Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ijho.2020.110003Article Views : 4Article Downloads : 8
A Personalized short-time Immunotherapy with Subcutaneous very low-dose Il-2 plus the Pineal Hormone Melatonin in advanced cancer patients with Persistent Lymphocytopenia
Paolo Lissoni, Giusy Messina, Andrea Sassola, Giorgio Porro, Simonetta Tassoni* and Giuseppe Di Fede
Institute of Biological Medicine, Milan
*Effatà Institute, Lucca, Italy
*Corresponding Author: Paolo Lissoni, Email: paolo.lissoni@gmx.com
Article Information
Aritcle Type: Research Article
Citation: Paolo Lissoni, Giusy Messina, Andrea Sassola, et al. 2020. A Personalized short-time Immunotherapy with Subcutaneous very low-dose Il-2 plus the Pineal Hormone Melatonin in advanced cancer patients with Persistent Lymphocytopenia. Int J Hematol Oncol. 3: 01-07.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2020; Paolo Lissoni
Publication history:
Received date: 27 February, 2020Accepted date: 16 March, 2020
Published date: 18 March, 2020
Abstract
Today, it is known that the prognosis of human neoplasms depends not only on the genetic characteristics of tumor cells, but also on the immunobiological response of patients. Moreover, despite its complexity, at present it is known that the antitumor immunity is generally suppressed by the macrophage system, and stimulated by T lymphocytes, with the only exception of regulatory T cells, which in contrast inhibit the anticancer immunity through the release of immunosuppressive cytokines, the most important of them is TGF-beta. Finally, it is known that lymphocytopenia may predict a negative prognosis in the advanced neoplasms, and a more negative significance is played by the occurrence of abnormally low values of lymphocyte-to-monocyte ratio (LMR), whose decline could depend on both lymphocyte decrease and monocyte increase. At present, the only cytokine able to enhance lymphocyte count still remains IL-2. Therefore, because of the negative prognostic significance of cancer-related lymphocytopenia, the use of IL-2 to correct cancer-related lymphocytopenia could constitute a new strategy in the immunotherapy of cancer. On these bases, a study was planned to evaluate the effects of a neuro-immunotherapeutic combination with SC very-low dose IL-2 in association with the antitumor immunostimulating pineal hormone melatonin (MLT) on lymphocyte count, and their persistence on time in a group of untreatable lymphocytopenic advanced cancer patients. The study included 14 lymphocytopenic cancer patients, who were eligible for the only palliative therapy. IL-2 was SC injected at 1.8 MIU/day for 5 days/week for 2 consecutive weeks, in association with MLT at 100 mg/day orally during the dark period of the day. A normalization of lymphocyte count was achieved in 9/14 (64%) patients within the two weeks of therapy. Both lymphocyte and LMR mean values significantly increased on therapy with respect to the pre-treatment values. On the other hand, monocyte mean values diminished on therapy, without, however, significant differences. The median duration of lymphocyte count normalization was 160 days (range 39-240 days). These preliminary results would suggest a new possible clinical use of IL-2 immunotherapy to counteract advanced cancer-related lymphocytopenia, because of its well documented negative effects on the survival, in an attempt to control tumor growth by activating the natural antitumor immunobiological response, which is fundamentally an IL-2-dependent phenomenon, and which is altered in the advanced human neoplasms.
Keywords: Cancer immunotherapy, Interleukin-2, Lymphocytopenia, Immunosuppression, Melatonin, Pineal gland
Introduction
At present, the immunotherapeutic approach has been finally universally accepted as a new possible strategy in cancer cure because of the recent advances in the knowledgements of cytokine mechanisms responsible for the activation, or the suppression of the anticancer immunity [1-3]. The activation of the anticancer immunity is substantially mediated by TH1 lymphocytes (CD4+), cytotoxic T lymphocytes (CD8+), NK cells, and dendritic cells, whereas it is suppressed by two major systems, consisting of the monocyte-macrophage system through the release of inflammatory immunosuppressive cytokines, namely IL-6, IL-1 beta and TNF-alpha [4], and regulatory T lymphocytes (T reg) (CD34+CD25+) through the release of anti-inflammatory immunosuppressive cytokines, including TGF-beta and in a less manner IL-10 [5]. Being its capacity of self-proliferation due to the simultaneous stimulation of IL-2 secretion , which is the main T cell growth factor [1], and expression of IL-2 receptor, TH1 lymphocyte plays a fundamental role in the generation of an effective anticancer immunity, and most in general in the control of the overall immune functions [3]. However, it has to be remarked that at present two different cancer immunotherapeutic strategies may be recognized, consisting of a direct administration of antitumor cytokines, namely IL-2 itself, either alone or in association with other anticancer cytokines, such as IL-12 [6], or the injection of monoclonal antibodies against some particular cell surface molecules, the so-called immune checkpoints, namely PD-1 and its ligands, which are able to induce a suppression of the anticancer immunity [7]. Moreover, the recent advances in the area of psycho-neuro-endocrino-immunology (PNEI) have demonstrated that the in vivo immune responses, including the anticancer immunity, are physiologically under a modulatory neuroendocrine regulation [8]. In more detail, despite the complexity of the neuroimmunomodulation, the anticancer immunity has been proven to be inhibited by catecholamines, corticosteroids and mu-opioid agonists [9,10], whereas it is stimulated and amplified by brain cannabinoid system-pineal functional axis [11,12]. Within the great number of biomarkers capable of synthetizing the immune status of cancer patients, lymphocyte count was the first identified parameter capable of predicting a negative prognosis, since lymphocytopenia has appeared to be associated with a worse prognosis in advanced cancer patients [13]. In addition to the negative prognostic significance of lymphocytopenia, monocytosis has also appeared to predict a negative prognosis, since monocyte count has been shown to be correlated to the degree of tumor macrophage infiltration, which has been proven to stimulate tumor growth [14]. Then, the lymphocyte-to-monocyte ratio (LMR) has appeared to represent the most simple and inexpensive clinical biomarker capable of exploring the immune status of cancer patients, as well as of predicting the prognosis of the neoplastic disease [16], because of its correlation to the degree of activation of T reg regulatory system [17]. In fact, a progressive decline in LMR has appeared to be associated with a negative prognosis in terms of both responses to therapy and survival time [16]. On the contrary, both spontaneous and therapy-induced lymphocytosis has been shown to predict a control of the neoplastic progression [18]. At present, IL-2 immunotherapy of tumours would constitute the only anticancer therapy capable of inducing a clear increase in lymphocyte count [1-3], even though the efficacy of the more recent cancer immunotherapies with anti-PD-1 monoclonal antibodies would be also associated with an increase in lymphocyte number, whose degree, however, has been proven to be inferior with respect to that obtained with IL-2 [7]. Unfortunately, in the past years IL-2 was clinically employed as an active anticancer agent, able to allow a cytotoxicity-mediated cancer cell destruction irrespectively of the immune status of patients, rather than as an immunomodulating agent to correct cancer-related lymphocytopenia, which is one of the main factors responsible for cancer progression [13,18,19]. The reinterpretation of IL-2 cancer immunotherapy as an antitumor treatment carried out to indirectly control tumor growth by correcting cancer-related lymphocytopenia, could constitute a new immunotherapeutic approach to cancer cure. Then, dose and time of treatment of IL-2 anticancer immunotherapy would have to be established and personalized in relation to the response of the single cancer patient in terms of both lymphocyte increase and duration of a normal lymphocyte count after its normalization on IL-2 treatment. At present, the neuro-immunotherapy with subcutaneous (SC) low-dose IL-2 in association with high-dose pineal hormone melatonin (MLT) would constitute the most simple and effective schedule reported in the literature to correct cancer-related persistent lymphocytopenia [20]. This finding is not surprising, since the pineal hormone MLT has been proven to play a fundamental neuroendocrine role in the generation of an effective anticancer immune response, namely by stimulating both TH1 and dendritic cell functions [21], with a consequent increase in the blood levels of both IL-2 and IL-12, whose fundamental role in the human anticancer immunity has been well demonstrated [1,6]. On these bases, a study was started with SC very low-dose of IL-2 in association with high-dose MLT to evaluate its effects and time duration on lymphocyte count in a group of advanced cancer patients with persistent lymphocytopenia.
Materials and Methods
The study included 14 consecutive lymphocytopenic untreatable advanced cancer patients, who were suitable for the only palliative therapy (M/F: 9/5; median age 63 years, range 45-71). Eligibility criteria were as follows: histologically proven solid tumor, measurable lesions, no availability of other conventional anticancer therapies, persistent lymphocytopenia for at least 3 consecutive months, and no chronic therapy with corticosteroids, because of their lymphocytopenic effects. Tumor histotypes were as follows: lung adenocarcinoma:2; colorectal cancer:2; breast cancer:2; pancreatic adenocarcinoma:2; glioblastoma:2; hepatocarcinoma:1; melanoma:1; soft tissue sarcoma:1; ovarian cancer:1. The experimental protocol was explained to each patient, and written consent was obtained. IL-2 was SC injected at a dose of 1,8 MIU/day for 5 days/week for 2 consecutive weeks. MLT was orally given at a dose of 100 mg/day during the dark period of the day according to its physiological light/night production, every day without interruption forthe whole duration of the study, by starting 7 days prior to IL-2 administration. All patients followed the treatment as a home therapy. For the immune evaluation, venous blood samples were collected in the morning after an overnight fast, before the onset of treatment, and at 7-days intervals for the first month, then at monthly intervals for 5 months. Normal values observed in our laboratory (95% confidence limits) were more than 1,500/mm3 for lymphocytes, below 400/mm3 for monocytes, and more than 2.1 for LMR values. The clinical response was evaluated according to WHO criteria. Data were reported as mean +/- SE, a statistically evaluated by the Student’s t test, the chi-square test, and the analysis of variance, as appropriate.
Results
In addition to lymphocytopenia, all patients had abnormally low values of LMR prior to therapy. A normalization of lymphocyte count was achieved in 5/14 (36%) patients within the first week of therapy, and in 9/14 (64%) patients within the two weeks of treatment. On the same way, a normalization of LMR values was achieved in 4/14 (29%) and in 8/14 (57%) patients, respectively after the first and the second week of therapy. As far as the response of the monocyte-macrophage system is concerned, abnormally high pre-treatment values of monocyte count were seen in 11/14 (79%) patients, which became within the normal range after the two weeks of treatment in only 2/11 (18%) patients with abnormally high monocyte count prior to therapy. No tumor regression was observed. However, a stable disease (SD) was achieved in 6/14 (43%) patients, with a median duration of 7 months (range 3-11), whereas the remaining 8/14 (57%) patients had a progressive disease (PD). Moreover, the percentage of SD observed in patients with normalization of lymphocyte count was statistically significantly higher than that found in patients with PD (5/9 (56%) vs 1/5 (20%), P<0.05). Finally, the median duration of IL-2-induced lymphocyte count normalization was 160 days (range 30-240 days), therefore more than 5 months. No IL-2-related toxicity was observed, and fever less than 38° C occurred in only 3/14 (21%) patients. Figure 1 illustrates changes in lymphocyte, monocyte and LMR mean values observed on immunotherapy. As shown, lymphocyte mean count significantly increased with respect to the pre-treatment values within the first 14 days of therapy (P<0.05). On the contrary,monocyte mean number decreased on therapy, without, however, statistically significant differences. Then, LMR mean values progressively increased on therapy, by reaching the statistical significance within the 2 weeks of therapy (P<0.05 after 14 and 21 days; P<0.01 after 30 days).
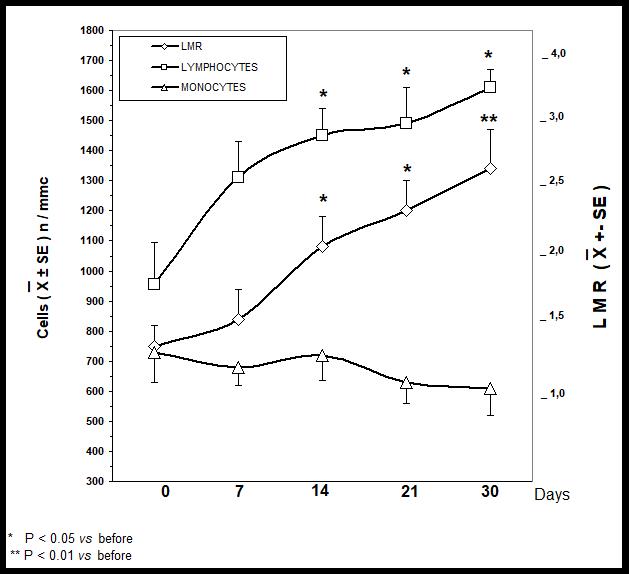
Figure 1: Lymphocyte, monocyte and LMR mean values in cancer patients therapy on immunotherapy with IL-2 plus melatonin.
Discussion
The results of this phase-2 preliminary study show that a short-time therapy with low-dose IL-2 in association with the immune-stimulating pineal hormone MLT is a well tolerable treatment, which may be sufficient to normalize lymphocyte count in most advanced cancer patients, irrespectively of their tumor histotype. IL-2 immunotherapy-induced normalization of lymphocyte count would not represent an epiphenomenon only, because of its association with a control of tumor growth. Therefore, this preliminary study would suggest a possible new clinical use of IL-2 in cancer immunotherapy, carried out to control tumor growth by stimulating lymphocyte system to maintain a normal number of lymphocytes, whose fundamental role in tumor cell destruction is well known (1-3), rather than as a simple direct anticancer agent. Moreover, it has to be remarked that IL-2 therapy had been administered according to well defined schedules, which were planned independently of the immune response of patients, then in a similar manner to that of the classical chemotherapeutic regimens. On the contrary, thispreliminary study would suggest the possibility to personalize the time duration of IL-2 injection by monitoring changes inlymphocyte count and LMR ratio, by planning a further IL-2 cycle in the presence of a new decline in lymphocyte count. On the other hand, the profile of changes in monocyte count is more complex to be understood, since a normalization of monocyte count was observed only in few patients. Because of the immunosuppressive activity of the monocyte-macrophage system, the lack of an evident decline in monocyte count on IL-2 therapy would counteract the antitumor activity of IL-2 itself. In fact, advanced cancer-related decline in LMR values has been proven to depend on both lymphocyte decrease and monocyte increase (20,21). Then, a greater stimulation of the anticancer immunity could be achieved by counteracting cancer-related macrophage system hyper-activation. The macrophage system may be inhibited by both cytokines and neuroactive agents, such as pineal hormones other than MLT (22) and cannabinoids (12). Macrophage-mediated chronic inflammatory status may be reduced by the anti-inflammatory cytokines TGF-beta and IL-10, but they cannot be clinically employed in cancer because of their suppressive effects on the anticancer immunity (5,17). The only cytokine, which may control macrophage system hyper-activation without suppressing the anticancer immunity, could be represented by IL-3 (23), but its clinical use is still at the beginning. Therefore, a control of macrophage function by neurohormones and neuro-mediators under IL-2 immunotherapy could amplify the activity of IL-2 itself. Finally, a greater immune activation could be obtained through an association between IL-2 and anti-PD-1 monoclonal antibodies, as suggested by preliminary experimental researches (19), since IL-2 may improve the activity of the anti-PD-1 agents by enhancing therapy-induced lymphocyte increase, and anti-PD-1 drugs may increase IL-2 efficacy by abrogating the immunosuppressive action of T reg cell system.
Conclusions
The results of this preliminary study would suggest a new possible clinical use of IL-2 immunotherapy in human neoplasms, carried out to control tumor growth by simply correcting cancer-related lymphocytopenia, by personalizing dose, time, and duration of administration on the basis of lymphocyte count values, instead of planning an already established schedule of therapy, irrespectively of the biological response of cancer patients, as occurred in the past years of IL-2 immunotherapy of cancer.
References
1. Grimm EA, Mazumder A, Zhang HZ, et al. 1982. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J ExpMed. 155: 1823-1841. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/6176669
2. Rosenberg SA. 1992. The immunotherapy and gene therapy of cancer. J ClinOncol. 10: 181-191. Ref.: https://bit.ly/3d25khe
3. Lissoni P. 2017. Therapy implications of the role of interleukin-2 in cancer. Exp Rev Clin Immunol. 13: 491-498. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/27782752
4. Grivennikov SI, Greten FR, Karin M. 2010. Immunity, inflammation, and cancer. Cell. 295:883-889.
5. Connolly EC, Freimuth J, Akhurst RJ. 2012. Complexity of TGF-beta targeted cancer therapy. Int J Biol Sci. 8: 964-978. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/22811618
6. Banks RE, Patel PM, Selby PJ. 1995. Interleukin-12: a new clinical player in cytokine therapy. B J Cancer. 71: 655-659. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/7710924
7. Chen J, Jiang CC, Jin L, et al. 2016. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 27: 409-416. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/26681673
8. Rubinow DR. 1990. Brain, behaviour and immunity: an interactive system. J Natl Cancer Inst Monogr. 10: 79-82. Ref.: https://bit.ly/2IRuf98
9. Lewis JW, Shavit Y, Terman GV. 1983. Apparent involvement of opioid peptides in stress-induced enhancement of tumor growth. Peptides. 4: 653-658. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/6686324
10. Manfredi B, Sacerdote P, Bianchi M. 1993. Evidence for an opioid inhibitory tone on T cell proliferation. J Neuroimmunol. 44: 43-46. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/8388406
11. Lissoni P, Resentini M, Mauri R, et al. 1996. Effects of tetrahydrocannabinol on melatoninsecretion in man. HormMetab Res. 18: 77-78. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/3005151
12. Grotenhermen F. 2004. Pharmacology of cannabinoids. NeuroendocrinolLett. 25: 14-23. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/15159677
13. Riesco A. 1970. Five-year cancer cure: relation to total amount of peripheral lymphocytes and neutrophils. Cancer 25: 135-140. Ref.: https://bit.ly/2x1dhm0
14. Mantovani A, Allavena P, Sica A, et al. 2008. Cancer-relatedinflammation. Nature 454: 436-444. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/18650914
15. Lissoni P, Messina G, Rovelli F, et al. 2018. Low lymphocyte-to-monocyte ratio is associated with an enhanced regulatory T lymphocyte function in metastatic cancer patients. Int J RecAdv Multi Res. 5: 3353-3356.
16. Gu L, Li H, Chen L, et al. 2016. Prognostic role of lymphocyte-to-monocyte ratio for patients with cancer: evidence from a systematic review and meta-analysis. Oncotarget. 3: 7876-7881. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/26942464
17. Zou W. 2006. Regulatory T cells, tumor immunity and immunotherapy. Nat Rev Immunol. 6: 295-307. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/16557261
18. Fumagalli L, Lissoni P, Di Felice G, et al. 1999. Pre-treatment serum markers and lymphocyteresponse to interleukin-2 therapy. Br J Cancer. 80: 407-411. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/10408846
19. West EE, Jin HT, Rasheed AU, et al. 2013. PD-L1 blockade sinergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest. 123: 2611-2615. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/23676462
20. Lissoni P, Rovelli F, Porro G, et al. 2018. Treatment of advanced cancer-related lymphocytopenia: comparison among the effects of subcutaneous low-dose interleukin-2, high-dose pineal hormone melatonin and checkpoint inhibitors. J Cancer Res Oncobiol. 1: 112-114. Ref.: https://bit.ly/2x4bXyL
21. Maestroni GJM. 1993. The immunoneuroendocrine role of melatonin. J Pineal Res. 14: 1-10. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/8483103
22. Sze SF, Ng TB, Liu WK. 1993. Antiproliferative effects of pineal indoles on cultured tumor cell lines. J Pineal Res. 14: 27-33. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/8483104
23. Lissoni P, Pittalis S, Brivio F, et al. 1993. In vitro modulatory effects of interleukin-3 on macrophage activation induced by inerleukin-2. Cancer. 71: 2076-2081.




















