornbos JF. 1990. The role of kilovoltage irradiation in the treatment of keloids. 18: 833-839.
56 Duan Q. 2015. Postoperative brachytherapy and electron beam irradiation for keloids: A single institution retrospective analysis. 3: 550-554. Ref.: https://pubmed.ncbi.nlm.nih.gov/26137265/ DOI: https://doi.org/10.3892/mco.2015.498
57 Flickinger. 2011. A radiobiological analysis of multicenter data for postoperative keloid radiotherapy. 79: 1164-1170. Ref.: https://pubmed.ncbi.nlm.nih.gov/20472370/ DOI: https://doi.org/10.1016/j.ijrobp.2009.12.019
58 Song C. 2014. Adjuvant single-fraction radiotherapy is safe and effective for intractable keloids. 55: 912-916. Ref.: https://pubmed.ncbi.nlm.nih.gov/24801475/ DOI: https://doi.org/10.1093/jrr/rru025
59 Ragoowansi R. 2003. Treatment of keloids by surgical excision and immediate postoperative single-fraction radiotherapy. 111: 1853-1859. Ref.: https://pubmed.ncbi.nlm.nih.gov/12711944/ DOI: https://doi.org/10.1097/01.prs.0000056869.31142.de
60 Wang LZ. 2014. Forty-five cases of chest keloids treated with subcutaneous super-tension-reduction suture combined with postoperative electron-beam irradiation. 40: 1378-1384. Ref.: https://pubmed.ncbi.nlm.nih.gov/25357171/ DOI: https://doi.org/10.1097/dss.0000000000000163
61 Bertiere M. 1990. Value of interstitial irradiation of keloid scars by Iridium 192. Apropos of 46 cases. In Annales de chirurgie plastique ET esthetique.
62 Escarmant P. 1993. The treatment of 783 keloid scars by iridium 192 interstitial irradiation after surgical excision. 26: 245-251. Ref.: https://pubmed.ncbi.nlm.nih.gov/8491682/ DOI: https://doi.org/10.1016/0360-3016(93)90204-9
63 Van Leeuwen. 2015. Surgical excision with adjuvant irradiation for treatment of keloid scars: a systematic review. 3.
64 Yang X. 2019. A Novel Radiotherapy Approach for Keloids with Intrabeam. BioMed research international. 2019. Ref.: https://pubmed.ncbi.nlm.nih.gov/31428636/ DOI: https://doi.org/10.1155/2019/4693528
65 Shaffer JJ. 2002. Cook-Bolden, Keloidal scars: a review with a critical look at therapeutic options. 46: S63-S97. Ref.: https://pubmed.ncbi.nlm.nih.gov/11807470/ DOI: https://doi.org/10.1067/mjd.2002.120788
66 Darzi MA. 1992. Evaluation of various methods of treating keloids and hypertrophic scars: a 10-year follow-up study. 45: 374-379. Ref.: https://pubmed.ncbi.nlm.nih.gov/1638291/ DOI: https://doi.org/10.1016/0007-1226(92)90008-l
67 Griffith BH. 1970. A follow-up study on the treatment of keloids with triamcinolone acetonide. 46: 145-150. Ref.: https://pubmed.ncbi.nlm.nih.gov/5423478/ DOI: https://doi.org/10.1097/00006534-197008000-00006
68 Chowdri NA. 1999. Keloids and hypertrophic scars: results with intra?operative and serial postoperative corticosteroid injection therapy. 69: 655-659. Ref.: https://pubmed.ncbi.nlm.nih.gov/10515339/ DOI: https://doi.org/10.1046/j.1440-1622.1999.01658.x
69 Kill JJ. 1977. Keloids treated with topical injections of triamcinolone acetonide. 11: 169-172. Ref.: https://pubmed.ncbi.nlm.nih.gov/345427/ DOI: https://doi.org/10.3109/02844317709025514
70 Cronin TD. 1961. The use of a molded splint to prevent contracture after split skin grafting on the neck. 27: 7-18. Ref.: https://pubmed.ncbi.nlm.nih.gov/24545569/ DOI: https://doi.org/10.1097/00006534-196101000-00002
71 Baur P. Ultrastructural analysis of pressure-treated human hypertrophic scars. 16: 958-967. Ref.: https://pubmed.ncbi.nlm.nih.gov/1003586/ DOI: https://doi.org/10.1097/00005373-197612000-00004
72 Kadouch DJ. 2010. Postoperative Pressure Therapy of Ear Keloids Using a Custom?Made Methyl Methacrylate Stent. 36: 383-385. Ref.: https://pubmed.ncbi.nlm.nih.gov/20100260/ DOI: https://doi.org/10.1111/j.1524-4725.2009.01449.x
73 Russell RN. 2001. Gault, Zimmer splintage: a simple effective treatment for keloids following ear-piercing. 2001. 54: 509-510. Ref.: https://pubmed.ncbi.nlm.nih.gov/11513513/ DOI: https://doi.org/10.1054/bjps.2001.3649
74 Alster TS. 1997. Treatment of scars: a review. 39: 418-432. Ref.: https://pubmed.ncbi.nlm.nih.gov/9339286/ DOI: https://doi.org/10.1097/00000637-199710000-00014
75 Lahiri AD. 2001. Experience with difficult keloids. 54: 633-635. Ref.: https://pubmed.ncbi.nlm.nih.gov/11583502/ DOI: https://doi.org/10.1054/bjps.2001.3665
76 Alster TS. 2003. Hypertrophic scars and keloids. 4: 235-243. Ref.: https://pubmed.ncbi.nlm.nih.gov/12680802/ DOI: https://doi.org/10.2165/00128071-200304040-00003
77 Layton AJ. 1994. A comparison of intralesional triamcinolone and cryosurgery in the treatment of acne keloids. 130: 498-501. Ref.: https://pubmed.ncbi.nlm.nih.gov/8186117/ DOI: https://doi.org/10.1111/j.1365-2133.1994.tb03385.x
78 Berman B. 1997. Recurrence rates of excised keloids treated with postoperative triamcinolone acetonide injections or interferon alfa-2b injections. 37: 755-757.
79 Conejo Mir. 1998. Linares, Carbon dioxide laser ablation associated with interferon alfa-2binjections reduces the recurrence of keloids. 39: 1039-1040. Ref.: https://pubmed.ncbi.nlm.nih.gov/9843032/ DOI: https://doi.org/10.1016/s0190-9622(98)70295-6
80 Granstein RD. 1990. A Controlled Trial of Intralesional Recombinant Interferon-? in the Treatment of Keloidal Scarring: Clinical and Histologic Findings. 126: 1295-1302. Ref.: https://pubmed.ncbi.nlm.nih.gov/2121104/
81 AL?Khawajah. 1996. Failure of interferon?alpha 2b in the treatment of mature keloids. 35: 515-517. Ref.: https://pubmed.ncbi.nlm.nih.gov/8809610/ DOI: https://doi.org/10.1111/j.1365-4362.1996.tb01671.x
82 Wong TW. 1994. Intralesional interferon α?2b has no effect in the treatment of keloids. 130: 683-684. Ref.: https://pubmed.ncbi.nlm.nih.gov/8204485/ DOI: https://doi.org/10.1111/j.1365-2133.1994.tb13125.x
83 Fitzpatrick RE. 1999. Treatment of inflamed hypertrophic scars using intralesional 5?FU. 25: 224-232. Ref.: https://pubmed.ncbi.nlm.nih.gov/10193972/ DOI: https://doi.org/10.1046/j.1524-4725.1999.08165.x
84 Stern JC. 1989. Carbon dioxide laser excision of earlobe keloids: a prospective study and critical analysis of existing data. 115: 1107-1111. Ref.: https://pubmed.ncbi.nlm.nih.gov/2765229/ DOI: https://doi.org/10.1001/archotol.1989.01860330097026
85 Sherman R. 1988. Experience with the Nd: YAG laser in the treatment of keloid scars. 21: 231-235. Ref.: https://pubmed.ncbi.nlm.nih.gov/2975929/ DOI: https://doi.org/10.1097/00000637-198809000-00007
86 Apfelberg DB. 1989. Failure of carbon dioxide laser excision of keloids. 9: 382-388. Ref.: https://pubmed.ncbi.nlm.nih.gov/2503668/ DOI: https://doi.org/10.1002/lsm.1900090411
87 Apfelberg DB. 1984. Preliminary results of argon and carbon dioxide laser treatment of keloid scars. 4: 283-290. Ref.: https://pubmed.ncbi.nlm.nih.gov/6438418/ DOI: https://doi.org/10.1002/lsm.1900040309
88 Alster TS. 1993. Alteration of argon laser–induced scars by the pulsed dye laser. 13: 368-373. Ref.: https://pubmed.ncbi.nlm.nih.gov/8515676/ DOI: https://doi.org/10.1002/lsm.1900130314
89 Alster TS. 1994. Improvement of erythematous and hypertrophic scars by the 585-nm flashlamp-pumped pulsed dye laser. 32: 186-190. Ref.: https://pubmed.ncbi.nlm.nih.gov/8192370/ DOI: https://doi.org/10.1097/00000637-199402000-00015
90 Alster TS. 1998. Pulsed dye laser treatment of hypertrophic burn scars. 102: 2190-2195. Ref.: https://pubmed.ncbi.nlm.nih.gov/9811021/ DOI: https://doi.org/10.1097/00006534-199811000-00060




















