Indexing & Abstracting
Full Text
Review ArticleDOI Number : 10.36811/ijdsc.2019.110002Article Views : 154Article Downloads : 107
Skin Aging & Modern Edge Anti-Aging Strategies
Abdul Kader Mohiuddin
Department of Pharmacy, World University of Bangladesh, 151/8, Green Road, Dhanmondi, Dhaka, Bangladesh
*Corresponding Author: Abdul Kader Mohiuddin, Assistant Professor, Department of Pharmacy, World University of Bangladesh, 151/8, Green Road, Dhanmondi, Dhaka-1205, Bangladesh, Tel: +8801716477485; Email: trymohi@gmail.com
Article Information
Aritcle Type: Review Article
Citation: Abdul Kader Mohiuddin. 2019. Skin Aging & Modern Edge Anti-Aging Strategies. Int J Dermatol Skin Care. 1: 08-62.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Abdul Kader Mohiuddin
Publication history:
Received date: 27 May, 2019Accepted date: 24 June, 2019
Published date: 11 July, 2019
Background
Skin is the barrier that segregates the body from the outer environment. Besides protecting the body from water loss and microorganism infection, it has an important cosmetic role. Young and beautiful appearance may have a positive influence on people’s social behavior and reproductive status. Cleopatra, the Egyptian queen is said to have indulged in daily donkey-milk baths, a practice which apparently required over 700 donkeys to accomplish. The alpha hydroxy acids in the milk is believed to be anti-aging and skin-softening agents. Tang-dynasty ruler and sole female emperor of China, Wu Zetia, maintained a lifelong interest in skincare formulas. She mixed her “fairy powder” (made of carefully harvested and prepared Chinese motherwort) with cold water in order to wash her face each morning. The empress was a famed beauty well into her old age. The most hair-raising entrant in this list, 16th century Hungarian countess Elizabeth Bather is infamous for being one of the world’s first documented female serial killers. Most of her life is shrouded in mystery and legend-the most famous story being that she would regularly bathe in the blood of her female victims. Mary, Queen of Scots, the ill-fated and attractive adversary of Elizabeth I, spent her sixteenth-century happier days on her estate in Edinburgh, Scotland, where her beauty regimen was said to include white-wine baths. In addition to wine’s antiseptic alcohol content, it was also was thought to improve complexion in general. Crème Celeste, a favorite product of empress Elisabeth (Sisi) of Austria, was a concoction of spermaceti (a wax found in the head of sperm whales), sweet almond oil, and rosewater. She would apply this daily and at night, she was known to coat her face in raw veal and crushed strawberries, kept in place with a custom-made leather mask. The skin folds are indicative of an aged personality, but not youthfulness. So, everyone wants to look younger for whole of the life, which lead to the discovery of many surgical and non-surgical treatment modalities to improve the youthfulness. Since the introduction of Botox in 2002 after FDA approval more aesthetic procedures using Botox were performed by aestheticisms involving plastic surgeons and dermatologists. However, many scientists are now starting to view physical aging as a disease process. The cellular and molecular mechanisms involved in aging reveal an intricate series of signals, markers, and pathways, all of which are programmed to monitor and control the lifespan of a cell as it ages. By studying these molecular events and pathways, the field of anti-aging will be furthered by the use of more and more cosmetics.
Abstract
As the most voluminous organ of the body that is exposed to the outer environment, the skin suffers from both intrinsic and extrinsic aging factors. Skin aging is characterized by features such as wrinkling, loss of elasticity, laxity, and rough-textured appearance. This aging process is accompanied with phenotypic changes in cutaneous cells as well as structural and functional changes in extracellular matrix components such as collagens and elastin. With intrinsic aging, structural changes occur in the skin as a natural consequence of the biological changes over time and produce a certain number of histological, physiological, and biochemical modifications. Intrinsic aging is determined genetically (influence of gender and ethnic group), variable in function of skin site, and also influenced by hormonal changes. Visually it is characterized by fine wrinkles. By comparison, “photoaging” is the term used to describe the changes occurring in the skin, resulting from repetitive exposure to sunlight. The histological, physiological, and biochemical changes in the different layers of the skin are much more drastic. From a mechanical point of view, human skin appears as a layered composite containing the stiff thin cover layer presented by the stratum corneum, below which are the more compliant layers of viable epidermis and dermis and further below the much more compliant adjacent layer of subcutaneous white adipose tissue. Upon exposure to a strain, such a multi-layer system demonstrates structural instabilities in its stiffer layers, which in its simplest form is the wrinkling. These instabilities appear hierarchically when the mechanical strain in the skin exceeds some critical values. Their appearance is mainly dependent on the mismatch in mechanical properties between adjacent skin layers or between the skin and subcutaneous white adipose tissue, on the adhesive strength and thickness ratios between the layers, on their bending and tensile stiffness as well as on the value of the stress existing in single layers. Gradual reduction of elastic fibers in aging significantly reduces the skin’s ability to bend, prompting an up to 4-fold reduction of its stability against wrinkling, thereby explaining the role of these fibers in skin aging. Anti-aging medicine is practiced by physicians, scientists, and researchers dedicated to the belief that the process of physical aging in humans can be slowed, stopped, or even reversed through existing medical and scientific interventions. This specialty of medicine is based on the very early detection and prevention of age-related diseases. Physicians practicing anti-aging medicine seek to enhance the quality of life as well as its length, limiting the period of illness and disability toward the end of one’s life. Anti-aging medicine encompasses lifestyle changes (diet and exercise); hormone replacement therapies, as needed, determined by a physician through blood testing (DHEA, melatonin, thyroid, human growth hormone, estrogen, testosterone); antioxidants and vitamin supplements; and testing protocols that can measure not only hormone levels and blood chemistry but every metabolic factor right down to the cellular level.
Keywords: Skin Care; Anti-Aging; Photoaging; Wrinkles; Antioxidants; Keratinocytes; Retinoids

Figure 1: Evergreen Monica Bellucci [227,228]. One of the hottest Italian beauties, although she is 54 years old, starts taking a cold shower to the day. Cold shower, the skin maintains the elasticity and argues that tightens. She uses thermal water and revitalizing spray for her face. The actress is totally against all sorts of plastic surgery, but don’t forget to constantly clean and moisturize the skin. She says, noting that eating and drinking can be anything, the main thing in small amounts and never blame themselves for the food. She never denied that sport is important for health and toned figure. Drinking plenty of water is another good thing that Bellucci follows as her regular activities.
Introduction
Skin aging is a complex biological process influenced by a combination of endogenous or intrinsic and exogenous or extrinsic factors. Because of the fact that skin health and beauty is considered one of the principal factors representing overall “well-being” and the perception of “health” in humans, several anti-aging strategies have been developed during the last years. In contrast to thin and atrophic, finely wrinkled and dry intrinsically aged skin, premature photoaged skin typically shows a thickened epidermis, mottled discoloration, deep wrinkles, laxity, dullness and roughness. Gradual loss of skin elasticity leads to the phenomenon of sagging. Slowing of the epidermal turnover rate and cell cycle lengthening coincides with a slower wound healing and less effective desquamation in older adults. This fact is important when esthetic procedures are scheduled. On the other side, many of these features are targets to product application or procedures to accelerate the cell cycle, in the belief that a faster turnover rate will yield improvement in skin appearance and will speed wound healing. A marked loss of fibrillin-positive structures as well as a reduced content of collagen type VII (Col-7), may contribute to wrinkles by weakening the bond between dermis and epidermis of extrinsically age skin. Sun-exposed aged skin is characterized by the solar elastosis. The sparse distribution and decrease in collagen content in photoaged skin can be due to increased collagen degradation by various matrix metalloproteinases, serine, and other proteases irrespective of the same collagen production. The overall collagen content per unit area of the skin surface is known to decline approximately 1%/year. Glycosaminoglycans (GAGs) are among the primary dermal skin matrix constituents assisting in binding water. In photo-aged skin, GAGs may be associated with abnormal elastotic material and thus be unable to function effectively. The total hyaluronic acid (HA) level in the dermis of skin that age intrinsically remains stable; however, epidermal HA diminishes markedly. Decreased estrogen levels may play a role in skin aging in women and compounds stimulating estrogen receptors could potentially counteract some of the visible signs of aging. As people live longer, women spend a larger portion of their lives in a post-menopausal state, with a deficiency of estrogen as compared to their younger selves. Changes in diet and increasing exercise, together with a regimen of antioxidants, nutritional supplements, and growth factors, can alter how the genes express themselves. Both factors can greatly enhance the healing capability of the skin and can improve the results of cosmetic surgeries.

Figure 2: Desired effect of anti-aging treatment [2],[246]. The desired therapeutic anti-aging effect of the skin is continuous, step-by step process, which combines various methods of the skin bio-revitalization and rejuvenation, augmentation, restoration of each skin layer individually and in the light of many other factors-from a style of the life to the immune, genetic, emotional and health status in general.
The aging processes
Aging can be viewed as the accumulation of changes in cells and tissues resulting from a greater disorderliness of regulatory mechanisms that result in reduced robustness of the organism to encountered stress and disease. The notion of greater disorderliness in aging is illustrated by the erosion of the orderly neuroendocrine feedback regulation of the secretion of luteinizing hormone (LH), follicle stimulating hormone (FSH), adrenocorticotropic hormone (ACTH) and growth hormone (GH). These changes are manifested as menopause, andropause, adreno-pause, and somato-pause. Skin aging is part of the slow decline in appearance and function that appears to be attributed in large part to the drastic decline of hormones in the body after adulthood. At the cellular level, several processes are involved in the physiology of aging and the development of some age-related diseases. The process of apoptosis signifies the process of nontraumatic and noninflammatory cell death. Dysregulation of apoptosis has been implicated in the increased incidence of cutaneous malignancies that are more prevalent in older individuals, such as basal cell carcinoma, squamous cell carcinoma, and malignant melanoma. Cell senescence limits cell divisions in normal somatic cells and may play a central role in age-related diseases. Telomeres are thought to play a role in cellular aging and might contribute to the genetic background of human aging and longevity. It has been speculated that the limited proliferation potential of human cells is a result of the telomere shortening that occurs during DNA synthesis at each cell division. Photoaging may accelerate the shortening of telomeres and push cells into senescence sooner. That could be the reason why various growth factors may affect the speed and quality of wound healing. Biochemical insults also arise within aging cells, in part from the action of reactive oxygen species generated and scavenged incompletely throughout the cell cycle. Aging-associated changes also occur between and among cells via alterations in the intercellular matrix, the intercellular exchange of trophic factors, the release of inflammatory cytokine mediators, and the degree of infiltration by other associated cell types. In addition, the quantity and distribution of various growth factors may affect wound healing. Decline of DNA repair in combination with loss of melanin increases the risk of photo-carcinogenesis and can also cause the decline of enzymatically active melanocytes (10-20% each decade) that contributes to increased sensitivity to UV radiation. However, it is not known why free radical damage does not adversely affect all of the body’s cells (e.g., gonadal germ cells) [1].
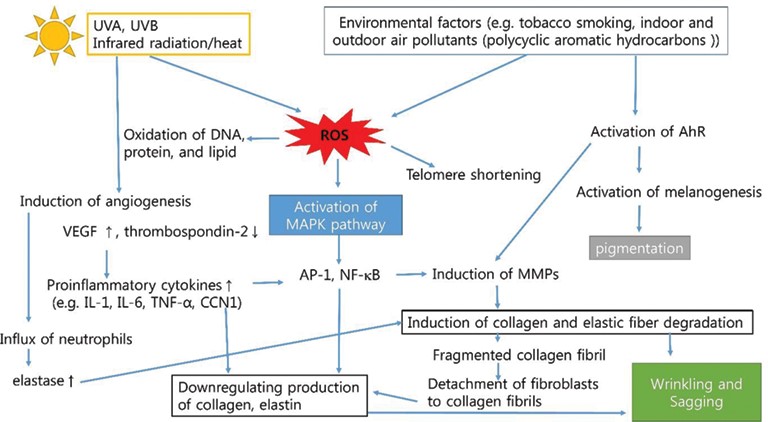
Figure 3: Schematic representation of pathogenesis of premature/extrinsic skin aging [226]. ROS: reactive oxygen species, AhR: aryl hydrocarbon receptor, NF-kB: nuclear factor kappa?B, IL-1: interleukin?1, TNF-α: tumor necrosis factor, CCN1: cysteine-rich protein 61, MAPK: mitogen?activated protein kinase, AP?1: activator protein 1, and MMPs: matrix metalloproteinases.
Factors involved in skin aging
Skin aging is a complex biological process influenced by combination of endogenous or intrinsic (genetics, cellular metabolism, hormone and metabolic processes) and exogenous or extrinsic (chronic light exposure, pollution, ionizing radiation, chemicals, toxins) factors. These factors lead together to cumulative structural and physiological alterations and progressive changes in each skin layer as well as changes in skin appearance, especially, on the sun-exposed skin areas [2]. Facial skin wrinkles can be considered as a marker for intrinsic aging (See wrinkle classification in Table 1). The major perceived risk factors are unhealthy eating habits, stress, less exercise, dehydration, diseased state and sleeping habits. Though the main factor responsible for extrinsic aging is UVR [3]. Beyond sun damage factors such as smoking and atmospheric pollution have also been studied and considered in extrinsic aging. Studies have shown a clear correlation between these factors and the appearance of melanosis and wrinkles. Both of these factors contribute to aging through a common mechanism called oxidative stress that has a negative impact on cellular processes, such as DNA replication. In addition to the UV region of solar radiation that contributes to cellular injury, visible radiation has an oxidative effect similar to that of infrared radiation via heat generation. The effects of comorbidities, such as metabolic illnesses common in the elderly, nutritional deficiencies, and the use of drugs such as corticosteroids, and even cancer treatments, should be assessed by dermatologists attending to skin conditions associated with aging [4]. Good skin condition can be maintained to some extent by changes in modifiable lifestyle factors such as smoking and sunscreen use [5]. Human skin cells respond to instructions from highly specialized proteins or hormones referred to as growth factors. The growth differentiation factor GDF11, a TGF-β family member, has been associated with the maintenance of youth phenotypes in different human tissues and organs, and in the skin has been related to an inhibition of the inflammatory response. The production of elastin and collagen dermal connective fibers slows, and, with age, the regenerative rates of GAGs become delayed [6.7].
| Table 1: Pierid Classification of Wrinkles [26]. |
|
? Atrophic wrinkles develop in exposed and non-exposed skin, disappear with skin traction, change in orientation with body posture, and are due to atrophy of the extracellular matrix. ? Elastotic wrinkles develop in sun exposed skin, Table solar elastosis, become progressively permanent, and do not disappear with perpendicular traction. ? Expressional wrinkles due to subdermal muscle contraction, become permanent with repeated wrinkling. ? Gravitational wrinkles due to skin sagging in response to gravitational forces and inelasticity. |
Photodamage
Chronic repetitive exposure of human skin to solar UV rays causes marked morphological, histological, biochemical, and biophysical changes that are described as photoaging. the clinical signs of photoaging are fine and coarse wrinkles, actinic keratoses, solar elastosis, yellowing, pigmentation disorders and premalignant lesions, skin atrophy, senile purpura, freckles, solar comedowns, telangiectasia, laxity, roughness, and extreme dryness [8]. UV damage can also cause significant changes in some of the mechanical properties of the stratum corneum, reducing its cell cohesion and mechanical integrity; the UV radiation also affects the molecular structure of cell proteins and lipids [4]. According to Lecia et.al, 2019, at the cellular level DNA damage is the main event following UV exposure. The kind of lesions produced depends on the wavelength and the energy profile of the radiation, with different photoproducts being formed as a result. Although endogenous DNA repair mechanisms are somewhat effective in repairing DNA, some DNA damage persists and can accumulate with chronic exposure [9]. Through ROS formation, UVB induces activator protein-1 (AP-1) overexpression along with the upregulation of collagen-degrading enzymes like matrix metalloproteinases (MMPs) (Figure 4). Overall, UVB stimulates collagen degradation and inhibits procollagen biosynthesis resulting in loss of collagen content and wrinkle formation, thus inducing skin photoaging, as reported by Karapatan et.al, 2019 [10]. Sun damage also creates a state of chronic inflammation, with the release of proteolytic enzymes by the inflammatory system, disrupting the dermal matrix [8]. UV protection strategies, such as sunscreen use, are important in limiting further DNA damage [9]. Exposure to UV radiation is the primary factor of extrinsic skin aging; it accounts for about 80% of facial aging [11].

Figure 4: A model proposed to explain the mechanism of inflammation in skin [11]. (A) UV radiation induces oxidative stress in epidermal cells, resulting in damaged cells with oxidized lipids. Oxidation-specific epitopes on damaged cells and oxidized lipids activate complement systems and cause inflammation, leading to infiltration and activation of macrophages. Activated macrophages release MMPs to degrade extracellular matrix. (B) Repeated UV radiation over-activates the complement system, causing damage to the dermis–epidermis junction, on which they deposit, and macrophages are overburdened with oxidized lipids. Overburdened macrophages release proinflammatory cytokines and ROS, the former of which cause chronic inflammation and long-term damage to the dermis, while the latter triggers the oxidative stress-induced damages to the dermal extracellular matrix.
Effects of UVR on the Dermal white adipose tissue (dWAT) in vitro: UVR can significantly modulate sWAT metabolism. This effect is observable not only in chronically sun-damaged human skin, but even after a single UV exposure of a non-damaged skin. Free fatty acid and triglyceride content in sWAT of sun-exposed skin (forearm) is significantly lower than in the buttocks (sun-protected area) of the same subjects. At the same time, young subjects did not demonstrate such differences, which points to the UV-induced effect and not just to the regional variations in fat metabolism. Additionally, both chronic and single UVR exposure significantly reduces master audiogenic factors such as peroxisome proliferator-activated receptor γ (PPARγ); this reduction was rapid and remained stable for at least 72 h after acute UVR exposure. From this point of view dWAT content correlates with a much more pronounced extrinsic aging process in the dorsal hand comparing to the palm area. Chronological skin aging demonstrates similar but not as pronounced differences in aging processes in palmar and dorsal regions of the hand. This can be an indication that UVR accelerates the processes of skin aging, whereas their basic components are determined by some other factors, one of which could be the local dWAT content. This can make skin aging not only body area dependent, but also spatially heterogeneous in the same body area, since dWAT can have a spatially heterogeneous structure [78].

Figure 5: Possible role of adipocyte-myofibroblast transition in extrinsic aging [78]. Absorption of UV radiation in the skin causes acute enlargement of the dWAT layer. However, upon chronic overexposure to UV radiation, it causes the depletion of dWAT and a concurrent development of cutaneous fibrosis, presumably through adipocyte-myofibroblast transition (AMT). Replacement of dWAT volume with fibrosis leads to production of mechanically heterogeneous skin structures and to the loss of the effective skin volume.
Environmental factors beyond UV radiation
Infrared radiation and heat: Visible light (400–740 nm) and IR radiation have long been considered to minimally impact the skin, apart from the heat sensation provided by IR radiation [12]. IR radiation accounts for approximately 40% of the solar radiation energy reaching the earth's surface, subsequently generating heat and increasing skin temperature. IR thermogenic radiation can reach the dermis (65%) and hypodermis (10%), and its capacity to induce metalloproteinase expression in the dermis is well known along with its oxidative role. In human skin, IR radiation and heat can lead to macrophage recruitment like UVR. Heat can induce various cytokines in human skin and was found to increase trophoblastic mRNA and protein expression in the epidermis and in the dermis. Both IR and heat-induced acute stress increase in the number of mast cells and expression of tryptase. Chronic IR and heat exposure each induce cutaneous angiogenesis and inflammatory cellular infiltration, disrupts the dermal extracellular matrix by inducing matrix metalloproteinases, and alters dermal structural proteins, thereby adding to premature skin aging [4], [13]. Erythema ab igne, a cutaneous rash characterized by a reticulated pattern of erythema and hyperpigmentation, is caused by repeated exposure of direct heat or infrared radiation to a person’s skin, often from occupational exposures or use of heating pads [14].
Pollution: The damaging effects of skin exposure to pollutants may result in skin disorders and pathologies, including xerotica skin, sensitive skin, premature skin aging and accelerated aging symptoms, such as wrinkle formation, abnormal pigmentation and skin dryness. Pollutants may also be involved in acne, eczema, skin rashes and skin cancers. Prolonged and repetitive daily exposure to high levels of pollutants impairs the skin’s natural defense capacity to some extent. Moreover, some pollutants (e.g., ozone) can induce damage via signal transduction mechanism even when there is no percutaneous penetration to deeper skin layers [230]. There is solid evidence that skin pathologies such as premature aging, atopic dermatitis (AD), and psoriasis are associated with pollutant exposure; all of these skin conditions are also associated with an altered redox status. Some of the most noxious pollutants that humans are exposed to include ozone (O3), particulate matter and cigarette smoke. Perorally et.al, 2019 reported that increased levels of 4-hydroxy-2-nonenal (HNE) in the skin, in response to pollutants, likely accelerates skin aging and exacerbates existing skin inflammatory conditions [15]. When ozone exposure precedes UV exposure, there is an enhancement of UV-induced depletion of protective vitamin E from the skin’s stratum corneum [16]. Even in indoor conditions, particulate matter (PM2.5) exposure levels were positively associated with skin aging manifestation. Particles can serve as carriers for organic chemicals and metals that are capable of localizing in mitochondria and generating ROS directly in mitochondria leading to collagen degradation in human skin [17]. In line with this, cosmetic anti-pollution products containing anti-oxidants, but also aryl hydrocarbon receptor (AHR) antagonists are effective in reducing or preventing increase in skin pigmentation [18].
Lifestyle-related factors
Smoking: It is now well established that smoking has an aggravating effect on skin aging. Even external exposure to cigarette smoke (secondhand cigarette smoke) prematurely ages the skin [4]. Particularly owing to nicotine, smoking negatively affects the dermal microvasculature and hinders the healing process. It also has a toxic effect on keratinocytes and fibroblasts by increasing the expression of metalloproteins and trophoblastic. Furthermore, smoking increases the expression of small proteoglycans and reduces the synthesis of procollagen. The clinical manifestations of these phenomena are pale and wrinkled skin; DNA mutations also result from oxidative effects or direct toxic damage [8]. Smoking provokes elastosis, telangiectasia, skin roughness, and premature wrinkles on facial skin due to the vascular constriction of nicotine. A clear dose-response relationship has been observed between smoking and wrinkling [4]. Park et.al, 2018 reported that cigarette smoke induces both ROS production (oxidative stress) and autophagy [19]. It has been observed that the skin of smoking addicts at the age of 40 years resembles skin of non-smoking 70-year-old adults. Skin damage due to tobacco smoke is irreversible, where further damage can be avoided by stopping smoking [20]. Wang et.al, 2018 reported that application of tobacco extracts to skin and oral fibroblasts in vitro triggered several hallmarks of senescence including premature cell cycle arrest, oxidative DNA damage, secretion of inflammatory cytokines and MMPs, and downregulation of cell junction proteins E-cadherin and Zonula occludens-1 (ZO-1, tight junction protein) [21].
Sleep: Restricted sleep affects facial appearance negatively and decreases others' willingness to socialize with the sleep-restricted person [22]. An estimated 50–70 million American adults suffer from one or more sleep disorders [23]. Sleep is important for growth and renewal of multiple physiological systems. Overtaken-White et.al, 2015 reported that good sleepers had significantly lower intrinsic skin ageing scores (by SCINEXATM). Sleep deprivation is associated with increased signs of intrinsic skin aging (fine lines, uneven pigmentation, reduced elasticity), with much slower recovery rates after skin barrier disruption and lower satisfaction with appearance [24,25]. The sleep deprived individuals were noted to have hanging eyelids, swollen eyes, darker circles and more droopy corners of the mouth [23]. Wrinkles occur where fault lines develop in aging skin. Those fault lines may be due to skin distortion resulting from facial expression or may be due to skin distortion from mechanical compression during sleep. Expression wrinkles and sleep wrinkles differ in etiology, location, and anatomical pattern. Compression, shear, and stress forces act on the face in lateral or prone sleep positions (Figure 6) [26].

Figure 6: External forces (including compression, tension, and shear) act on facial tissue in lateral or prone sleep positions [26]. During side or stomach sleeping, facial tissue is subject to shear, compression, and tensile mechanical forces. The skin is stretched and pulled in all directions with changes in sleep position. These forces become significant when we consider the amount of time spent in sleep and sleep position.
Diet and Nutrition: Rhytids, sagging of skin, and loss of elasticity are all related to changes in the collagen and elastic fibers of the skin, which are themselves impacted by diet. Ingestion of sugar, in particular, can accelerate these signs of aging, as it promotes cross-linking of collagen fibers. This process is accelerated by hyperglycemia. Research indicates that once established, the body is unable to repair these cross-links. With accumulation of advanced glycation end products (AGEs), structural changes in the skin can occur, resulting in increased stiffness and reduced elasticity. Cooking processes that lead to higher levels of AGEs include grilling, frying, and roasting. Herbs and spices, such as oregano, cinnamon, cloves, ginger, and garlic, as well as substances found naturally in certain fruits and vegetables, such as lipoic acid inhibit the production of AGEs [27]. Frequently researched antioxidants such as carotenoids, tocopherols and flavonoids, as well as vitamins (A, C, D and E), essential omega-3-fatty acids, some proteins and lactobacilli have been referred as agents capable of promoting skin health and beauty [28]. The WHO and Food and Agriculture of the UN reports recommend adults to consume at least five servings of fruits and vegetables per day excluding starchy vegetables [29]. National Health and Nutrition Examination Surveys (NHANES) 2007-2010 indicate that among US population 75% consumed less fruit and 87% consumed fewer vegetables than recommended [30]. The accumulation of glycoxidation products such as carboxymethyl lysine (CML) and pentosidine in cutaneous collagen promotes skin aging. Braganza et.al, 2019 reported that chronic caloric restriction decreased the glycation rate of skin proteins, resulting in the reduction of age-related accumulation of these metabolites in cutaneous collagen [31]. Medic et.al, 2019 reported that better adherence to the Dutch Healthy Diet Index (DHDI) was significantly associated with less wrinkles among women but not in men. In women, a red meat and snack-dominant PCA pattern was associated with more facial wrinkles, whereas a fruit-dominant principal component analysis (PCA) pattern was associated with fewer wrinkles [32]. Higher intakes of vitamin C and linoleic acid and lower intakes of fats and carbohydrates are associated with better skin-aging appearance [33].
Inappropriate/Harsh soaps: Dry skin often occurs in the elderly and tends to worsen in association with hot baths and the use of standard alkaline bar soaps [4]. Skin dryness, scaling and roughness-lipid solvents such as acetone, alcohols and even nonionic surfactants can cause dryness of the skin [34]. Each cleansing agent, even normal tap water, influences the skin surface. The increase of the skin pH irritates the physiological protective 'acid mantle', changes the composition of the cutaneous bacterial flora and the activity of enzymes in the upper epidermis, which have an acid pH optimum. The dissolution of fat from the skin surface may influence the hydration status leading to a dry and squamous skin [35]. Accordingly, in order to lowering the skin damage, cleansings with neutral pH and pH close to 5.5 are recommended [36].
Systemic morbidities
From a biochemical standpoint, chronological aging induces increased markers of oxidation, glycoxidation, lip oxidation, and glycation in skin collagen. In particular, skin collagen’s cross-linking lysine residues undergo significant oxidative changes with age. Lysine oxidase, a copper-dependent enzyme, converts lysine to ally sine at all ages. Recently it has been shown that allysine is further oxidized to a stable end product, 2-aminoadipic acid. This oxidative change results in significant accumulation of 2-aminoadipic acid in collagen of aged skin; increased oxidative end product is also seen in diabetes, renal failure, and sepsis. Obesity and overweight are risk factors for various disorders, including diabetes [38].
Diabetes mellitus (DM): Yoon et.al, 2002 reported that elasticity of facial skin was decreased in patients with diabetes. Decrease of the fine flakes of the diabetes patients reflect that irritation and xerotica changes are aggravated in skins of diabetic patients [44]. DM is among the most common aging-related comorbidities, and the generation of advanced glycation end products is intimately related to dermal damage since it changes the properties of collagen types I and IV. Clinically, reductions in flexibility and rigidity and an increase in susceptibility to mechanical stimulation are observed [4]. 30-70% of patients with DM, both type 1 and type 2, will present with a cutaneous complication of DM at some point during their lifetime. The prevalence of ichthyosiform changes of the shins (“fish scale” skin) in those with type 1 diabetes has been reported to be between 25-50%. Xerosis is one of the most common skin presentations (abnormally dry skin) in patients with diabetes and has been reported to be present in as many as 40% of patients with diabetes [37]. Ruska et.al, 2019 reported a two-way relationship between insulin resistance and AGE accumulation in the skin in people with Type 1 diabetes [39] which is related with increased stiffness and reduced elasticity. Moreover, not only collagen, but also elastin, is affected by AGEs, resulting in a reduction of skin elasticity. Pigeon et.al., 2014 reported that the imbalance between synthesis and degradation that results from glycation, may contribute to skin aging [40]. Noord am et.al, 2013 reported higher glucose levels are associated with a higher perceived age among non-diabetic subjects also. Several studies have shown that culturing human fibroblasts under hyperglycemic conditions results in both an increased amount of ROS at a cellular level as well as an increased induction of premature cellular senescence which in turn may cause premature skin aging and a higher perceived age (Figure 7) [41-43]. People can use medication to resolve skin problems, but managing blood sugars is usually the best way to prevent and treat skin problems that relate to diabetes [247].
Obesity: A hyperglycemic state is common in obesity and is associated with peripheral resistance to insulin and a higher risk of glycation [45]. Also, Sami et.al, 2015 reported that skin of the patients with massive weight loss is weak due to lower density and thickness of collagen fibers and damage to its elastic fibers. It usually occurs because of damage of collagen and elastin, which allows for no skin retraction after weight loss [46]. Striae distensile (striae or stretch marks) is a common dermatosis in patients with obesity, representing linear atrophic plaques which are created due to tension and skin stretching from expanding fat deposits. Due to excessive sweating and increased friction between skin surfaces, a number of skin infections are more frequent in obesity including oppositional intertrigo (inflammation-rash in body folds), candidiasis, candida folliculitis, folliculitis and less often cellulitis, erysipelas or fasciitis [47]. Ibukun et.al, 2017 reported that obese-diabetes patients have decreased stratum corneum hydration, increased trans epidermal water loss, higher skin advanced glycation end-products and decreased dermal collagen fiber density compared with normal-weight subjects. These results indicate that the ordinary age-related physiological skin changes seen in the elderly can also occur in obese-diabetes patients aged in their 40s [48].

Figure 7: ROS-mediated senescence [42]. Besides causing DNA damage and mitochondria dysfunction, OS activates p53 that, in turn, induces prooxidant genes and imbalances antioxidant genes induction. The set of alterations caused by ROS lead to induction of cell senescence, which, in turn, can develop both positive and negative effects; miR34a expression increases with aging in many tissues downregulating SIRT1 protein activity (a longevity promoting factor) and PNUT protein (a DNA protecting factor which prevents telomere attrition and is involved in tissues repairs).
Menopause: The effects of estrogen deficiency on the skin are an important endogenous cause of aging skin in women. Estrogen’s key role in maintaining the skin’s structural and functional integrity is well established with evidence that shows that estrogens are essential for skin hydration, sebum production, improved barrier function of the stratum corneum, and increased collagen and elastin content [49]. Following menopause many women detect a swift commencement of skin aging; skin becomes thinner with decreased collagen content, decreased elasticity, increased wrinkling and increased dryness [50]. Reduced estrogen levels during menopause affect skin components with estrogen receptors, particularly in epidermal cells and sebaceous glands. By contrast, androgenic hormone levels do not decline significantly during this period [4]. Accordingly, dermal cellular metabolism is influenced by the hypoestrogenism state of menopause leading to changes in the collagen content, alterations in the concentration of glycosaminoglycans and most importantly the water content. Consequently, changes in these basic components leads to an alteration in function compatible with skin aging. Changes in the skin collagen leads to diminished elasticity and skin strength. Collagen content may be measured by various methods such as direct skin biopsy, skin blister assessment for collagen markers and skin thickness measurement. All these variables indicate a reduction in collagen content following menopause. This may be reversed with the administration of estrogen given both topically and systemically. A reduction in hydrophilic glycosaminoglycans leads to a direct reduction in water content, which influences the skin turgor [51]. A study of elderly males and females has confirmed that administration of topical estrogen increases keratinocyte proliferation and epidermal thickness after only two weeks. In estrogen deficient women skin thickness is reduced by 1.13% and collagen content by 2% per postmenopausal year. Type I and III skin collagen is thought to decrease by as much as 30% in the first five years after menopause. This decrease in skin thickness and collagen content in elderly females correlates with the period of estrogen deficiency rather than chronological age [50]. The highest loss (of up to 30%) is observed in the first 5 years, followed by a 1%–2% loss of collagen annually [171].
Acne scarring: Skin with acne scarring has reduced elasticity due to scar fibrosis and shows a worsened appearance of furrows and wrinkles. Atrophic facial acne scarring is a widely prevalent condition that can have a negative impact on a patient's quality of life. The appearance of these scars is often worsened by the normal effects of aging. Facial aging often exacerbates the effects of acne scarring. Inflammation associated with moderate to severe acne can result in dermal collagen and fat loss, leading to atrophic scarring. Both acne scarring and the normal aging process can result in the loss of dermal collagen and facial lipoatrophy, such that patients already suffering from the negative impact of facial acne scarring may find the appearance of these scars worsening over time as they approach their 40s and 50s [52].
Emotional stress and depression: Evidence suggests that chronic psychological stress stimulates the autonomic nervous system, renin-angiotensin system, and the hypothalamic-pituitary-adrenal axis when the body attempts to resolve perceived threats to homeostasis. Prolonged activation of these pathways can result in chronic immune dysfunction, increased production of ROS, and DNA damage, which are known to contribute to the again of skin and other tissues [53]. Maruf et.al, 2019 reported similar observation of aberrant barrier dysfunction, characterized by decreased epidermal lipid and structural protein production, decreased stratum corneum hydration and increased trans epidermal water loss [54]. Liu et.al, 2018 reported that early life adversity is associated with both persistent disruptions in the hypothalamic-pituitary-adrenal (HPA) axis and psychiatric symptoms. Glucocorticoid receptors (GRs), which are encoded by the NR3C1 gene, bind to cortisol and other glucocorticoids to create a negative feedback loop within the HPA axis to regulate the body's neuroendocrine response to stress. Excess methylation of a promoter sequence within NR3C1 that attenuates GR expression, however, has been associated with both early life adversity and psychopathology. As critical regulators within the HPA axis, GRs and their epigenetic regulation may mediate the link between early life adversity and the onset of psychopathology [55].
Hormone and metabolic processes
All endocrine glands are affected by the global aging process. A few direct consequences interfere with skin aging. They are mostly related to the declined activity of the pituitary gland, adrenal glands, ovaries, and testes [56]. The most important endocrine compound produced by the skin is vitamin D, which is a regulator of the calcium metabolism and Tables other systemic effects as well. Vitamin D3 and its analogues regulates several physiological processes in the skin-like proliferation, differentiation, and apoptosis of keratinocytes and maintenance of normal skin barriers and immune system [57]. Extension of health-span in experimental animals and analysis of survival curves suggest that in the absence of Growth hormone (GH), aging is slowed down or delayed. The peripheral effects of GH are mainly exerted by insulin-like growth factor (IGF), produced by the liver upon GH stimulation. The circulating IGF-1 is bioavailable and functionally active depending upon its binding with the IGF-binding proteins (IGF-BPs) [58]. Eto et.al, 2018 reported severe GH deficiency results in early aging, such as wrinkling and dryness of skin [59]. Hypopituitary adults are usually described as having dry and thin skin, an increase in skin thickness was demonstrated after GH treatment in normal elderly males selected on the basis of low IGF-I levels [60]. The progressive decline in dehydroepiandrosterone (DHEA) serum concentration with age, and conversely its supplementation has not demonstrated prominent effects on the skin except on sebum production [56]. DHEA is the major steroid produced by the adrenal zona reticularis and, in contrast to cortisol and aldosterone, its secretion declines with ageing [61]. DHEA and its sulfate (DHEA-S) are the most abundant steroids in humans whose low levels are related to aging, greater incidence of various cancers, immune dysfunction, atherosclerosis, and osteoporosis [62]. Calvo et.al, 2008 strongly suggested the possibility that DHEA could exert an anti-aging effect in the skin through stimulation of collagen biosynthesis, improved structural organization of the dermis while modulating keratinocyte metabolism [63]. Estrogen, alone or together with progesterone, prevents or reverses skin atrophy, dryness, and wrinkles associated with chronological aging or photoaging. Estrogen and progesterone stimulate proliferation of keratinocytes while estrogen suppresses apoptosis and thus prevents epidermal atrophy. Estrogen also enhances collagen synthesis, and estrogen and progesterone suppress collagenolytic by reducing MMP activity in fibroblasts, thereby maintaining skin thickness. Estrogen maintains skin moisture by increasing hyaluronic acid levels in the dermis; progesterone increases sebum excretion [64]. Several reports suggest positive correlations between the levels of circulating estrogens and: (1) perceived age, (2) attractiveness, (3) enhanced skin health, and (4) facial coloration in women [65]. Topical corticosteroids have been shown to reduce cutaneous CD44 expression, correlated with skin atrophy if there’s a CD44 deficiency. Corticosteroids can also induce dermatoporotic changes through modulating gene expression of collagen I, collagen III, collagen IV, and matrix metalloproteinases (MMPs) [66]. The corticosteroid-induced atrophy can be one of the most severe forms of skin aging corresponding to demetaphorizes.
| Table 3: Neuroendocrine Receptors Active in the Skin [56] |
| ? Adrenergic receptors ? Androgen and estrogen receptors ? Calcitonin gene-related peptide receptor ? Cholinergic receptors ? Corticotropin-releasing hormone and urocortin receptors ? Glucocorticoid and mineralocorticoid receptors ? Glutamate receptors ? Growth hormone receptor ? Histamine receptors ? Melanocortin receptors ? Miscellaneous neuropeptide receptors ? Miscellaneous receptors ? Neurokinin receptors ? Neutrophin receptors ? Opioid receptors ? Parathormone and PTH-related protein receptors ? PRL and LH-CG receptors ? Serotonin receptors ? Thyroid hormone receptors ? Vasoactive intestinal peptide receptor ? 21. Vitamin D receptor |
*CGRP-R, calcitonin gene-related peptide receptor; CRH-R, corticotropin-releasing hormone and urocortin receptors; GH-R, growth hormone receptor; MC-R, melanocortin receptors; NK-R, neurokinin receptors; NT-R, neutrophil receptors; PTH, parathormone; PTHrP, PTH-related protein receptors; LH/CG-R, PRL and LH-CG receptors; VIP-R, vasoactive intestinal peptide receptor; VDR, vitamin D receptor.
| Table 4: Hormones and Neurotransmitters Produced by the Skin [56] |
| ? Hypothalamic and pituitary hormones ? Neuropeptides and neurotrophies ? Neurotransmitters/neurohormones ? Other steroid hormones ? Parathormone-related protein ? Sex steroid hormones ? 7. Thyroid hormones |
Other Intrinsic Issues of aging
Anatomical Skin Sites: Large variations in some skin properties (hydration, trans epidermal water loss, epidermal lipids, sebum secretion, and mechanical properties) have been observed with respect to the studied body site. There are also large differences in skin thickness in function of the body site, ranging from very thin on the eyelids to more than 5 mm on the sole of the feet. A regional variation is clearly observed when considering the quantity and composition of lipids in the stratum corneum. Because of thickness and sebum secretion, the viscoelastic properties of the skin is very different at the forehead, nose, and cheeks compared with the forearm [8]. Human skin retains water mostly through the outermost stratum corneum layer. Loss of hydration in aged skin, due to a decline in function of the stratum corneum, results in a sagging and wrinkling appearance [77].
Ethnicity: Campeche et.al, 2019 reported that Africans from the African continent show delayed signs of aging compared to Caucasians [67]. Facial wrinkles and ne lines appear later in African Americans than in Caucasians and may not appear until late in the fifth or sixth decade. White women self-reported more signs of moderate and severe facial aging than Asian and Hispanic women beginning in the fourth decade. When comparing the severity of facial features against photo-numeric rating scales, the mean severity of crow’s feet lines was most severe in Fitzpatrick skin type I and least severe in Fitzpatrick skin types IV and V [68]. Asians are a population with various skin photo types, ranging from type III to IV Fitzpatrick's classification in Chinese and Japanese to type IV and V in Indian and Pakistani people. Chan et.al, 2019 reported that Asian skin tends to present post-inflammatory hyperpigmentation, melasma, lentigines and freckles, nevus of Ota, and Hori nevus. The main skin diseases reported in Asians are acne, atopic dermatitis, and viral infections. Wrinkles and skin thickness, early signs of aging in Caucasians, are less evident in Asian skin. However, pigmentary changes occur earlier [69]. Asian and black skin has thicker and more compact dermis than white skin, with the thickness being proportional to the degree of pigmentation. This likely contributes to the lower incidence of facial rhytids in Asians and blacks [70]. Signs of facial aging in individuals with skin of color tend to be most pronounced in the periorbital and midface region with less prominent features of skin aging in the upper third of the face and a decreased tendency toward perioral rhytids and radial lip lines [71]. Darker skin types are better protected regarding sun exposure due to the higher melanin content in their skin. In fair-skinned persons the skin appears severely atrophic with multiple telangiectasias and a variety of premalignant lesions such as actinic keratosis, whereas in dark-skinned persons deep furrows and severe solar elastosis occur [72].
Gender: Sugawara et.al, 2019 reported cauliflower-shaped sebaceous glands in male while young females had somewhat more cylindrical and smaller sebaceous glands than the young males [73]. There are significant morphological differences according to sex: total skin thickness is greater for men on most skin sites [56]. Also, increased sebum and decreased skin elasticity were mostly correlated with facial pore development in male [74]. Reproving et.al, 2018 reported SC rehydration capacity in sun-exposed aged female subjects was significantly lower than that of age-matched male subjects. The skin parameters of hydration, trans epidermal water loss, sebum, microcirculation, pigmentation, and thickness are generally higher in men but skin pH is higher in women [75]. Trojan et.al, 2015 reported that changes in skin elasticity, wrinkling, sagging, and yellowness seem to be caused by additional extrinsic ageing in women. Intrinsic ageing has a very strong influence on facial skin characteristics in Caucasian women in general [76].
Skin Aging Prevention and Therapy
Anti-aging in dermatology primarily focuses on the prevention of skin aging with UV protection (clothing and sunscreens), free radical scavengers (synthetic or botanic), and cell-protecting agents such as vitamin B3. For the correction of signs of early skin aging, retinoic acid derivatives in dermatological prescriptions are the best studied substances. Topical hormonal prescriptions are also an option if UV damage has not been the leading culprit for aging. Chemical peeling leads to a marked increase in collagen formation, the deeper the better. Ingredients in cream preparations can reduce superficial skin folds (polyphenols, amino acid peptides). Modulators of regular pigmentation are important for anti-aging preparations [79]. There are no proven effective topical antiaging ingredients/or treatment that completely eliminates the symptoms of skin photoaging, but there are products and treatments that can visibly reduce or slow down these symptoms: it is more correct to consider reduction of the appearance of aged skin. Many cosmetic products claim to reduce the clinical signs of photoaged skin; however, there are very few scientific, randomized, double-blind, placebo-controlled, clinical studies to support these claims. Generally speaking, the quality control testing on ingredients and safety testing are of good quality, and the used ingredients are mostly safe. However, these ingredients may not be as efficient as claimed, and the concentrations used in these formulations will not necessarily correspond to an “effective” concentration. This can be the case with many plant extracts with antioxidant properties [8]. Indeed, product testing may also be warranted by the companies to document claimed efficacy and to support marketing. Finally, many antiaging claims are based on in vivo testing on cells or simple skin models but not in vivo on a sufficient number of human subjects.
|
Table 5: Skin antiaging approaches [2] |
|
|
Cosmetologically care |
Daily skin care, Correct sun protection, Aesthetic non-invasive procedures, Chemical peelings, visible light devices, intense pulsed light (IPL), ablative and non-ablative laser photo–rejuvenation, radiofrequency (RF) |
|
Topical medical agents or topical agents |
Antioxidants, Cell regulators |
|
Invasive procedures |
Injectable skin bio-stimulation and rejuvenation, prevention of dynamic wrinkles, correction of static, anatomical wrinkles, restoration (redistribution) of fat and volume loss, skin augmentation and contouring, restoration (redistribution) of fat and volume loss, skin augmentation and contouring |
|
Systemic agents |
Hormone replacement therapy (HRT) |
|
Avoiding of exogenous factors of aging, correction of life style and habits |
Smoking, Pollution, Solar UV irradiation. Stress, Nutrition, diet restriction and alimentary supplementation, Physical activity, Control of general health |
|
Preventive medicine |
|
Cosmetologically care
Daily skin care: Healthy and functioning skin barrier is important protector against dehydration, penetration of various microorganisms, allergens, irritants, reactive oxygen species and radiation. The skin barrier may be specifically adjusted to allow penetration. For this reason, daily skin care may increase skin regeneration, elasticity, smoothness, and thus temporarily change the skin condition [2]. Protection, prevention, cleansing, and moisturizing are the key components of an effective skincare routine. Because most sun damage results from every day, incidental UV exposure, rather than occasional bursts while on vacation, dermatologists recommend daily use of sunscreens. In general, gel-based and bar cleansers are best for oily complexions, whereas cream or lotion-based ones are better for normal to dry skin. Moisturizers supply humectant agents, which draw water into the stratum corneum from the environment and dermis below. Moisturizers also include occlusive agents that act as a barrier to trans-epidermal water loss. In almost all cases, products contain both humectants, like hyaluronic acid, urea, and allantoin, and occlusive, including petrolatum, mineral oil, and lanolin. The classical moisturizers are used for treating dryness in the photoaged skin: polyols (glycerin, propylene glycol, butylene glycol and sorbitol), urea, lactic acid and salts, hyaluronic and salts, pyrrolodone-5-carboxylic acid and salts, panthenol, amino acids and proteins (collagen and proteins from wheat, rice, silk, soybean, and oat). More sophisticated peptides and proteins are presently used as moisturizers. It concerns generally more lipophilic quaternary N-alkyl derivatives of proteins or small polypeptides with long side chains (ester binding) to increase the lipophilic character: binding to the horny layer and a better percutaneous absorption. Recently, the use of small peptides, which mimic the amino acid sequence of collagen or enzymes (biomimetic peptides), has been proposed as moisturizers [56]. Surface-smoothing silicone derivatives or filmgoer proteins such as quaternate proteins or silk, rice and oat, and skin feel agents are used in antiaging products. The high adsorption to the skin surface provokes a smoothing of the skin surface and is at the same time humectant. For a better percutaneous penetration, small fragments of hyaluronic acid were also suggested. Humectants are present in the water phase of a formula; occlusive are in the oil phase. Oil in water formulations tend to be lightweight gels, lotions, and serums and are best suited for normal to dry skin. Water in oil formulations may be ointments or creams and offer superior hydration for dry skin [80].
Correct Sun-Protection: Singer et.al, 2019 reported that avoidance of sun exposure at peak times and textile sun protection are important pillars of a modern prophylactic approach. Besides, antioxidants and DNA repair enzymes may be added to topical sunscreens in order to enhance the protection before and even after sun exposure [81]. The FDA regulates sunscreen as an over-the-counter medication. Currently, 16 UV filters are listed, 14 organic filters and two nonorganic filters, including zinc oxide and titanium dioxide. The FDA has changed its guidelines to address broad-spectrum sunscreen use, which involves UVA and UVB coverage; water resistance, to indicate the time duration the sunscreen is effective; and sun protection factor (SPF). SPF-30 or higher is recommended and can be labeled as reducing the risk of skin cancer and early skin aging [82,83]. Nutritional antioxidants act through different mechanisms and in different compartments, but are mainly FR scavengers: (a) they directly neutralize free radicals (b) they reduce the peroxide concentrations and repair oxidized membranes (c) they quench iron to decrease ROS production (d) via lipid metabolism, short-chain free fatty acids and cholesteryl esters neutralize ROS. The most important source of antioxidants is provided by nutrition. To the most known systemic antioxidants belong vitamin C, vitamin E, carotenoids, and from the trace elements copper and selenium. There are also studies demonstrating that vitamins C and E combined with ferulic acid impart both a sunscreen and an anti-oxidant effect [2].
Aesthetic non-invasive procedures: Noninvasive skin tightening has become one of the most common cosmetic aesthetic procedures being performed today. According to the American Society for Aesthetic Plastic Surgery (ASAPS) surveys released in 2014 and 2015, there has been a 12% increase in the demand for cosmetic procedures, with Americans spending more than $12 billion and having 10 billion procedures in 2014 [84]. A noninvasive device combines multipolar RF and PEMFs and is referred as (MP)2, which stands for “Multipolar Magnetic Pulse.” The device was introduced for the non-ablative treatment of skin laxity and cellulite [85]. Lee et.al, 2014 reported that combined multi-polar radiofrequency and pulsed electromagnetic field device is safe and effective for rejuvenating aged skin in Korean subjects [88].

Figure 8: The Venus Legacy noninvasive skin tightening device. [86,87]. The medical device is used in non-invasive body shaping, cellulite reduction, skin tightening, and wrinkle reduction for the face and body. The device is powered by (MP)2 technology, which combines Multi-Polar Radio Frequency and Pulsed Electro Magnetic Fields, and features the advanced technology that induces lipolysis, allows for increased blood circulation, and stimulates lymphatic drainage in the treatment area.
Pulsed electromagnetic fields (PEMFs): are induced by short pulses of electrical current that penetrates into the skin and results in the stimulation of molecular and cellular activities. It has been used in medicine for bone growth, wound healing, cardiovascular disease, and other conditions. Pulsed electromagnetic fields increase collagen fiber production by dermal fibroblasts and stimulate angiogenesis, leading to wound-healing effects. Radiofrequency (RF) devices remain a dominant technology in the noninvasive management of skin aging, as it is a safe and effective treatment for a broad range of skin conditions. It can induce wrinkle reduction, cellulite improvement, laxity and body, and skin contouring improvement. When radiofrequency is applied by an alternating current, an electric field is generated, which achieves skin tissues, generating thermal energy. The heat is not diminished by tissue diffraction or absorption by epidermal melanin and is then appropriate for treatment of all skin types [85,86]. RF with micro-needling is effective and safe in improving skin laxity and texture. Pairing skincare cosmeceutical products pre- and post-procedure is beneficial as it enhances patient results, patient experience, and reduces patient downtime. Zahra et.al, 2019 reported that combining the multi-ingredient anti-aging facial moisturizer pre- and post-RF micro needling was safe and tolerable for the patients [229].
|
Table 6: Classification of Noninvasive Body-Contouring Devices According to Energy Used [89] |
|
|
Energy |
Device (Company) |
|
Mechanical suction |
Enterology (LPG Systems) |
|
Mechanical suction and thermal |
Reactive (Cynosure); Smooth Shapes (Cynosure) |
|
Radiofrequency |
Vela Shape (Syneron Candela); Vela Smooth (Syneron Candela); Thermae (Solta Medical); Accent (Alma Lasers); TiteFX (Invasix); Vanquish (BTL Industries, Inc); Exilis (BTL Industries, Inc) |
|
Ultrasound |
Ultrashape (Ultrashape); Liposonix (Solta Medical); VASERShape (Solta Medical) |
|
Cryolipolysis |
Coolsculpting (Zeltia) |
|
Low-level light laser |
Zerona (Echonian Medical, Inc) |
|
Energy |
Device (Company) |
|
Mechanical suction |
Enterology (LPG Systems) |
|
Mechanical suction and thermal |
Reactive (Cynosure); Smooth Shapes (Cynosure) |
|
Radiofrequency |
VelaShape (Syneron Candela); VelaSmooth (Syneron Candela); Thermae (Solta Medical); Accent (Alma Lasers); TiteFX (Invasix); Vanquish (BTL Industries, Inc); Exilis (BTL Industries, Inc) |
|
Ultrasound |
Ultrashape (Ultrashape); Liposomes (Solti Medical); VASERShape (Solta Medical) |
|
Cryolipolysis |
Coolsculpting (Zeltiq) |
|
Low-level light laser |
Zerona (Echonian Medical, Inc) |

Figure 9: Improvements in skin condition [85]. Photographs of selected patients before (a) and after eight sessions (b) of treatment with RF and PEMFs.
Topical anti-aging preparations
Retinoids
Topical vitamin A has the ability to diminish the signs of aging by decreasing fine lines and wrinkling. In addition, there is a normalization and enhancement of elasticity. Improvement of skin tone and texture is a benefit of vitamin A, which enhances skin lightening when used in conjunction with skin lighteners [95]. The most widely utilized ones include retinol, retinyl esters (e.g., retinyl acetate, retinyl propionate, and retinyl palmitate), and retinaldehyde. Through endogenous enzymatic reactions, all of these are converted ultimately to trans-retinoic acid (trans-RA), which is the active form of vitamin A in skin. Specifically, retinyl esters are converted to retinol via esterase’s. Retinol (ROL) is then converted to retinaldehyde by retinol dehydrogenase. And finally, retinaldehyde is oxidized to RA by retinaldehyde oxidase. Retinol and retinal must be metabolized in the skin to the active trans-retinoic acid. The incorporation of retinol and probably also retinal in cosmetic preparations poses the problem of stability (slow oxidation of retinol in function of time) [8], [90]. Topical natural retinoic acid precursors such as retinaldehyde or ROL are less irritant than acidic retinoids. Retinoids may be combined with other compounds with complementary actions against ageing, nutritional deficiency and cancer, such as antioxidants, to potentiate their beneficial effects in the skin [100].

Figure 10: Chemical structures of retinoids [91-93]. First generation retinoids include tretinoin (all-trans RA), isotretinoin (13-cis-retinoic acid), and alitretinoin (9-cis RA). Second generation retinoids include etretinate and acitretin. Third generation retinoids include adapalene, tazarotene, and bexarotene. Kim et.al, 2005 designed synthetic retinoid, gelatinoid G, by using computer-aided molecular modeling, and investigated its effects on the expression of extracellular matrix proteins in human skin in vivo.
The molecular mechanisms by which retinoids improve aged human skin have been difficult to investigate largely due to lack of appropriate in vitro models. Shao et.al, 2017 reported that topical application of 0.4% ROL to aged human skin leads to remarkable skin changes in both epidermis and dermis through affecting three major types of skin cells, epidermal keratinocytes, dermal endothelial cells and fibroblasts. Topical ROL significantly increases epidermal thickness by stimulating epidermal keratinocytes proliferation, which involves c-Jun transcription factor, a major deriving force for keratinocyte proliferation. In addition to epidermal changes, topical ROL significantly improves dermal ECM microenvironment; increasing dermal blood vessel formation by stimulating endothelial cells proliferation and ECM production by activating fibroblasts. Topical ROL also stimulates TGF-β/CTGF pathway, the major regulator of ECM homeostasis, and thus increased the deposition of mature collagen in aged human skin in vivo. Additionally, the restoration of dermal ECM may provide a better, more permissive environment for the proliferation of dermal endothelial cells and epidermal keratinocytes, and activation of dermal fibroblasts (TGF-β/CTGF pathway). Coupling of the proliferation of keratinocytes and endothelial cells, and dermal fibroblasts activation forms a self-enforcing environment, which might explain the remarkable anti-aging effects of ROL in aged human skin [94]. Kong et.al, 2016 reported that ROL anti?aging effects include the inhibition of UV?induction of matrix metalloproteinases, and the promotion of collagen synthesis in photoaged skin.5, 10 In clinical studies, topical retinol treatment significantly improved fine wrinkles.11 and affected markers of photoaging, including matrix metalloproteinase, collagenase, and collagen.12 Retinol was effective in producing retinoid?mediated histological changes, such as keratinocyte proliferation [96]. Bagatti et.al, 2018 reported that treatments with adapalene 0.3% gel and tretinoin 0.05% cream in cutaneous photoaging did not differ significantly regarding clinical evaluation of the following criteria: global cutaneous photoaging, periorbital wrinkles, opheliids/melanosis, forehead wrinkles, and actinic keratosis. They concluded that adapalene 0.3% gel is a safe and effective option for the treatment of mild or moderate photoaging [97]. Tretinoin is a prescription strength retinoid approved by the US FDA for acne and for the mitigation of fine facial wrinkles, mottled hyperpigmentation, and tactile roughness of facial skin. Topical application of tretinoin inhibits AP-1, thus suppressing the expression of MMPs and preventing the degradation of collagen. An increase in epidermal thickness and anchoring fibrils is observed, and intrinsically aged skin may also benefit from the topical application of retinoids. Prescription strength tretinoin affords the most potent retinoid effects, but often results in limited utility and decreased adherence due to irritation reactions (ie, burning, scaling, and dermatitis) [11], [91], [98]. Bakuchiol is a meroterpene phenol abundant in seeds and leaves of the plant Psoralen coprolalia. Chaudhuri et.al, 2014 reported that bakuchiol, having no structural resemblance to retinoids, can function as a functional analogue of retinol. Volcano plots showed great overall similarity of retinol and bakuchiol effects on the gene expression profile [101]. Dhaliwal et.al, 2019 reported that demonstrates that bakuchiol is comparable with retinol in its ability to improve photoaging and is better tolerated than retinol. Bakuchiol is promising as a more tolerable alternative to retinol (bakuchiol 0·5% cream twice daily or retinol 0·5% cream daily) [102]. Kwon et.al, 2018 reported that retinaldehyde 0.1% and 0.05% creams used to treat photoaged skin both were well tolerated and improved skin hydration and texture. Retinaldehyde 0.1% cream improved the melanin index as well [99]. An improvement of the photoaged dermal matrix by topical application of a cosmetic “antiaging” product containing a lipoentapeptide, white lupin, and retinyl palmitate was reported by Watson et. al, 2008 [142]. Also, synthetic retinyl-N-formyl aspartame has also been demonstrated to improve skin roughness and wrinkles. However, studies of retinyl esters, such as retinyl palmitate and retinyl propionate fail to show good efficacy [105].
α-Hydroxy Acids (AHAs): Hydroxy acids, also called fruit acids, are among non-organic acids which have been used in the treatment of skin disorders since about 50 years ago. They are some of the most widely used and studied anti-aging skincare compounds. AHAs act on both the epidermal and the dermal levels. When applied to the skin, AHAs stimulate the exfoliation of epidermal cells in the stratum corneum by interfering with the ionic bonding between these cells. This results in the sloughing off dull and rough skin and promotes cellular renewal. Initially used for treatment of hyperkeratosis and other skin conditions affecting subcutaneous turnover, AHAs were found to promote softer, smoother skin, faded wrinkles, lightened age spots, and decreased blemishes. AHAs also improve the subcutaneous barrier function, increase epidermal proliferation and thickness, and restore hydration and pursiness through an increase in hyaluronic acid. The well-known benefits of AHA’s include exfoliation, moisturization, reduction of fine lines and wrinkles, collagen synthesis, firming and skin lightening. Although these naturally occurring organic acids are often referred to as fruit acids because they are found in many common fruits such as citrus fruits (citric acid), apples (malic acid), and grapes (tartaric acid), the two most widely used AHAs are not components of fruit. Glycolic acid (GA) is a sugar cane derivative, and lactic acid (LA) is derived from milk [95,103].
Glycolic acid (GA): Tang et.al, 2019 demonstrated that GA reduced UVB-induced type-I procollagen expression and secretory collagen levels, when applied topically onto human keratinocytes and the C57BL/6J mice dorsal skin. The UV-induced MMP-9 level and activity were reduced by GA pre-treatment. Concomitantly, GA reverted mitogen-activated protein kinase (MMP-9) activation and inhibited the extracellular signal-regulated kinase activation (p38, pERK) triggered by UVB. Finally, GA triggers the transient receptor potential vanilloid-1 (TRPV-1) channel to initiate the anti-photoaging mechanism in keratinocytes. These findings clearly indicated that the mechanisms of GA promote skin protection against UVB-induced photoaging and wrinkle formation [104]. Application of 5% GA cream for 3 months has been shown to improve skin texture and discoloration of photoaged skin. In another study, 8% (glycolic acid or L-lactic acid) for 22 weeks, the majority of patients (76% for glycolic acid; 71% for lactic acid) reported a noticeable improvement in the appearance and smoothness of photoaged skin [105]. In a study of 50% GA peels by Newman et al, there was improvement in mild photoaging of skin. Other significant improvements were noted, including decreases in rough texture and fine wrinkling, fewer solar keratoses, and slight lightening of solar lentigines. Histologic analysis showed thinning of the stratum corneum, granular layer enhancement, and epidermal thickening. Some specimens showed an increase in collagen thickness in the dermis. GA peels do not affect deep wrinkles or deep pigmentations [106].
Lactic Acid (LA): Lactic acid (as sodium lactate) is a well-known part of the skin’s natural moisturizing complex, and is considered to be an excellent moisturizer. LA also contributes to the cell cycle in human keratinocytes [107]. Treatment with 12% LA resulted in increased epidermal and dermal firmness and thickness and clinical improvement in skin smoothness and in the appearance of lines and wrinkles. Both the lactic and glycolic acid peelings were effective in reducing fine wrinkles on the external-lateral region of the eyes, after three applications (85% LA versus 70% GA) [109]. Recently more attention has been drawn to alpha hydroxy and polyhydroxy acids (AHA and PHA) due to their excellent moisturizing and antioxidant properties. Algiert-Zieli?ska et.al, 2019 reported maintenance of the epidermal barrier integrity during application of lactic acid (LA) and lactobionic acid and the opportunity to use them on sensitive skin types including coupe rose skin [112]. One of the reasons lactic acid is widely used as exfoliator and chemical peeling agent is its profound effect on desquamation of the skin. Desquamation is due to the dissociation of the cellular adhesions, which occurs as a result of reduced calcium ion concentration in the epidermis by chelating action of AHAs [113]. Yamamoto et.al, 2006 also showed that LA not only increased the production of ceramide in the stratum corneum, but also appeared to improve the ratio of ceramide 1?linoleate to oleate as compared to vehicle following 1?month topical application of 4% L?lactic acid. The increased ratio of ceramide 1?linoleate to oleate has been suggested to play an important role in increasing skin barrier function [114].
β-Hydroxy Acids (BHAs): Beta Hydroxy Acids (BHAs), such as salicylic acid, are very similar to AHAs except for difference in their solubility. In the other hands, they are lipid-soluble in contrast to water solubility of AHAs. This structure allows them to penetrate into the skin through sebaceous follicles, making it appropriate for patients with oily skin and open comedowns. In addition to prove anti-inflammatory effect of BHAs (e.g. salicylic acid), the skin irritancy effect of them have also been proved to be less than AHAs. Beta hydroxy acid found in skin-care products works best in a concentration of 1-2% [103]. Salicylic acid (SA) is a BHA, which has action to normal keratinization, decreases inflammation, and reduces sebum production with a comedolytic effect. The concentration of salicylic to treat acne is 0.5–5% [116]. SA has been used in the treatment of photoaging with in-office peels of 20–30%. These can be quite helpful in patients who are unable to tolerate AHAs since irritancy levels tend to be less with salicylic acid. In addition, it can be quite useful to combine or alternate both AHAs and BHAs since their mechanisms of action differ, and using both may be quite beneficial [95]. Vender et.al, 2019 reported that daily use of a ceramide containing cleanser and cream that also has SA offers an effective, easy and comfortable option for dry skin conditions. After treatment subjects reported a significant improvement in the quality of their professional life, self-image, and social life. The products were shown to be safe, comfortable, and well tolerated [115]. Shalmaneser et.al, 2018 reported that salicylates activate adenosine monophosphate?activated kinase (AMPK), which is now considered as a promising target to slow down aging and prevent age?related diseases in humans [116]. A topical combination containing 10.4% L-lactic acid, 2% salicylic acid and alpha-hydroxy acid/retinoate conjugate (ethyl lastly retinoate) was used in the topical treatment of females of ages 20 to 58. After 4 weeks, improvement was achieved, which remained continuous and cumulative in the eighth week [97]. 2% supramolecular salicylic acid has a similar efficacy with 5% benzoyl peroxide 0.1% adapalene in mild to moderate acne treatment. The skin barrier (skin hydration value and TEWL value), skin brightness (L* value) and erythema (a* values) indicators showed similar statistical improvement [118].
Ascorbic Acid (AA): Vitamin C is a water-soluble antioxidant which protects skin from oxidative damage and rejuvenates photo-aged skin. It has been utilized as a skin lightener (e.g., via tyrosinase inhibition and/or its antioxidant effect). It also has been reported to have anti-inflammatory properties since it reduces the erythema associated with post-operative laser resurfacing. In addition, AA also serves as an essential co-factor for the enzymes lysyl hydroxylase and prolyl hydroxylase, both of which are required for posttranslational processing in collagen (Types I and III) biosynthesis. Thus, by stimulating these biosynthetic steps, ascorbic acid will increase the production of collagen which will lead to wrinkle reduction [90]. Vitamin C deficient individuals may experience easy bleeding, bruising, and poor wound healing [130]. In addition, topical vitamin C increases levels of tissue inhibitors of collagen-degrading matrix metalloproteinase-1 (MMP-1) [95]. Normal skin contains high concentrations of vitamin C, which supports important and well-known functions, stimulating collagen synthesis and assisting in antioxidant protection against UV-induced photodamage. Vitamin C uptake from the plasma and transport across the skin layers is mediated by specific sodium-dependent vitamin C transporters (SVCTs) that are present throughout the body and are also responsible for transport into other tissues. Interestingly, cells in the epidermis express both types of vitamin C transporter, SVCT1 and SVCT2 (Figure 8) [131].

Figure 11: Delivery of nutrients to the skin [131]. The location of the vitamin C transport proteins SVCT1 and SVCT2 are indicated. Red arrows depict nutrient flow from the blood vessels in the dermis to the epidermal layer. Nutrients delivered by topical application would need to penetrate the barrier formed by the stratum corneum.
Garre et.al, 2018 reported that topical serum containing L-Ascorbic acid, soluble proteoglycans, low molecular weight hyaluronic acid, and a tripeptide protected against oxidative damage and dermal protein loss caused by photo- and chronological aging in human skin explants. In-vivo, the serum hydrated skin for 6 hours, and users perceived increased skin brightness, hydration, and fewer wrinkles [126]. Zasada et.al, 2019 reported that 2.5 ml of serum containing 20% L-ascorbic acid with hydrate from strawberries was used topically in every of 4 treatments. The impact of active substance on skin firmness and elasticity as well as the degree of hydration and skin tone was more efficient after micro-needle mesotherapy [127]. Wang et.al, 2019 reported 2-O-β-d-glucopyranosyl-l-ascorbic acid (AA-2βG), a unique AA derivative identified in Lyceum barb arum, Tableed enhanced free radical scavenging activity compared with AA and its synthetic derivative AA-2αG. AA-2βG protected hydrogen peroxide-induced cell death in murine macrophage RAW264.7 cells. Treatment with AA-2βG eliminated oxidative stress and the ratio of cellular glutathione to glutathione disulfide more effectively than AA and AA-2αG [128]. G?gotek et.al, 2019 reported three times higher antioxidant properties of than rutin, measured by the cation radical scavenging activity by the ferric-reducing activity of plasma (FRAP) test. However, the mixture of ascorbic acid and rutin (Ascorbic A. + Rutin) had approximately 20% higher antioxidant properties compared to Ascorbic A alone. The F–C test showed that AA + Rutin acted two times stronger than AA. or Rutin alone [129]. Crisan et.al, 2015 reported topically applied vitamin C (concentration of 5% and a pH of 5.5 in a novel complex with Rosa moschata, the musk rose oil and proteoglycans) is highly efficient as a rejuvenation therapy, inducing significant collagen synthesis in all age groups with minimal side effects [132].
Garre et.al, 2018 reported that topical serum containing L-Ascorbic acid, soluble proteoglycans, low molecular weight hyaluronic acid, and a tripeptide protected against oxidative damage and dermal protein loss caused by photo- and chronological aging in human skin explants. In-vivo, the serum hydrated skin for 6 hours, and users perceived increased skin brightness, hydration, and fewer wrinkles [126]. Zasada et.al, 2019 reported that 2.5 ml of serum containing 20% L-ascorbic acid with hydrate from strawberries was used topically in every of 4 treatments. The impact of active substance on skin firmness and elasticity as well as the degree of hydration and skin tone was more efficient after micro-needle mesotherapy [127]. Wang et.al, 2019 reported 2-O-β-d-glucopyranosyl-l-ascorbic acid (AA-2βG), a unique AA derivative identified in Lyceum barb arum, Tableed enhanced free radical scavenging activity compared with AA and its synthetic derivative AA-2αG. AA-2βG protected hydrogen peroxide-induced cell death in murine macrophage RAW264.7 cells. Treatment with AA-2βG eliminated oxidative stress and the ratio of cellular glutathione to glutathione disulfide more effectively than AA and AA-2αG [128]. G?gotek et.al, 2019 reported three times higher antioxidant properties of than rutin, measured by the cation radical scavenging activity by the ferric-reducing activity of plasma (FRAP) test. However, the mixture of ascorbic acid and rutin (Ascorbic A. + Rutin) had approximately 20% higher antioxidant properties compared to Ascorbic A alone. The F–C test showed that AA + Rutin acted two times stronger than AA. or Rutin alone [129]. Crisan et.al, 2015 reported topically applied vitamin C (concentration of 5% and a pH of 5.5 in a novel complex with Rosa moschata, the musk rose oil and proteoglycans) is highly efficient as a rejuvenation therapy, inducing significant collagen synthesis in all age groups with minimal side effects [132].
Vitamin E
The very properties that make alpha-tocopherol such a powerful antioxidant causes it to break down in the presence of oxygen or upon exposure to light. For that reason, α-tocopherol acetate, which is the more stable esterified form, is used in cosmetics. Since α-tocopherol acetate is not an antioxidant and has no antioxidant activity, it must first convert to its active alpha-tocopherol form. Years of debate questioned the ability of alpha-tocopherol acetate to be delivered to the skin and bio-converted to an active form. Finally, in 1990, the bioconversion of alpha-tocopherol acetate to free alpha-tocopherol was able to be demonstrated. The use of vitamin E in skin care has anti-aging benefits based on its moisturization properties but mostly on its protective capabilities. Vitamin E enhances the photoprotective to protective effects of sunscreen, and when combined with vitamin C, the two are even stronger as photo protectants [95]. Unfortunately, oral supplementation of vitamin C and E has proven insufficient in preventing skin aging owing to their poor solubility, inefficient skin permeability, or instability during storage [136]. Topical vitamin E (α-tocopherol) used as a component of skin products has anti-inflammatory and antiproliferative effects in concentrations between 2 and 20%. It acts by smoothing the skin and increasing the ability of the stratum corneum to maintain its humidity, to accelerate the epithelialization, and contribute to photoprotection of the skin. The effects are not as strong as with vitamins C and B3 [133]. Most of the OTC antiaging creams contain 0.5%–1% of vitamin E. Topical application of the gel containing 2% phytonadione, 0.1% retinol, 0.1% vitamin C, and 0.1% vitamin E has been seen to be fairly or moderately effective in reducing dark under-eye circles, especially in cases of hemostasis. Topical application of vitamin E can rarely cause contact dermatitis, erythema multiforme, and xanthomata’s reaction [134]. The interaction of vitamins E and C has led to the idea of “vitamin E recycling”, where the antioxidant function of oxidized vitamin E is continuously restored by other antioxidants (Figure 9). This “antioxidant network” depends upon the supply of aqueous antioxidants and the metabolic activity of cells [135].
|
Table 7: Skin ailments, their causes and evidence from in vitro and in vivo studies for association with vitamin C levels [131]. |
|||
|
Type of Skin Damage |
Cause |
Skin Structure Affected |
Evidence of Protection by Vitamin C |
|
Sunburn |
Acute and excessive UV exposure. |
Cell death of all skin cells, with associated inflammation. |
Improving skin vitamin C and vitamin E levels can improve resistance to UV exposure. |
|
Photoaging, oxidant-induced damage |
Chronic UV overexposure, cigarette smoking. |
Damaged collagen and elastin matrix, thinning of the epidermal layer. |
Decreased signs of aging with higher fruit and vegetable intake. Protection inferred from studies with acute UV exposure. |
|
Hyperpigmentation |
Chronic UV exposure and environmental stresses. |
Excessive pigment formation and propagation of melanocytes in the epidermis. |
Nutrition studies showing improved skin color with higher fruit and vegetable intake. |
|
Wrinkle formation |
Natural aging, oxidative stress, UV exposure, smoking, medical treatments. |
Dermal layer changes, deterioration of collagen and elastic fibers. |
Lessening of wrinkle depth following vitamin C supplementation. Increased collagen formation by fibroblasts in cell culture. |
|
Skin sagging |
Natural aging, oxidative stress damage, extreme weight loss. |
Loss of elastin and collagen fibers, thinning of skin layers, loss of muscle tone. |
Improved skin tightness in individuals with higher fruit and vegetable intake. |
|
Loss of color |
Natural aging, UV exposure, illness. |
Thinning of skin layers, loss of melanocytes or decreased melanin formation, loss of vasculature in dermis. |
Improved skin tone with high fruit and vegetable intake. |
|
Surface roughness |
Chemical and UV exposure, physical abrasion, allergy and inflammation. |
Stratum corneum, loss of skin moisture barrier function. |
Vitamin C enhances production of barrier lipids in cell culture. |

Figure 12: The interdependence of vitamins E and C, and glutathione, in the scavenging of free radicals and regeneration of the reduced antioxidants [131]. Vitamin E is in the lipid fraction of the cell, whereas vitamin C and glutathione are water-soluble and present in the cytosol. Vitamin C is only one player in the antioxidant arsenal that includes enzymatic defenses (catalase, glutathione peroxidase and superoxide dismutase) as well as other non-enzymatic defenses (vitamin E, glutathione, uric acid and other putative antioxidants such as carotenoids).
Vitamin E is a promising chemo-preventive and pharmacologically safe agent, which can be exploited or tested against skin cancer [137]. Experimental evidence suggests that topical and oral vitamin E has anticarcinogenic, photoprotective, and skin barrier-stabilizing properties [138]. The topical use of resveratrol, a polyphenol from red grapes with great antioxidant activity in skin care formulation Farris et.al, 2014 reported that significant improvement in fine lines and wrinkles, skin firmness, skin elasticity, skin laxity, hyperpigmentation, radiance, and skin roughness over baseline in 12 weeks after using a topically applied proprietary blend containing 1% resveratrol, 0.5% baicalin, and 1% vitamin E. Ultrasound measurements in the periorbital area showed an average improvement of 18.9% in dermal thickness suggesting significant dermal remodeling [139]. Combination of vitamin E, vitamin C, and ferulic acid can reduce the incidence of oxidative stress-induced tumors, and their antioxidant effects are much better than the use of vitamin C alone [140]. Burns et.al, 2013 demonstrated that topical 5% alpha tocopherol may actually promote carcinogenesis when applied on chronically UVB-damaged skin while treating with a more stable antioxidant compound may offer therapeutic benefits [141].
Coenzyme Q10: Coenzyme Q10 (a ubiquinone) is a powerful free radical inhibitor that inhibits lipid peroxides from forming in plasma membranes. Q10 plays a very important role in cellular energy production and works in the mitochondrial ATP energy-producing pathway of the cell. Q10 levels diminish with age, as does cellular energy production, which may improve by adding Q10 [95]. Additionally, UVR, which leads to oxidative damage, significantly reduces skin's Q10 levels. Approximately 46% of total Q10 was found to be present in the reduced form in human epidermis. Q10 scavenges ROS and protects cells against oxidative stress. Zhao et.al, 2019 concluded that suppression of the PKA-ERK 1/2 signaling pathway may be one of the important mechanisms by which Q10 protects astrocytes from UVB-induced oxidative damage [149]. Knott et.al, 2015 reported that quinone values on the skin surface were significantly increased after treatment with Q10?containing formulas demonstrating that the powerful antioxidant Q10 can be delivered directly to the uppermost layer of the skin [143]. Q10 is an insoluble, poorly permeable antioxidant with great biological value which acts as anti-aging and anti-wrinkle agent. Q10 nano-structured lipid carrier (Q10-NLC) had greater antioxidant properties and topical skin penetration than the Q10-emulsion [144]. Also, El-Leithy et.al, 2018 reported Q10 nano-emulsion having enhanced solubility and permeability with improved anti-wrinkle efficiency [146]. The concentration of Vitamin E and Q10, which together with squalene, play a key role against external oxidative insult, has been shown to decrease significantly during ageing. Topical application was found to be more effective than oral administration in terms of sebum levels of lipophilic antioxidants and squalene [145]. Also, Žmitek et.al, 2017 reported oral supplementation with CoQ10 did not significantly affect skin hydration and dermis thickness [148]. As an effective fat-soluble antioxidant and an essential element of the mitochondrial respiratory chain, Q10 may have healing effects on wound tissues by decreasing oxidative stress and improved mitochondrial efficiency. Choi et. al, 2009 reported the anti-inflammatory and wound healing effect of Q10 in mice [146]. Despite the lack of evidence, large numbers of people in the population are taking oral Q10 and other vitamins and cofactors in the hope that these agents will slow senescence and expand longevity [150].
α-Lipoic Acid: Lipoic acid is a very powerful antioxidant that has the unusual advantage of being both water and fat soluble and is an important cofactor in mitochondrial dehydrogenases. α-lipoic acid (ALA) is a sulfhydryl compound found naturally in virtually all plant and animal species and in both prokaryotic and eukaryotic cells. In the human body, it is bonded to lysine residues and acts as a cofactor in various multienzyme complexes. Nevertheless, there is often little or no free ALA in tissues, so a topical antioxidant formulation containing this natural antioxidant could be used to protect the skin against the effects of ultraviolet rays, such as photoaging and skin cancer [156]. Studies have shown the ease with which lipoic acid is able to penetrate the skin, after which it converts into its active byproduct dihydrolipoic acid. Topical application of 3% lipoic acid has demonstrated its ability to decrease UVB-induced erythema, which demonstrates its photoprotective and anti-inflammatory properties. Also, a 12 -week study demonstrated that using a topical cream containing 5% ALA was quite effective in treating signs of photoaging [95]. ALA and its reduced form, dihydrolipoic acid, are powerful antioxidants that have many physiological functions, including free radical scavenging of reactive oxygen species, generation of cellular antioxidants, chelation of metal ions, and inflammatory suppression (when given orally) [155]. Though ALA is normally administered in oral or injection, it is rarely used topically because of its bad penetration. Kubota et.al, 2019 developed novel nanocapsule of ALA, named α-lipoactive (nLA), to improve skin permeability. In in vivo experiments, it was found that nLA is very effective for improving UV-induced pigmentation and epidermal thickening [151]. Sherif et.al, 2019 demonstrated application of topical 30% poloxamer gel loaded with ALA cubosomes. Reduction in facial lines, almost complete resolution of fine lines in the periorbital region and upper lip area and overall improvement in skin color and texture in most volunteers. There were no instances of irritation, peeling or other apparent adverse side effects [152]. In a similar study with 5% Cubosomal ALA significantly increased epidermal thickness with effective and safe modality for improving aging face [154]. Lin et.al, 2004 were unable to detect protection using ALA alone or together with vitamins C and E. According to them, a commercial formulation of ALA provided no protection [153]. Isaac et.al, 2015 reported that rheological features, such as viscosity, thixotropy, and compliance, and the presence of a hydrophilic polymer strongly influenced the release of ALA from topical emulsion dosage form [156].
β-Glucans:
β-Glucan is a dietary fiber, found in many natural sources, and controls chronic metabolic diseases effectively. The in vivo cholesterol binding and reduction in the skin thickness by β-glucan were highly encouraging [160]. Although isolated from different sources, including oat, barley, and reishi mushrooms, the most biologically active are isolated from cell membranes of baker’s yeast (Saccharomyces cerevisiae). In the epidermis, where macrophage-derived cells include both keratinocytes and Langerhans cells, β-Glucans act to stimulate the protective qualities of these cells as our first line of defense. Topical β-Glucans can accelerate wound healing and increase resistance to infection by enhancing macrophage-mediated phagocytosis. Studies have also demonstrated that β-Glucans have photoprotective properties similar to those of vitamin E by their ability to sustain levels of reduced glutathione in the skin following UVR. β-Glucans are extremely soothing and calming to the skin through their reinforcement of skin macrophages, which have implications in minimizing irritancy potential of products. The potential uses of β-Glucans in dermatology are numerous. In personal-care products for shaving, where nicks and cuts, razor burn, irritation and folliculitis are problematic, the protective, wound-healing, anti-irritating effects of β-Glucans can be quite helpful. The photoprotective effects of β-Glucans as well as their ability to soothe, moisturize, and protect the skin from potential irritation that can occur with other treatment products, makes them quite useful in anti-aging skin regimens [95]. Topical application of β-glucans is increasing, since their pluripotent activity (antioxidant, anti-inflammatory and regenerative effects, immunomodulation, radioprotection, moisturization and rejuvenation) might help as a complementary therapy in managing various skin diseases and conditions. Macrophages, keratinocytes and fibroblasts are considered the main target cells of β-glucans during wound healing. β-glucans enhance wound repair by increasing the infiltration of macrophages, which stimulates tissue granulation, collagen deposition and re-epithelialization [157].

Figure 13: Schematic depiction of β-glucan pluripotent mechanisms in wound healing [157].
A long-term use of glucan showed reduction of wrinkle depth, height and overall roughness, which is probably caused by stimulation of fibroblast and increase production of collagen. A cell turnover and regenerative extract of βeta-glucan is believed to support healthy immunosurveillance [158]. Dammarane ginsenosides are considered to play a major role in the antiwrinkle activities of ginseng. These compounds are strongly linked with cellulose, pectin, or β-glucan [159]. Jesenak et.al, 2016 investigated the immunomodulatory and anti-inflammatory activity of an Imunoglukan P4H® cream, containing β-glucans (pleuran), in patients suffering from atopic dermatitis, where use of β-glucan-based cream as a supportive complementary therapy [161]. The topical application of Imunoglukan P4H® showed significant improvements in both subjective and objective symptoms of atopic dermatitis and a significant decline in disease severity; exacerbation was observed [162]. Sensitive skin is frequently complaint in dermatology consultation with cutaneous manifestations such as stinging, redness, dryness, and burning sensation that affect the quality of life. Its pathogenesis is mainly related to dysfunction of neurosensory, skin barrier, and also immune activity. Wang et.al, 2018 confirmed the effectiveness, tolerance and antisensitive function of a new complex cream composed by Yunnan Portulaca oleracea extract, Prinsepia utilis oil, beta-glucan, and sodium hyaluronate extracted from mushroom. The proposed daily care safe moisturizer provided a statistically significant improvement in clinical grading scores for dryness, roughness, and erythema at 28 days compared to baseline [163].
| Table 8: List of the Plant Extracts Mostly Used in Commercial Antiaging Cosmetics [8]. |
| Sesamum indium, Prunus Amygdales dulcin, Phyllanthus umbilical, Siegesbeckia orientalism, Theobroma cacao, Pittosporum parkin, Mangier Indica, Mentha piperada, Aleurits moluccana, Glycurrhiza glabra, Arcostaphylos uva, Imperata cylindrica, Centella asiatica, Echinacea purpurea, Camelia sinensis, Thea sinensis, Hordeum vulgare, Crithium maritimum, Plantago lanceolata, Phellodendron amurense, Spirea ulmaria, Artemisia vulgaris, Santalum album, Rosmarinus officinalis, Centella asiatica, Curcuma longa, Aloe vera, Arnica calendula, Ginkgo biloba, various algae such as Fucus vesiculosus, Laminaria flexicaulis, Ascophyllan nodosum. |
Ceramide: The stratum corneum is comprised of corneocytes surrounded by inter-celluar lipids including ceramides, free fatty acids, and cholesterol. Ceramide predominant moisturizers have become a mainstay of treatment of skin disease. Ceramides constitute (on a weight basis) approximately 47% of the SC lipids [186]. Moisturizing treatment involves a four-step process: a) repairing the skin barrier, b) increasing water content, c) reducing TEWL and d) restoring the lipid barriers’ ability to attract, hold and redistribute water. Interestingly, a statistically significant higher ceramide/cholesterol ratio was found for men than for women, as reported by Vozella et.al, 2019 [183]. Jensen et.al, 2005 reported reduced activities of ceramide-generating epidermal acid sphingomyelinase (SMase) and ceramide synthase in the inner epidermis of aged skin, explaining its reduced capacity in barrier repair [182]. The effect of Ceramide cream on enhancing skin barrier function and hydration might be explained by its unique ingredients. Ceramide cream increases skin hydration and improves barrier function which may make it suitable for use on dry skin [179]. Several studies have demonstrated that ceramides play an essential role in both the barrier and water-holding functions of healthy stratum corneum, suggesting that the dysfunction of the stratum corneum associated with ageing as well that observed in patients with several skin diseases could result from a ceramide deficiency. A 2-week topical application of a sonicated Streptococcus thermophilus preparation led to significant and relevant increase of stratum corneum ceramide levels [180]. Drawls et.al, 2018 demonstrates that a proprietary combination of ceramide PC-104, palmita ide MEA, glycyrrhizin acid, and grape seed extract in a glycerin, dimethazone, and petrolatum vehicle was effective in reducing the signs and symptoms of mild-to-moderate atopic dermatitis and other types of pruritic dermatoses (e.g., senile itch, cosmetic intolerance syndrome) in children and adults [184]. Yazdanparast et.al, 2018 reported skin-identical ceramide complex cream improved contact dermatitis with a decrease in Three-Item Severity (TIS) and an increase in skin hydration, implying a repair of the skin barrier [185]. Advancements in cosmetic chemistry have resulted in the development of bio-identical synthetic ceramides that are commonly incorporated into skin care products (notably CER-1, CER-3, and CER-6), which have been shown to function similar to natural ceramides [186]. Zhang et.al, 2015 reported limited penetration of ceramide species into SC and accumulation on to the skin, suggesting that topical replenishment of CER may not be an effective approach to improve the barrier properties of healthy skin [187].

Figure 14: The molecular structures of the ceramides (CER) present in human stratum corneum [225], indicated according to the numbering system (based on chromatographic migration) and according to their structures. A, α-hydroxy fatty acid; H, 6-hydroxysphingosine; N, monohydroxy fatty acid; P, Phyto sphingosine; S, sphingosine.
Nicotinamide: Niacin (vitamin B3) has two potential forms that can be used in cosmeceuticals: niacinamide (nicotinamide) and nicotinic acid [193]. Topical nicotinamide (the active form of vitamin-B3) has been shown to improve fine lines and wrinkles, hyperpigmented spots, red blotchiness and sallowness (yellowing), as well as elasticity. In addition, nicotinamide has been demonstrated to increase the skin’s production of collagen and ceramides, and to stimulate keratinocyte differentiation, leading to improved barrier function and skin appearance [95], [105]. Nicotinamide cream is a more effective moisturizer than white petrolatum on atopic dry skin, and may be used as a treatment adjunct in atopic dermatitis [188]. Ashkan et.al, 2015 reported its anti-inflammatory, antioxidant, and immunomodulatory properties, as well as an epithelization inducing action. Nicotinamide also improved tissue regeneration through the increment of fibroblast proliferation, collagen synthesis, and vascularization [189]. Nicotinamide and clindamycin gels were significantly more efficacious in oily and non-oily skin types, respectively. Skin type is a significant factor in choosing between topical nicotinamide and clindamycin in patients with acne vulgaris [196]. Because topical clindamycin, like other antimicrobials, is associated with emergence of resistant microorganisms, nicotinamide 4% gel is a desirable alternative treatment for acne vulgaris [190]. Niacinamide 4% induces a decrease in pigmentation, inflammatory infiltrate, and solar elastosis. Niacinamide is a safe and effective therapeutic agent for melasma, compared to 4% hydroquinone. Niacinamide was effective in approximate 40% of patients, showing outstanding clinical results [191]. In ageing skin, topical application of niacinamide improves the surface structure, smoothens out wrinkles and inhibits photo-carcinogenesis. It is possible to demonstrate anti-inflammatory effects in acne, rosacea and nitrogen mustard-induced irritation [192]. Nicotinamide also increases the production of the epidermal proteins keratin, filaggrin, and involucrum [194]. Nicotinamide increases collagen production in fibroblast cultures and reduces the increased dermal glycoaminoglycosides in photodamaged skin. The glycation between protein and sugar resulting in formation of cross-linked products gives a yellow color to the skin. As nicotinamide is a precursor of antioxidant NADPH, it has antiglycation effects, thus preventing shallowing of skin. In a double-blinded, split face, randomized controlled trial, 5% nicotinamide cream was compared to “vehicle only cosmetic” in 30 Japanese women on face for 8 weeks. There was a significant decrease in wrinkles and skin roughness with nicotinamide [195].
Zinc: The skin is the third most zinc (Zn)-abundant tissue in the body. Zn is a cofactor for over 1000 enzymatic reactions and is necessary for over 2000 transcription factors. Zn-finger proteins function for DNA interaction, RNA packaging, activation of transcription, regulation of apoptosis, folding and assembly of protein, and lipid binding. Zn also functions as an intracellular signaling molecule, like calcium, by transducing extracellular stimuli into intracellular signaling. Additionally, about 10% of human proteins binds to Zn. affects 17% of the world’s population who are in the condition of general malnutrition due to starvation, severe illness, alcohol addiction [214]. The importance of zinc for humans was acknowledged in the Middle East (Iran, Egypt), in the early 1960s, in patients with growth retardation, hypogonadism, hepatomegaly, splenomegaly, dry and wrinkled skin, and severe iron deficiency anemia [215]. A Zn-deficient diet alters the expression of keratin polypeptides in rats because of impaired keratinolytic enzyme activity. Zn is required for the proliferation of keratinocytes and the suppression of inflammation in Keratinocytes. Zn facilitates the melanocyte proliferation and the autophagy. Zn promotes lipogenesis and glucose transport via its insulin-like effects on 3T3-L1 fibroblasts and adipocytes [214]. Topical preparations like zinc oxide, calamine, or zinc parathion have been in use as photo protecting, soothing agents or as active ingredient of antidandruff shampoos. Its use has expanded manifold over the years for a number of dermatological conditions including infections (leishmaniasis, warts), inflammatory dermatoses (acne vulgaris, rosacea), pigmentary disorders (melasma), and neoplasia’s (basal cell carcinoma) [216].
Anti-pollution preparations
Fernández et.al, 2018 demonstrated that SIG-1273 reduced cell death by 66%, outperforming niacinamide, ascorbic acid, and α-tocopherol, commonly used actives in antipollution skin-care products [232]. Addor et.al, 2019 reported Cryptomphalus asperse secretion with regenerative (hyaluronic acid, peptides) and antioxidant ingredients (ecotone, coffeeberry oil, and olive oil), according to the type and area of the face, on the improvement of signs of skin aging. Ingredients from formulations studied have been shown to reduce the signs of skin aging by the multiple extrinsic factors known today as ultraviolet, visible, and infrared solar radiation; pollutants; aridity conditions; or even endogenous factors, such as dietary factors [233]. A film-forming exopolysaccharide (EPS) called as alter monas ferment extract was included in the formulation for its anti-adhesion effect. EPS significantly reduced particle adhesion to skin and protected keratinocyte membranes from lipid peroxidation, preserved cell integrity, and normalized the collagen network in skin exposed to heavy metals, hydrocarbons, and particulate matter. Nard et.al, 2018 reported that daily application of the facial cream containing an EPS, carnosine, and niacinamide over 5 days had a protective effect against pollution-induced changes [234]. Giacomelli et.al, 2018 reported that clinical application of a multicomponent powder, including three naturally occurring standardized extracts rich in polyphenols (grape seed extract, green tea extract, oak wood/bark extract) allows the prevention of any metal deposition within the SC following exposure in a polluted environment and plays an effective role in counteracting skin damages induced by air pollution [235].
Systemic Anti-aging preparations
Collagen supplementation
In 2016, the collagen market was valued at an estimated 3.71 billion USD and is projected to reach 6.63 billion USD by 2025. Collagen supplements, originating from various sources (e.g., porcine, bovine, marine) and available in numerous formulations (e.g., protein, gelatin, hydrolysate, peptides), are marketed as improving skin integrity and modulating skin aging. When denatured by heat, collagen forms gelatin, which has been used for centuries as a food source and traditional medicine in Europe and China. Further enzymatic hydrolysis of gelatin produces collagen hydrolysates (CH) composed of peptides of varying lengths, conveniently formulated into liquid drinks and jelly sticks for oral consumption. In the past decade, CHs have gained popularity as a nutraceutical supplement. Choi et.al, 2019 reported promising preliminary results for the short and long-term use of oral collagen supplements for wound healing and skin aging. Oral collagen supplements also increase skin elasticity, hydration, and dermal collagen density. However, even with this increase in patient interest and market share, the use of collagen supplementation in dermatology remains controversial due to the lack of regulation on quality and quantity of ingredients in OTC collagen supplements [164], [171]. Maria et.al, 2019 reported improvement of general skin conditions, acting in different mechanisms by oral supplementation and topical application of hydrolyzed proteins [165]. Proksch et.al, 2014 reported significant improvement in after 8 weeks of supplementation in women aged 35-55 years but study failed to reach a level of statistical significance with regard to skin moisture and skin evaporation [166]. Oral administration of Low-molecular-weight Collagen peptide (LMWCP), which is a fish-derived collagen hydrolysate, promotes recovery of collagen fibers and normal elastic fibers in the skin from degraded collagen and abnormal elastic fibers caused by UVB irradiation in hairless mice [167]. Kim et.al, 2018 reported that LMWCP is a safe health functional food ingredient with anti-skin photoaging efficacy which can effectively improve hydration, elasticity, and wrinkling in human skin at the dose of 1000 mg once daily [168].

Figure 15: Collagen fiber structure, absorption/metabolism and deposition into skin cells and dermal layers [174]. It is generally thought that collagen (derived products) are hydrolyzed into amino acids in the GIT prior to being abssorbed into the blood circulation, which are then deposited into the skin cells and/or utilized as building block components for extracellular matrix proteins produced by fibroblasts. ALA = Alanine, HYP = Hydroxyproline, GLY = Glycine, PRO = Proline, and Ser = Serine.
Oral supplementation with collagen bioactive peptides (hydrolyzed fish collagen) combined with chondroitin sulphate, glucosamine, L-carnitine, vitamins, and minerals significantly improved the clinical parameters related to skin aging and joint health [169]. Lee et.al, 2019 reported that orally administering collagen peptide NS (CPNS) to rats, the plasma concentrations of Gly-Pro and Pro-Hyp increased dramatically. The CPNS consumption significantly attenuated UVB-induced wrinkle formation, transepidermal water loss, and epidermis thickness, and increased skin hydration [170]. An association between oral administration of collagen peptides combined with vitamin C and extracts of Hibiscus sabdariffa and Aristotelia chilensis was observed by Addor et.al, 2018. Female adult patients received an oral nutritional supplement from a sachet and were instructed to consume 1 sachet diluted in 200 mL of water once daily for 12 weeks. Clinical evaluation by high frequency ultrasound and cutometry showed significant improvement of firmness and elasticity and an increase in dermal thickness by ultrasound after 3 months of use [171]. Zague et al, 2018 reported that collagen peptides modulate the metabolism of extracellular matrix proteins by human dermal fibroblasts (in culture) that were derived from sun-protected and sun-exposed body sites [172]. Song et al, 2017 examined the effects of collagen hydrolysates from sliver carp skin on UV-induced photo-aging in mice and found that LMW peptides exerted beneficial effects when compared to high molecular weight CHs on HA levels and moisture content of the skin [173].
|
Table 10: Summary of natural compounds and minerals used as supplement for skin health [174] |
|
|
Natural Compound or Mineral |
Mechanism of Action(s) Involved in Maintaining Skin Health |
|
Building block of collagen and elastin fibers-improves skin and nail health; inhibits matrix metalloproteinases (MMPs); stimulates fibroblast function |
|
|
2. Ceramides |
Provides the major component of the lipid “mortar” of the stratum corneum essential in the structure and maintenance of skin barrierintegrity; also involved in cell proliferation, differentiation and apoptosis |
|
3. Beta Carotene |
Provitamin A molecule, acts as an antioxidant, anti-inflammatory agent and blocks ROS formation and/or ability to quench free radicals; prevents cellular damage, premature skin aging and skin cancer |
|
4. Astaxanthin |
Potent antioxidant; anti-inflammatory agent; prevents DNA damage & enhance mitochondrial function, provides UV protection; activates the Nrf2 pathway toto stimulate production of other antioxidants; inhibits MMPs; stimulates collagen production and wound healing |
|
5. Coenzyme Q10 |
Antioxidant; anti-aging properties-enhances collagen; potential treatment for psoriasis; accelerates generation of ATP levels after irradiation of fibroblasts |
|
6. Colostrum |
Contains, growth factors and other immune regulatory factors that promote growth of keratinocytes and wound healing |
|
7. Zinc |
Importance for skin morphogenesis, repair and maintenance such as wound healing |
|
8. Selenium |
Acts as a cofactor for glutathione peroxidase (GPX) removing harmful peroxides; involved in DNA synthesis and repair; prevents oxidative stress and UVB-radiation; also acts as an antioxidant |
Probiotics
Lactic acid bacteria consist of 26 genera now, and play an essential role in the food industry in the manufacture of many fermented products (cheese, yogurt, fermented vegetables, etc.). Application of these organisms is now being extended to the area of health improvement, as their probiotic activities become known. Lactococcus lactis H61 improved skin status in Japanese women with oral intake of heat?killed or live cells. With regard to live cells in fermented milk made by strain H61, the reported effects are attractive and it is expected that consumption of H61?fermented milk will increase [175]. It is also reported that oral intake of Lactobacillus rhamnosus SP1 improves the appearance of adult acne [176]. Oral intake of yoghurt made by using Lactobacillus delbrueckii subsp. bulgaricus 2038 plus Streptococcus thermophilus 1131 for 4 weeks improved skin elasticity and the degree of dryness in cheeks of women [177]. Mori et al, 2016 also reported that the intake of fermented milk containing Bifidobacterium breve strain Yakult plus galactooligosaccharides for 4 weeks increased hydration levels of the stratum corneum in women [178].

Figure 16: Bio-actives from probiotics for dermal applications [113].
Astaxanthin
Astaxanthin is ubiquitous in nature, especially found in the marine environment as a red-orange pigment common to many aquatic animals such as salmonids, shrimp, and crayfish. The ROS lead to skin aging via oxidative damage that are induced by UVR. Therefore, topical formulations which have antioxidant effect could reduce aging level [200]. Eren et.al, 2019 reported that topical formulations of astaxanthin-loaded algae extract could be suggested as topical anti-aging formulations [199]. Comparative studies examining the photoprotective effects of carotenoids have demonstrated that astaxanthin is a superior antioxidant, having greater antioxidant capacity than canthaxanthin and β-carotene in human dermal fibroblasts [200]. In particular, astaxanthin inhibits ROS formation and modulates the expression of oxidative stress-responsive enzymes such as heme oxygenase-1 (HO-1), which is a marker of oxidative stress and a regulatory mechanism involved in the cell adaptation against oxidative damage [205].

Figure 17: The proposed mechanism by which astaxanthin inhibits oxidative stress-induced mitochondrial dysfunction, and development and progression of diseases [211].
Astaxanthin exerts significant antioxidant activities not only via direct radical scavenging, but also by activating the cellular antioxidant defense system through modulation of the nuclear factor erythroid 2-related factor (Nrf2) pathway. Fang et.al, 2017 demonstrated that astaxanthin protected against early burn-wound progression by attenuating ROS-induced oxidative stress in a rat deep-burn model [201]. In vitro, astaxanthin effectively suppresses cell damage caused by free radicals and induction of MMP-1 in skin after UV irradiation [202]. Chou et.al, 2016 reported that an enriched astaxanthin extract from H. pluvialis increased collagen content through inhibition of MMP-1 and MMP-3 expression in human dermal fibroblasts [203]. Meephansan et.al, 2017 reported that astaxanthin-treated wounds in mice showed significantly increased expression of wound healing biological markers such as collagen type I α 1 (Col1A1) and basic fibroblast growth factor (bFGF) [204]. The immunomodulatory action of astaxanthin has been also reported in dogs and cats, enhancing both cell-mediated and humoral immune responses. In these studies, astaxanthin increased natural killer (NK) cell cytotoxic activity, suggesting that astaxanthin may regulate NK cells that serve as an immunosurveillance system against tumors and virus-infected cells [206,207]. Astaxanthin is reported to improve the DNA repair capacity of cells exposed to UV radiation. In particular, astaxanthin was capable of minimizing DNA damage and influencing the kinetics of DNA repair [208]. Human cells possess multiple protection mechanisms against UV-induced ROS, either by preventing damage or by damage repair. Camera et.al, 2009 reported that astaxanthin inhibits the UV-induced DNA damage and increases the expression of oxidative stress-responsive enzymes [209]. Tominaga et.al, 2017 suggested that long-term prophylactic astaxanthin supplementation may inhibit age-related skin deterioration and maintain skin conditions associated with environmentally induced damage via its anti-inflammatory effect [210].
Colostrum
Colostrum is the initial milk or “first milk” that is produced by mammals (including humans) immediately following parturition. As expected, colostrum was more effective than milk with the total lipid, linoleic acid, linolenic acid, ganglioside, and glycolipid contents were higher in colostrum when compared to milk. In addition, with further analysis, the fat globule fraction provided the strongest stimulation for wound repair that contained Epidermal Growth Factors. The milk fluid produced by all female mammalian species after birth has the function to meet the complete nutritional requirements of the neonate and, at the same time, provide all of the biochemical needs and support the many biological functions of the immature newborn to help the newborn survive and develop. Starting in the 1980s and through the mid-1990s, supplemented cell culture medium with milk or colostrum was reported to improve the growth rate of many cell types including skin (fibroblasts). Peptides from milk protein hydrolysates improved the growth of human keratinocytes in culture. Medium supplemented with 300 μg/mL for 12 days where the average molecular weight of 800 Da containing a high concentration of amino acids promoted the growth of the keratinocytes by 108% [174]. Colostrum is the only known natural source of the enzyme, telomerase, which may help to slow down the aging of DNA. In fact, there is evidence that short telomeres and a lack of telomerase can exert a longevity-promoting effect via prevention of cancer [212]. Colostrum also includes EGF and IGF-1, which are known to assist in the repair and regeneration of cells. EGF and IGF-1 play essential roles in wound healing, which makes colostrum an important potential adjunct to the skin’s repair following a surgical cosmetic procedure. Let’s not forget about the lactoferrin in colostrum, either. Lactoferrin helps manage the immune response in the skin cells, which means supplementing with lactoferrin may potentially help a person increase his or her skin’s anti-inflammatory response [213].
Selenium
Selenium (Se) is an essential trace element in the human body and plays an important role in the body via selenoprotein, which contains selenium. Selenoproteins (glutathione peroxidase, thioredoxin reductase, methionine sulfoxide reductase-1 and endoplasmic reticulum-selenoproteins, etc.) have antioxidant effects and are involved in regulating antioxidant activities [217]. Se and the selenoproteins are essential for keratinocyte function and skin development. A lack of selenoenzymes in the mouse epidermis leads to abnormalities in the skin and hair follicles, premature skin aging, and premature death. Additionally, several studies have shown that Se pretreatment can drastically protect keratinocytes, melanocytes, and fibroblasts from UV-induced cytotoxicity. Low doses of Se were very potently protective against UVA-induced cytotoxicity in young keratinocytes, whereas the aged keratinocytes require four times more Se than the young keratinocytes to be protected from UVA-induced cytotoxicity [218,219]. Se protects keratinocyte stem cells (KSCs) against senescence via preservation of their stemness phenotype through adhesion to the basement membrane [219]. Wang et. al, 2017 showed that Vitamin C (250 mg/kg), vitamin E (250 mg/kg) and Se (0.2 mg/kg) exerted antioxidant effects and consequently may prevent skin damage caused by streptozotocin-induced diabetes (65 mg/kg) in Swiss albino rats [220].
Hyaluronic Acid
Hyaluronic acid (HA) is part of the body's connective tissues, and is known to cushion and lubricate. Aging destroys HA. Diet and smoking can also affect your body's level of HA over time. Skin care products with HA are most frequently used to treat wrinkled skin although they don't replace anything the body has naturally lost. These are very effective moisturizers [119]. UV radiation damage causes initially a mild form of wound healing and is associated at first with an increase of dermal HA. As little as 5 min of UV exposure in nude mice caused enhanced deposition of HA, indicating that UV radiation induced skin damage is an extremely rapid event. The initial redness of the skin following exposure to UV radiation may be due to a mild edematous reaction induced by the enhanced HA deposition and histamine release. Repeated and extensive exposures to UV ultimately simulate a typical wound healing response with deposition of scarlike type I collagen, rather than the usual types I and III collagen mixture that gives skin resilience and pliability [120]. HA based formulations (i.e., gels, creams, intra-dermal filler injections, dermal fillers, facial fillers, autologous fat gels, lotion, serum, and implants, etc.) Table remarkable anti-wrinkle, anti-nasolabial fold, anti-aging, space-filling, and face rejuvenating properties. This has been achieved via soft tissue augmentation, improved skin hydration, collagen and elastin stimulation, and face volume restoration. HA, alone or in combination with lidocaine and other co-agents, showed promising efficacy in skin tightness and elasticity, face rejuvenation, improving aesthetic scores, reducing the wrinkle scars, longevity, and tear trough rejuvenation [125]. Sparavigna et.al, 2019 reported significant improvement of wrinkles' grade around the eyes, vertical lip lines and wrinkles' severity of nasolabial folds after the first injection and the effect increased after the second injection. Aging/photoaging grade and surface microrelief improved 2 months after the first injection procedure. The treatments were very well tolerated by the volunteers as determined by the self-grading score [121]. Lee et.al, 2019 reported that Cross-linked hyaluronic acid (CLHA) patches were not an irritant, whereas a clinical study showed that application of single CLHA patches significantly improved skin hydration at the periorbital region for 3 days and at the nasolabial fold for 6 days. Patch application also improved superficial wrinkles at the periorbital region for 3 days and at the nasolabial fold for 1 day. The absence of side effects indicated that application of these CLHA microstructure patches is both safe and convenient for moisturization and anti-wrinkle effects [122]. Jeon et.al, 2019 reported that CTP-EGF has a superior ability, compared with natural EGF, to permeate skin and induce HA synthesis and collagen formation. Thus, it has great potential to be used in cosmetics and therapeutic agents to improve wrinkles and health of the skin [123]. There exist many different types of HA gel fillers that differ in their HA concentration, particle size, cross-linking density, duration, and presence of lidocaine. High-density, large-particle fillers are recommended for deep dermal injections while the low-density, small-particle fillers are recommended for fine lines. HA gel is used by several healthcare professionals include the plastic surgeon, primary care provider, dermatologist, nurse practitioner and the internist to enhance cosmesis. HA fillers are injected to restore volume lost due to age or disease, provide facial contour, and help maintain a youthful appearance. Filler injection has become one of the most commonly performed procedures in a dermatology cosmetic practice [124].
Poly-L-lactic acid
Poly-L-lactic acid (PLLA) is an injectable filler used for restoring facial fat volume loss. Polydioxanone Cog thread and poly-L-lactic acid (PLLA) thread have been used clinically for lifting and antiaging purposes [221]. PLLA is an effective treatment for patients seeking to correct volume loss due to aging. Although the US FDA has approved PLLA for use in people with the HIV in 2004, it is well-suited for patients seeking cosmetic treatment. By 2009 PLLA was FDA-approved for the correction of nasolabial fold contour deficiencies and other lines and wrinkles. There have since been limited but promising results with off-label use of PLLA for non-facial volumization as well, including the hands, neck/décolleté, abdomen, and gluteal area [222]. PLLA is a safe, biodegradable volumizer used to reverse the signs of aging by gradually correcting volume loss. Patients should be aware of possible adverse reactions during the course of treatment [221]. Injection of PLLA in the deep dermis or subcutaneous tissue may cause an immediate augmentation of the treated tissue. This is a temporary but immediate response that is due to tissue edema and fluid from the reconstitution of the product. It will resolve within 2 to 3 days after injection. Once the carrier substance is absorbed, the poly-L-lactic acid particles induce an inflammatory response through phagocytosis by tissue macrophages. This is a similar process to suture reabsorption in the skin. The inflammatory response breaks down the poly-L-lactic acid into lactic acid monomers and is then metabolized to carbon dioxide and water while stimulating the production of new collagen type-I fibers in the skin. Approximately half of the product is digested within 6 months. The duration of action is 12 to 24 months [223]. Kapicio?lu et.al, 2019 reported that PLLA and Cog sutures were effective in facial rejuvenation (studied in female rats); both increased dermis thickness and stimulated collagen production [110]. Repeated PLLA treatments may improve skin quality in a time-dependent manner. Pigmentation, erythema, and pore size were significantly decreased, whereas radiance and smoothness were significantly increased at 12 months. No treatment-related adverse events occurred. Repeated PLLA treatments may improve skin quality in a time-dependent manner [111]. The process of hydration, loss of cohesion and molecular weight, and solubilization and phagocytosis of PLA by the host’s macrophages, degrades PLA into lactic acid microspheres and eliminates CO2 by way of respiratory excretion. Crystals are left behind to stimulate collagen and a granulomatous reaction. This inflammatory reaction elicits resorption and the formation of fibrous connective tissue about the foreign body, causing dermal fibroplasia that leads to the desired cosmetic effect [2]. Kim et.al, 2019 reported that powdered polydioxanone injection induces collagen formation more effectively than PLLA injection [223].
Hormone Replacement Therapy
In postmenopausal women, dermal collagen decreases, and skin becomes thinner [241]. Hormone replacement therapy (HRT) has been shown to be effective in alleviating menopausal symptoms. However, its use is controversial owing to potential health risks, such as thromboembolism and cancer. Bioidentical hormone therapy has also been used by dermatologists for its anti-aging effects on the skin, but little is known about efficacy and side effects of bioidentical hormones in this field [236]. Women’s Health Initiative (WHI) study showed a higher risk for breast cancer, stroke, cardiovascular disease, and thromboembolic events with combined treatment of estrogens and progestin. Synthetic progestins mostly used worldwide include medroxyprogesterone acetate (most frequently used in the US), norethidrone acetate, cyproteron acetate, norgestimate, norgestrel, and dydrogesterone [238]. The HRT impact on skin thickness and dermal density was demonstrated early when estrogens were initially administered to postmenopausal women. Such replenishment therapy was therefore considered as an attempt at alleviating in part skin atrophy and xerosis in postmenopausal women. Indeed, HRT controls in part the dermal thickness and laxity, and the collagen content and density, as well as the tissue mechanical reactivity to stress [237]. Physicians and patients have become extremely reluctant concerning HRT following the WHI study. Numbers of HRT prescriptions in the US rose from 58 million in 1995 to 90 million in 1999, corresponding to 15 million women per year. Numbers remained stable through to 2002. Within 3 months after publication of the results of the WHI study, prescriptions of various formulations of combined estrogens and progesterone dropped by 33% to 66% [238]. Vinogradova et.al, 2019 reported association between risk of venous thromboembolism and different types of HRT. Transdermal treatment was the safest type of hormone replacement therapy when risk of venous thromboembolism was assessed. Transdermal treatment appears to be underused, with the overwhelming preference still for oral preparations [239]. Both oral and transdermal estradiol caused a significant decrease in FSH while only transdermal resulted in a significant decrease in LH. Oral estradiol, though not transdermal estradiol, increased serum high density lipoprotein, thyroxine binding protein and growth hormone binding protein [240]. Applying estrogen cream to the skin after menopause improves the external appearance of facial skin [241]. There is strong evidence that transdermal estradiol has a cardioprotective effect [243]. Due to their lack of first-pass hepatic metabolism, transdermal products achieve clinical benefits while minimizing patient exposure to estrogens, which is consistent with the most recent clinical guidelines [244]. Also, by increasing skin collagen content, and increasing acid mucopolysaccharides and HA, estrogen therapy encourages the growth and development of vaginal epithelial cells which make up the thick layers of the vaginal wall, and condone a moist, supple and elastic environment [242]. Botelho et.al, 2014 reported that nanostructured formulation of progesterone (10%) combined with estriol (0.1%) + estradiol (0.25%) is safe and effective in re-establishing optimal serum levels of estradiol and follicle-stimulating hormone and relieving the symptoms of menopause [243]. Abdi et.al, 2017 also concluded that use of transdermal nano-formulations in hormone therapy can relieve climacteric symptoms and prevent other postmenopausal symptoms [245].
Epilogue
As more and more anti-ageing and antioxidant skin care products flood the market, there is growing concern about definitions and experimental proof of effectiveness. The physician has an important role in understanding which treatment options are appropriate for mild, moderate, and severe photoaging, and in educating patients on the risks and benefits of each. The choice of the right active compounds, the verification of their activity inside a cosmetic formulation, their stability and synergistic effects should be the first step toward the creation of modern and effective products. To be active inside the skin, the antioxidants have to penetrate into the living layers of the skin, where free radicals are generated and should be effective against ROS. This is possible only if the topical applied formulation holds the potential to be effective. Moisturizing and emollient products are gaining increasing importance in dry skin treatment, maintenance of daily care of normal skin as well as ancillary therapy of many skin diseases. Consumers are nowadays more focused on their health and appearance. As a result, there has been an increasing demand in topical antiaging formulations with natural and nutraceutical ingredients. Novel and innovative delivery systems are transforming the new product development in the cosmetic field because of consumer perceivable benefits and optimized sensory attributes. The applications of novel drug delivery systems can be found in many cosmetic products. Nanomaterials are nowadays used in almost all the major cosmetic industries. The truth is, there is no magic pill at present that will retard aging. But that is not to say there are not simple lifestyle and dietary adjustments that can make people live longer. A cosmetic product that produces clinically objective effects on the most-reported signs of aging is an attractive option for those unable to avoid extrinsic aging factors but wishing to improve their appearance without resorting to more invasive measures.
Article Summary
Skin care products with antioxidative and anti-aging claims are one of the most fast-growing market for cosmetics worldwide. Anti-aging in dermatology primarily focuses on the prevention of skin aging with UV protection (clothing and sunscreens), free radical scavengers (synthetic or botanic), and cell-protecting agents. Many synthetic and natural products have been reported to enhance levels of antioxidant enzymes, which make them therapeutic candidates to mitigate UV-mediated damage and to prevent the health consequences of UV exposure. Topical hormonal prescriptions are also an option if UV damage has not been the leading culprit for aging. Chemical peeling leads to a marked increase in collagen formation, the deeper the better. Ingredients in cream preparations can reduce superficial skin folds (polyphenols, amino acid peptides). Modulators of regular pigmentation are important for anti-aging preparations. New approaches are being designed to exploit the signaling pathways to delay or even prevent free-radical induced symptoms of aging. There are too many products on the market, from so many brands, with more and more ingredients, and at various price points. Selecting the right anti-aging product is definitely a daunting task, but this guide is meant to simplify the process and help to choose the right anti-aging skin care products for an individual skin.
Article Highlights
1. Skin aging is a complex biological process influenced by combination of endogenous or intrinsic (genetics, cellular metabolism, hormone and metabolic processes) and exogenous or extrinsic (chronic light exposure, pollution, ionizing radiation, chemicals, toxins) factors.
2. Skin aging is characterized by features such as wrinkling, loss of elasticity, laxity, and rough-textured appearance.
3. Anti-aging medicine encompasses lifestyle changes, hormone replacement therapies, as needed, determined by a physician through blood testing; antioxidants and vitamin supplements; and testing protocols that can measure not only hormone levels and blood chemistry but every metabolic factor right down to the cellular level.
4. Cell senescence limits cell divisions in normal somatic cells and may play a central role in age-related diseases.
5. The major perceived risk factors are unhealthy eating habits, stress, less exercise, dehydration, diseased state and sleeping habits, though the main factor responsible for extrinsic aging is UVR.
6. Exposure to UVR is the primary factor of extrinsic skin aging, it accounts for about 80% of facial aging.
7. IR radiation and heat can lead to macrophage recruitment like UVR.
8. Even in indoor conditions, particulate matter (PM2.5) exposure levels were positively associated with skin aging manifestation.
9. Smoking provokes elastosis, telangiectasia, skin roughness, and premature wrinkles on facial skin due to the vascular constriction of nicotine.
10. Sleep deprivation is associated with increased signs of intrinsic skin aging (fine lines, uneven pigmentation, reduced
elasticity), with much slower recovery rates.
11. Cooking processes that lead to higher levels of advanced glycation end product (AGEs) include grilling, frying, and roasting.
12. Among US population 75% consumed less fruit and 87% consumed fewer vegetables than recommended.
13. Higher intakes of vitamin C and linoleic acid and lower intakes of fats and carbohydrates are associated with better skin-aging appearance.
14. In order to lowering the skin damage, cleansings with neutral pH and pH close to 5.5 are recommended.
15. 30-70% of patients with DM, both type 1 and type 2, will present with a cutaneous complication of DM at some point during their lifetime.
16. Obese-diabetes patients have decreased stratum corneum hydration, increased trans-epidermal water loss, higher skin AGEs and decreased dermal collagen fiber density compared with normal-weight subjects.
17. Type I and III skin collagen is thought to decrease by as much as 30% in the first five years after menopause.
18. Africans from the African continent show delayed signs of aging compared to Caucasians. Darker skin types are better protected regarding sun exposure due to the higher melanin content in their skin.
19. The skin parameters of hydration, trans-epidermal water loss, sebum, microcirculation, pigmentation, and thickness are generally higher in men but skin pH is higher in women.
20. There is no proven effective product that completely eliminates the symptoms of skin photoaging, but there are products and treatments that can visibly reduce or slow down these symptoms.
Abbreviations: luteinizing hormone (LH); follicle stimulating hormone (FSH); adrenocorticotropic hormone (ACTH); growth hormone (GH); Transforming growth factor beta (TGF-β); matrix metalloproteinases (MMPs); activator protein-1 (AP-1); glycosaminoglycan (GAG); Reactive oxygen species (ROS); 4-hydroxy-2-nonenal (HNE); particulate matter (PM2.5); transepidermal water loss (TEWL); glycation end products (AGEs); National Health and Nutrition Examination Surveys (NHANES); Dutch Healthy Diet Index (DHDI); principal component analysis (PCA); carboxymethyl lysine (CML); dermal White Adipose Tissue (dWAT); Peroxisome Proliferator-Activated Receptor γ (PPARγ); hypothalamic-pituitary-adrenal (HPA); Glucocorticoid receptors (GRs); nuclear receptor subfamily 3 group C member 1 (NR3C1); Pulsed electromagnetic fields (PEMFs); Multipolar Magnetic Pulse (MP)2; sun protection factor (SPF); Epidermal growth factor (EGF); cytoplasmic transduction peptide (CTP); ferric-reducing activity of plasma (FRAP); sodium-dependent vitamin C transporters (SVCTs); matrix metalloproteinase-1 (MMP-1); epithelial/epidermal growth factor (EGF); insulin-like growth factor (IGF-1); nuclear factor erythroid 2-related factor (Nrf2); collagen type I α 1 (Col1A1); basic fibroblast growth factor (bFGF).
References
1. Quiroga RM. Chapter 1. Anti-Aging Medicine As It Relates to Dermatology. In: Cheryl M. Burgess.
2. Ganceviciene R, Liakou AI, Theodoridis A, et al. 2012. Skin anti-aging strategies. Dermatoendocrinol. 4: 08-19. Ref.: https://bit.ly/2xby971
3. Bhatt N, Agrawal S, Mehta K. 2019. Risk factors and self-perception for facial aging among Nepalese population. Ref.: https://bit.ly/2J9fMFd
4. Addor FAS. 2018. Beyond photoaging: additional factors involved in the process of skin aging. Clin Cosmet Investig Dermatol. Ref.: https://bit.ly/2IGiXoY
5. Asakura K, Nishiwaki Y, Milojevic A, et al. 2009. Lifestyle factors and visible skin aging in a population of Japanese elders. J Epidemiol. 19: 251-9. Ref.: https://bit.ly/2Y9sF8R
6. Tito A, Barbulova A, Zappelli C, et al. 2019. The Growth Differentiation Factor 11 is Involved in Skin Fibroblast Ageing and is Induced by a Preparation of Peptides and Sugars Derived from Plant Cell Cultures. Mol Biotechnol. 61: 209-220. Ref.: https://bit.ly/2J9q88j
7. Barone F, Bashey S, Woodin FW. 2019. Clinical Evidence of Dermal and Epidermal Restructuring from a Biologically Active Growth Factor Serum for Skin Rejuvenation. J Drugs Dermatol. 18: 290-295.
8. Clarys P, Barel AO. Chapter 27. New Trends in Antiaging Cosmetic Ingredients and Treatments: An Overview. In: André O. Barel, Marc Paye, Howard I. Maibach. Handbook of Cosmetic Science and Technology.
9. Leccia MT, Lebbe C, Claudel JP, et al. 2019. New Vision in Photoprotection and Photorepair. Dermatol Ther (Heidelb). 9: 103-115. Ref.: https://bit.ly/2FwrQzp
10. Karapetsas A, Voulgaridou GP, Konialis M, et al. 2019. Propolis Extracts Inhibit UV-Induced Photodamage in Human Experimental In Vitro Skin Models. Antioxidants (Basel). 8. Ref.: https://bit.ly/2FwIIWZ
11. Zhang S, Duan E. 2018. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 27: 729-738. Ref.: https://bit.ly/2J2hLeC
12. Krutmann J, Bouloc A, Sore G, et al. 2017. The skin aging exposome. J Dermatol Sci.152-161. Ref.: https://bit.ly/2zPhB70
13. Cho S, Shin MH, Kim YK, et al. 2009. Effects of infrared radiation and heat on human skin aging in vivo. J Investig Dermatol Symp Proc. 14: 15-9. Ref.: https://bit.ly/2w19pRe
14. Kettelhut EA, Traylor J, Roach JP. 2019. Erythema Ab Igne. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Ref.: https://bit.ly/2J3eonR
15. Pecorelli A, Woodby B, Prieux R, et al. 2019. Involvement of 4-hydroxy-2-nonenal in pollution-induced skin damage. Biofactors. Ref.: https://bit.ly/2FsCkzJ
16. Burke KE, Wei H. 2009. Synergistic damage by UVA radiation and pollutants. Toxicol Ind Health. 25: 219-24. Ref.: https://bit.ly/2IFmXpI
17. Ding A, Yang Y, Zhao Z, et al. 2017. Indoor PM (2.5) exposure affects skin aging manifestation in a Chinese population. 7: 29. Ref.: https://bit.ly/2X2pZNH
18. Schikowski T, Krutmann J. 2017. [Air pollution (particulate matter and nitrogen dioxide) and skin aging]. Hautarzt. 70: 158-162. Ref.: https://bit.ly/2RyIVgS
19. Park SY, Byun EJ, Lee JD, et al. 2018. Air Pollution, Autophagy, and Skin Aging: Impact of Particulate Matter (PM(10)) on Human Dermal Fibroblasts. Int J Mol Sci. 12; 19. Ref.: https://bit.ly/2IImXFF
20. Urba?ska M, Nowak G, Florek E. 2012. [Cigarette smoking and its influence on skin aging]. Przegl Lek. 69: 11-4. Ref.: https://bit.ly/2Leiufx
21. Wang AS, Dreesen O. 2018. Biomarkers of Cellular Senescence and Skin Aging. Front Genet. 9: 247. Ref.: https://bit.ly/2NbRfF4
22. Sundelin T, Lekander M, Sorjonen K, et al. 2017. Negative effects of restricted sleep on facial appearance and social appeal. 4: 918.
23. Walia HK, Mehra R. 2016. Overview of Common Sleep Disorders and Intersection with Dermatologic Conditions. Int J Mol Sci. 17. Ref.: https://bit.ly/2J8qT1e
24. Oyetakin-White P, Suggs A, Koo B, et al. 2015. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 40: 17-22. Ref.: https://bit.ly/2KzAz3q
25. Clatici VG, Racoceanu D, Dalle C, et al. 2017. Perceived Age and Life Style. The Specific Contributions of Seven Factors Involved in Health and Beauty. Maedica (Buchar). 12: 191-201. Ref.: https://bit.ly/2YaCEuw
26. Anson G, Kane MA, Lambros V. 2016. Sleep Wrinkles: Facial Aging and Facial Distortion During Sleep. Aesthet Surg J. 36: 31-40. Ref.: https://bit.ly/2YdjK64
27. Katta R, Desai SP. 2014. Diet and dermatology: the role of dietary intervention in skin disease. J Clin Aesthet Dermatol. 7: 46-51. Ref.: https://bit.ly/2B0cot0
28. Schagen SK, Zampeli VA, Makrantonaki E, et al. 2012. Discovering the link between nutrition and skin aging. Dermatoendocrinol. 4: 298-307. Ref.: https://bit.ly/2RyNuIe
29. Pem D, Jeewon R. 2015. Fruit and Vegetable Intake: Benefits and Progress of Nutrition Education Interventions- Narrative Review Article. Iran J Public Health. 44: 09-21. Ref.: https://bit.ly/2RzwOk0
30. Moore LV, Thompson FE, Demissie Z. 2017. Percentage of Youth Meeting Federal Fruit and Vegetable Intake Recommendations, Youth Risk Behavior Surveillance System, United States and 33 States, 2013. J Acad Nutr Diet. 117: 545-553. Ref.: https://bit.ly/2L8w1oS
31. Bragazzi NL, Sellami M, Salem I, et al. 2019. Fasting and Its Impact on Skin Anatomy, Physiology, and Physiopathology: A Comprehensive Review of the Literature. Nutrients.11. Ref.: https://bit.ly/31PQMvz
32. Meki? S, Jacobs LC, Hamer MA, et al. 2019. A healthy diet in women is associated with less facial wrinkles in a large Dutch population-based cohort. J Am Acad Dermatol. 80: 58-63. Ref.: https://bit.ly/2LfKXl0
33. Cosgrove MC, Franco OH, Granger SP, et al. 2007. Dietary nutrient intakes and skin-aging appearance among middle-aged American women. Am J Clin Nutr. 86: 25-31. Ref.: https://bit.ly/2PW9gUO
34. Mukhopadhyay P. 2011. Cleansers and their role in various dermatological disorders. Indian J Dermatol. 56: 2-6. Ref.: https://bit.ly/2WY2hNO
35. Gfatter R, Hackl P, Braun F. 1997. Effects of soap and detergents on skin surface pH, stratum corneum hydration and fat content in infants. Dermatology. 195: 258-262. Ref.: https://bit.ly/2E4Fnf5
36. Kulthanan K, Maneeprasopchoke P, Varothai S, et al. 2014. The pH of antiseptic cleansers. Asia Pac Allergy. 4: 32-6. Ref.: https://bit.ly/2LeEGWT
37. Rosen J, Yosipovitch G. Skin Manifestations of Diabetes Mellitus. [Updated 2018 Jan 4]. Ref.: https://bit.ly/2X6UQsw
38. Waller JM, Maibach HI. Chapter 23. A Quantitative Approach to Age and Skin Structure and Function: Protein, Glycosaminoglycan, Water, and Lipid Content and Structure. Ref.: https://bit.ly/2FvZt4x
39. Uruska A, Gandecka A, Araszkiewicz A, et al. 2019. Accumulation of advanced glycation end products in the skin is accelerated in relation to insulin resistance in people with Type 1 diabetes mellitus. 36: 620-625. Ref.: https://bit.ly/2YcyFgO
40. Pageon H, Zucchi H, Rousset F, et al. 2014. Skin aging by glycation: lessons from the reconstructed skin model. Clin Chem Lab Med. 52: 69-74. Ref.: https://bit.ly/2Fwgw6i
41. Kong JG, Park JB, Lee D, et al. 2015. Effect of high glucose on stress-induced senescence of nucleus pulposus cells of adult rats. Asian Spine J. 9: 55-61. Ref.: https://bit.ly/2RynYml
42. Davalli P, Mitic T, Caporali A, et al. 2016. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid Med Cell Longev. Ref.: https://bit.ly/2IL70P7
43. Noordam R, Gunn DA, Tomlin CC, et al. 2013. Leiden Longevity Study Group. High serum glucose levels are associated with a higher perceived age. Age (Dordr). 35: 189-95. Ref.: https://bit.ly/2X2Ctox
44. Yoon HS, Baik SH, Oh CH. 2002. Quantitative measurement of desquamation and skin elasticity in diabetic patients. Skin Res Technol. 8: 50-54. Ref.: https://bit.ly/2KDHYn4
45. Lyons TJ, Bailie KE, Dyer DG, et al. 1991. Decrease in skin collagen glycation with improved glycemic control in patients with insulin-dependent diabetes mellitus. J Clin Invest. 87: 10-15. Ref.: https://bit.ly/2XwjS3r
46. Sami K, Elshahat A, Moussa M, et al. 2015. Image analyzer study of the skin in patients with morbid obesity and massive weight loss. Eplasty.15: 4. Ref.: https://bit.ly/2X7WfyT
47. Kyrou I, Randeva HS, Tsigos C, et al. Clinical Problems Caused by Obesity. [Updated 2018 Jan 11]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. Ref.: https://bit.ly/2RyTIHU
48. Ibuki A, Kuriyama S, Toyosaki Y, et al. 2018. Aging-like physiological changes in the skin of Japanese obese diabetic patients. SAGE Open Med. 6: 62. Ref.: https://bit.ly/2FIq9zh
49. Rzepecki AK, Murase JE, Juran R, et al. 2019. Estrogen-deficient skin: The role of topical therapy. Int J Womens Dermatol. 5: 85-90. Ref.: https://bit.ly/2Y7Shmw
50. Thornton MJ. 2013. Estrogens and aging skin. Dermatoendocrinol. 5: 64-70. Ref.: https://bit.ly/31RBPsJ
51. Raine-Fenning NJ, Brincat MP, Muscat-Baron Y. 2003. Skin aging and menopause: implications for treatment. Am J Clin Dermatol. 4: 71-80. Ref.: https://bit.ly/31VxZ1Y
52. O'Daniel TG. 2011. Multimodal management of atrophic acne scarring in the aging face. Aesthetic Plast Surg. 35: 43-50. Ref.: https://bit.ly/2xfqvZe
53. Dunn JH, Koo J. 2013. Psychological Stress and skin aging: a review of possible mechanisms and potential therapies. Dermatol Online J. 19: 185. Ref.: https://bit.ly/2J2zrXw
54. Maarouf M, Maarouf CL, Yosipovitch G, et al. 2019. The impact of stress on epidermal barrier function: an evidence-based review. Br J Dermatol. Ref.: https://bit.ly/31SBUwl
55. Liu PZ, Nusslock R. How Stress Gets Under the Skin: Early Life Adversity and Glucocorticoid Receptor Epigenetic Regulation. Curr Genomics. 19: 653-664. Ref.: https://bit.ly/2IJQzT3
56. Pie´rard GE, Pie´rard-Franchimont C, Quatresooz P. Chapter 22. Skin Ageprint: The Causative Factors. In: André O. Barel, Marc Paye, Howard I. Maibach. Handbook of Cosmetic Science and Technology, 3rd Edition, published by CRC Press.
57. Umar M, Sastry KS, Chouchane AI. 2018. Role of Vitamin D Beyond the Skeletal Function: A Review of the Molecular and Clinical Studies. Ref.: https://bit.ly/2YbezDT
58. Bartke A. 2019. Growth Hormone and Aging: Updated Review. World J Mens Health. 37: 19-30. Ref.: https://bit.ly/2J6DHVV
59. Ito N, Seki S, Ueda F. 2018. Effects of Composite Supplement Containing Collagen Peptide and Ornithine on Skin Conditions and Plasma IGF-1 Levels-A Randomized, Double-Blind, Placebo-Controlled Trial. Mar Drugs. 16. Ref.: https://bit.ly/2ZKNkAo
60. Carroll PV, Christ ER, Bengtsson BA, et al. 1998. Growth hormone deficiency in adulthood and the effects of growth hormone replacement: a review. Growth Hormone Research Society Scientific Committee. J Clin Endocrinol Metab. 83: 382-95. Ref.: https://bit.ly/2Fy95f3
61. Arlt W. 2004. Dehydroepiandrosterone and ageing. Best Pract Res Clin Endocrinol Metab. 18: 363-380. Ref.: https://bit.ly/2X5I5hY
62. Lee KS, Oh KY, Kim BC. 2000. Effects of dehydroepiandrosterone on collagen and collagenase gene expression by skin fibroblasts in culture. J Dermatol Sci. 23: 103-110. Ref.: https://bit.ly/2XxdM31
63. Calvo E, Luu-The V, Morissette J, et al. 2008. Pangenomic changes induced by DHEA in the skin of postmenopausal women. J Steroid Biochem Mol Biol. 112: 186-93. Ref.: https://bit.ly/2KBF16e
64. Kanda N, Watanabe S. 2005. Regulatory roles of sex hormones in cutaneous biology and immunology. J Dermatol Sci. 38: 1-7. Ref.: https://bit.ly/2KCj1YZ
65. Lephart ED. 2018. A review of the role of estrogen in dermal aging and facial attractiveness in women. J Cosmet Dermatol. 17: 282-288. Ref.: https://bit.ly/31VQS4u
66. Dyer JM, Miller RA. 2018. Chronic Skin Fragility of Aging: Current Concepts in the Pathogenesis, Recognition, and Management of Dermatoporosis. J Clin Aesthet Dermatol. 11: 13-18. Ref.: https://bit.ly/2xc2ylJ
67. Alexis AF, Obioha JO. 2017. Ethnicity and Aging Skin. J Drugs Dermatol. 16: 77-80. Review. Ref.: https://bit.ly/2X6TcH8
68. Vashi NA, Maymone MB, Kundu RV. 2016. Aging Differences in Ethnic Skin. J Clin Aesthet Dermatol. 9: 31-38. Ref.: https://bit.ly/2XpgHuH
69. Chan IL, Cohen S, da Cunha MG, et al. 2019. Characteristics and management of Asian skin. Int J Dermatol. 58: 131-143. Ref.: https://bit.ly/2Jb3mwC
70. Campiche R, Trevisan S, Séroul P, et al. 2019. Appearance of aging signs in differently pigmented facial skin by a novel imaging system. J Cosmet Dermatol. 18: 614-627. Ref.: https://bit.ly/2J33oGZ
71. Alexis AF, Alam M. 2012. Racial and ethnic differences in skin aging: implications for treatment with soft tissue fillers. J Drugs Dermatol. 11: 30-32. Ref.: https://bit.ly/2KBMO46
72. Vierkötter A, Krutmann J. 2012. Environmental influences on skin aging and ethnic-specific manifestations. Dermatoendocrinol. 4: 227-231. Ref.: https://bit.ly/2XEOEHP
73. Sugawara T, Nakagawa N, Shimizu N, et al. 2019. Gender- and age-related differences in facial sebaceous glands in Asian skin, as observed by non-invasive analysis using three-dimensional ultrasound microscopy. Skin Res Technol. 25: 347-354. Ref.: https://bit.ly/31SFXsx
74. Kim BY, Choi JW, Park KC, et al. 2013. Sebum, acne, skin elasticity, and gender difference - which is the major influencing factor for facial pores? Skin Res Technol. 45-53. Ref.: https://tinyurl.com/yxkdalur
75. Rahrovan S, Fanian F, Mehryan P, et al. 2018. Male versus female skin: What dermatologists and cosmeticians should know. Int J Womens Dermatol. 4:122-130. Ref.: https://tinyurl.com/y285lvpt
76. Trojahn C, Dobos G, Lichterfeld A, et al. 2015. Characterizing facial skin ageing in humans: disentangling extrinsic from intrinsic biological phenomena. Biomed Res Int. Ref.: https://tinyurl.com/y2lc9mj4
77. Xiong ZM, O'Donovan M, Sun L, et al. 2017. Anti-Aging Potentials of Methylene Blue for Human Skin Longevity. Sci Rep. 7: 2475. Ref.: https://tinyurl.com/y39e38g4
78. Kruglikov IL, Scherer PE. 2016. Skin aging: are adipocytes the next target? Aging (Albany NY). 8: 57-69. Ref.: https://tinyurl.com/yxuqaw2l
79. Bayerl C. 2016[Skin aging and evidence-based topical strategies]. Hautarzt. 67:140-147. Ref.: https://tinyurl.com/y2cgsx3s
80. Rodan K, Fields K, Majewski G, et al. 2016. Skincare Bootcamp: The Evolving Role of Skincare. Plast Reconstr Surg Glob Open. Ref.: https://bit.ly/2Fy62Ue
81. Singer S, Karrer S, Berneburg M. 2019. Modern sun protection. Curr Opin Pharmacol. 46: 24-28. Ref.: https://bit.ly/2X4tZgG
82. AK Mohiuddin. 2019. Sunscreen and Suntan Preparations. ARC Journal of Pharmaceutical Sciences.
83. Gabros S, Zito PM. 2019. Sunscreens And Photoprotection. [Updated 2019 Jan 13]. In: StatPearls. Ref.: https://bit.ly/2Wvny5c
84. The American Society for Aesthetic Plastic Surgery (ASAPS) The American Society for Aesthetic Plastic Surgery Reports Americans Spent More Than 12 Billion in 2014: Procedures for Men Up 43% Over Five-Year Period. Ref.: https://prn.to/2RGsTlq
85. de Oliveira TC, Rocha SF, Ramos DG, et al. 2017. Effects of Multipolar Radiofrequency and Pulsed Electromagnetic Field Treatment for Face and Neck Rejuvenation. Dermatol Res Pract. Ref.: https://bit.ly/2xflq3a
86. Gold MH. 2015. Noninvasive Skin Tightening Treatment. J Clin Aesthet Dermatol. 8: 14-8. Ref.: https://bit.ly/2NdIgDg
87. Venus Legacy Body Shaping Featured on KTLA 5 News. Ref.: https://bit.ly/2Xd2AJG
88. Lee YB, Eun YS, Lee JH, et al. 2014. Effects of multi-polar radiofrequency and pulsed electromagnetic field treatment in Koreans: case series and survey study. J Dermatolog Treat. 25: 310. Ref.: https://bit.ly/2J91R1U
89. Nassab R. 2015. The evidence behind noninvasive body contouring devices. Aesthet Surg J. 35: 279-293. Ref.: https://bit.ly/2Ng0GTN
90. Bissett DL Chapter 11. Anti-aging Skin Care Formulations. In: Zoe Diana Draelos, Lauren A. Thaman. Cosmetic Formulation of Skin Care Products Cosmetic Science and Technology. Ref.: https://bit.ly/2ZQNo1z
91. Mukherjee S, Date A, Patravale V, et al. 2006. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 1: 327-348. Ref.: https://bit.ly/2X39uvR
92. Zito PM, Mazzoni T. 2019. Acitretin. [Updated 2018 Dec 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Ref.: https://bit.ly/2Yhy7GE
93. Kim MS, Lee S, Rho HS, et al. 2005. The effects of a novel synthetic retinoid, seletinoid G, on the expression of extracellular matrix proteins in aged human skin in vivo. Clin Chim Acta. 362: 161-169. Ref.: https://bit.ly/2X45Qlk
94. Shao Y, He T, Fisher GJ, et al. 2017. Molecular basis of retinol anti-ageing properties in naturally aged human skin in vivo. Int J Cosmet Sci. 39: 56-65. Ref.: https://bit.ly/2KDmoyV
95. Graf J. 2005. Chapter 2. Anti-Aging Skin Care Ingredient. In: Technologies Anti-Aging Medicine As It Relates to Dermatology. In: Cheryl M. Burgess. Cosmetic Dermatology, published by Springer Science & Business Media.
96. Kong R, Cui Y, Fisher GJ, et al. 2016. A comparative study of the effects of retinol and retinoic acid on histological, molecular, and clinical properties of human skin. J Cosmet Dermatol.15: 49-57. Ref.: https://bit.ly/2KF8H2o
97. Bagatin E, Gonçalves HS, Sato M, et al. 2018. Comparable efficacy of adapalene 0.3% gel and tretinoin 0.05% cream as treatment for cutaneous photoaging. Eur J Dermatol. 28: 343-350. Ref.: https://bit.ly/2NdWHY4
98. Fu JJ, Hillebrand GG, Raleigh P, et al. 2010. A randomized, controlled comparative study of the wrinkle reduction benefits of a cosmetic niacinamide/peptide/retinyl propionate product regimen vs. a prescription 0.02% tretinoin product regimen. Br J Dermatol. 162: 647-54. Ref.: https://bit.ly/2XBOm44
99. Kwon HS, Lee JH, Kim GM, et al. 2018. Efficacy and safety of retinaldehyde 0.1% and 0.05% creams used to treat photoaged skin: A randomized double-blind controlled trial. J Cosmet Dermatol. 17: 471-476. Ref.: https://bit.ly/2XAybUP
100. Chaudhuri RK, Bojanowski K. 2014. Bakuchiol: a retinol-like functional compound revealed by gene expression profiling and clinically proven to have anti-aging effects. Int J Cosmet Sci. 36: 221-230. Ref.: https://bit.ly/2Fv4pq7
101. Dhaliwal S, Rybak I, Ellis SR, et al. 2019. Prospective, randomized, double-blind assessment of topical bakuchiol and retinol for facial photoageing. Br J Dermatol. 180: 289-296. Ref.: https://bit.ly/2Yhl2gG
102. Moghimipour E. 2012. Hydroxy Acids, the Most Widely Used Anti-aging Agents. Jundishapur J Nat Pharm Prod. 7: 9-10. Ref.: https://bit.ly/2ZOlZxk
103. Tang SC, Tang LC, Liu CH, et al. 2019. Glycolic acid attenuates UVB-induced aquaporin-3, matrix metalloproteinase-9 expression, and collagen degradation in keratinocytes and mouse skin. Biochem J. 476: 1387-1400. Ref.: https://bit.ly/2J5yT36
104. Tran D, Townley JP, Barnes TM, et al. 2014. An antiaging skin care system containing alpha hydroxy acids and vitamins improves the biomechanical parameters of facial skin. Clin Cosmet Investig Dermatol. 8: 9-17. Ref.: https://bit.ly/2xbSJ7j
105. Sharad J. 2013. Glycolic acid peel therapy - a current review. Clin Cosmet Investig Dermatol. 281-288. Ref.: https://bit.ly/2X82JxF
106. Tang SC, Yang JH. Dual Effects of Alpha-Hydroxy Acids on the Skin. Molecules. 23. Ref.: https://bit.ly/2J7KpLk
107. Smith WP. 1996. Epidermal and dermal effects of topical lactic acid. J Am Acad Dermatol. 388-391. Ref.: https://bit.ly/2ZHVKbE
108. Prestes PS, Oliveira MM, Leonardi GR. 2013. Randomized clinical efficacy of superficial peeling with 85% lactic acid versus 70% glycolic acid. An Bras Dermatol. 88: 900-905. Ref.: https://bit.ly/31QB6rH
109. Kapicio?lu Y, Gül M, Saraç G, et al. 2019. Comparison of Antiaging Effects on Rat Skin of Cog Thread and Poly-L-Lactic Acid Thread. Dermatol Surg. 45: 438-445. Ref.: https://bit.ly/2Lo1IKK
110. Bohnert K, Dorizas A, Lorenc P, et al. 2019. Randomized, Controlled, Multicentered, Double-Blind Investigation of Injectable Poly-L-Lactic Acid for Improving Skin Quality. Dermatol Surg. 45: 718-724. Ref.: https://bit.ly/2X99QpN
111. Algiert-Zieli?ska B, Mucha P, Rotsztejn H. 2019. Lactic and lactobionic acids as typically moisturizing compounds. Int J Dermatol. 58: 374-379. Ref.: https://bit.ly/2Yd0fL6
112. Lew LC, Liong MT. 2013. Bioactives from probiotics for dermal health: functions and benefits. J Appl Microbiol. 114: 1241-53. Ref.: https://bit.ly/2Letyt7
113. Yamamoto Y, Uede K, Yonei N, et al. 2006. Effects of alpha-hydroxy acids on the human skin of Japanese subjects: the rationale for chemical peeling. J Dermatol. 33: 16-22. Ref.: https://bit.ly/2Nempf0
114. Vender RB, Andriessen A, Barankin B, et al. 2019. Cohort Using a Ceramides Containing Cleanser and Cream With Salicylic Acid for Dry, Flaking, and Scaling Skin Conditions. J Drugs Dermatol.18: 80-85. Ref.: https://bit.ly/2ZL2zJx
115. Kantikosum K, Chongpison Y, Chottawornsak N, et al. 2019. The efficacy of glycolic acid, salicylic acid, gluconolactone, and licochalcone A combined with 0.1% adapalene vs adapalene monotherapy in mild-to-moderate acne vulgaris: a double-blinded within-person comparative study. Clin Cosmet Investig Dermatol.12: 151-161. Ref.: https://bit.ly/2IMFvEQ
116. Shamalnasab M, Gravel SP, St-Pierre J, et al. 2018. A salicylic acid derivative extends the lifespan of Caenorhabditis elegans by activating autophagy and the mitochondrial unfolded protein response. Ref.: https://bit.ly/2KG4Xh5
117. Zheng Y, Yin S, Xia Y, et al. 2019. Efficacy and safety of 2% supramolecular salicylic acid compared with 5% benzoyl peroxide/0.1% adapalene in the acne treatment: a randomized, split-face, open-label, single-center study. Cutan Ocul Toxicol. 38: 48-54. Ref.: https://bit.ly/2FwMOOG
118. Understanding Skin Care Products. Ref.: https://wb.md/2KCzkFt
119. Papakonstantinou E, Roth M, Karakiulakis G. 2012. Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinol. 4: 253-258. Ref.: https://bit.ly/2xdnbOe
120. Sparavigna A, Tenconi B, Giori AM, et al. 2019. Evaluation of the efficacy of a new hyaluronic acid gel on dynamic and static wrinkles in volunteers with moderate aging/photoaging. Clin Cosmet Investig Dermatol. 12: 81-90. Ref.: https://bit.ly/2KDCAjK
121. Lee YJ, Kim HT, Lee WJ, et al. 2019. Anti-aging and hydration efficacy of a cross-linked hyaluronic acid microstructure patch. Dermatol Ther. 88. Ref.: https://bit.ly/2FxMbVk
122. Jeon YJ, Kim YH, Jeon YJ, et al. 2019. Increased synthesis of hyaluronic acid by enhanced penetration of CTP-EGF recombinant in human keratinocytes. J Cosmet Dermatol. Ref.: https://bit.ly/2IJa0v7
123. Walker K, Zito PM. Hyaluronic Acid. [Updated 2019 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Ref.: https://bit.ly/2X8hO2d
124. Skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int J Biol Macromol. 120: 1682-1695. Ref.: https://bit.ly/2NefF0o
125. Garre A, Narda M, Valderas-Martinez P, et al. 2018. Antiaging effects of a novel facial serum containing L-Ascorbic acid, proteoglycans, and proteoglycan-stimulating tripeptide: ex vivo skin explant studies and in vivo clinical studies in women. Clin Cosmet Investig Dermatol. 11: 253-263. Ref.: https://bit.ly/31QDMFL
126. Zasada M, Markiewicz A, Dro?d? Z, et al. 2019. Preliminary randomized controlled trial of antiaging effects of l-ascorbic acid applied in combination with no-needle and microneedle mesotherapy. J Cosmet Dermatol. 18: 843-849. Ref.: https://bit.ly/2RBYgxy
127. Wang SF, Liu X, Ding MY, et al. 2019. 2-O-β-d-glucopyranosyl-(l)-ascorbic acid, a novel vitamin C derivative from Lycium barbarum, prevents oxidative stress. Redox Biol. 24: 173. Ref.: https://bit.ly/2Lhq0X1
128. G?gotek A, Ambro?ewicz E, Jastrz?b A, et al. 2019. Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation. Arch Dermatol Res. 311: 203-219. Ref.: https://bit.ly/2ZL7slV
129. Abdullah M, Attia FN. 2019. Vitamin C (Ascorbic Acid). Ref.: https://bit.ly/2IK86KB
130. Pullar JM, Carr AC, Vissers MCM. 2017. The Roles of Vitamin C in Skin Health. Nutrients. 9. Ref.: https://bit.ly/2Fz5E7O
131. Crisan D, Roman I, Crisan M, et al. 2015. The role of vitamin C in pushing back the boundaries of skin aging: an ultrasonographic approach. Clin Cosmet Investig Dermatol. 8: 463-470. Ref.: https://bit.ly/2J4YYiR
132. Zhai H, Behnam S, Villarama CD, et al. 2005. Evaluation of the antioxidant capacity and preventive effects of a topical emulsion and its vehicle control on the skin response to UV exposure. Skin Pharmacol Physiol.18: 288-293. Ref.: https://bit.ly/2X7Vwco
133. Keen MA, Hassan I. Vitamin E in dermatology. Indian Dermatol Online J. 7: 311-315. Ref.: https://bit.ly/2LhrRer
134. Traber MG, Stevens JF. 2011. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 51: 10-13. Ref.: https://bit.ly/2WZKx4E
135. Cho S. The Role of Functional Foods in Cutaneous Anti-aging. J Lifestyle Med. 4: 8-16. Ref.: https://bit.ly/2FwE8rK
136. Rahman S, Bhatia K, Khan AQ, et al. 2008. Topically applied vitamin E prevents massive cutaneous inflammatory and oxidative stress responses induced by double application of 12-O-tetradecanoylphorbol-13-acetate (TPA) in mice. Chem Biol Interact.172: 195-205. Ref.: https://bit.ly/2LkRlrd
137. Thiele JJ, Hsieh SN, Ekanayake-Mudiyanselage S. 2005. Vitamin E: critical review of its current use in cosmetic and clinical dermatology. Dermatol Surg. 31: 805-813. Ref.: https://bit.ly/2FxZkh5
138. Farris P, Yatskayer M, Chen N, et al. 2014. Evaluation of efficacy and tolerance of a nighttime topical antioxidant containing resveratrol, baicalin, and vitamin e for treatment of mild to moderately photodamaged skin. J Drugs Dermatol. 13: 67-72. Ref.: https://bit.ly/2FAf9nj
139. Wang K, Jiang H, Li W, et al. 2018. Role of Vitamin C in Skin Diseases. Front Physiol. 9: 819. Ref.: https://bit.ly/2YhrWm6
140. Burns EM, Tober KL, Riggenbach JA, et al. 2013. Differential effects of topical vitamin E and C E Ferulic® treatments on ultraviolet light B-induced cutaneous tumor development in Skh-1 mice. PLoS One. 8: 63809. Ref.: https://bit.ly/2XxlHNM
141. Watson RE, Long SP, Bowden JJ, et al. 2008. Repair of photoaged dermal matrix by topical application of a cosmetic 'antiageing' product. Br J Dermatol. 158: 472-477. Ref.: https://bit.ly/2NoMA2K
142. Knott A, Achterberg V, Smuda C, et al. 2015. Topical treatment with coenzyme Q10-containing formulas improves skin's Q10 level and provides antioxidative effects. Biofactors. 41: 383-390. Ref.: https://bit.ly/2X6fNne
143. Yue Y, Zhou H, Liu G, et al. 2010. The advantages of a novel CoQ10 delivery system in skin photo protection. Int J Pharm. 392: 57-63. Ref.: https://bit.ly/2xbSIA7
144. Passi S, De Pità O, Grandinetti M, et al. 2003. The combined use of oral and topical lipophilic antioxidants increases their levels both in sebum and stratum corneum. Biofactors. 18: 289-297. Ref.: https://bit.ly/2RzDyhL
145. El-Leithy ES, Makky AM, Khattab AM, et al. 2018. Optimization of nutraceutical coenzyme Q10 nanoemulsion with improved skin permeability and anti-wrinkle efficiency. Drug Dev Ind Pharm. 44: 316-328. Ref.: https://bit.ly/2RD4ixD
146. Choi BS, Song HS, Kim HR, et al. 2009. Effect of coenzyme Q10 on cutaneous healing in skin-incised mice. Arch Pharm Res.32: 907-913. Ref.: https://bit.ly/2X9m2aa
147. Žmitek K, Poga?nik T, Mervic L, et al. 2017. The effect of dietary intake of coenzyme Q10 on skin parameters and condition: Results of a randomised, placebo-controlled, double-blind study. Biofactors. 43:132-140. Ref.: https://bit.ly/2LkHCBp
148. Zhao Q, Ma YM, Jing L, et al. 2019. Coenzyme Q10 Protects Astrocytes from Ultraviolet B-Induced Damage Through Inhibition of ERK 1/2 Pathway Overexpression. Neurochem Res. Ref.: https://bit.ly/2KB0TyI
149. Barcelos IP, Haas RH. 2019. CoQ10 and Aging. Biology (Basel). 8. Ref.: https://bit.ly/2Nf7CAv
150. Kubota Y, Musashi M, Nagasawa T, et al. 2019. Novel nanocapsule of α-lipoic acid reveals pigmentation improvement: α-Lipoic acid stimulates the proliferation and differentiation of keratinocyte in murine skin by topical application. Exp Dermatol. 28: 55-63. Ref.: https://bit.ly/2Xr0XHB
151. Sherif S, Bendas ER, Badawy S. The clinical efficacy of cosmeceutical application of liquid crystalline nanostructured dispersions of alpha lipoic acid as anti-wrinkle. Eur J Pharm Biopharm. 86: 251-259. Ref.: https://bit.ly/2RBaHJC
152. Lin JY, Lin FH, Burch JA, et al. 2004. Alpha-lipoic acid is ineffective as a topical antioxidant for photoprotection of skin. J Invest Dermatol. 123: 996-998. Ref.: https://bit.ly/2ZOrVGl
153. El-Komy M, Shalaby S, Hegazy R, et al. 2017. Assessment of cubosomal alpha lipoic acid gel efficacy for the aging face: a single-blinded, placebo-controlled, right-left comparative clinical study. J Cosmet Dermatol. 16: 358-363. Ref.: https://bit.ly/2KDt4wX
154. Kim GD, Kim TH, Jang AH, et al. 2011. α-Lipoic acid suppresses the development of DNFB-induced atopic dermatitis-like symptoms in NC/Nga mice. Exp Dermatol. 20: 97-101. Ref.: https://bit.ly/2xgVKmV
155. Isaac VL, Chiari-Andréo BG, Marto JM, et al. 2015. Rheology as a Tool to Predict the Release of Alpha-Lipoic Acid from Emulsions Used for the Prevention of Skin Aging. Biomed Res Int. 2015: 656. Ref.: https://bit.ly/2IOOAwN
156. Majtan J, Jesenak M. 2018. β-Glucans: Multi-Functional Modulator of Wound Healing. Molecules. 23. Ref.: https://bit.ly/2RDdDFR
157. Vetvicka V, Vannucci L, Sima P, et al. 2019. Beta Glucan: Supplement or Grug? From Laboratory to Clinical Trials. Molecules. 24. Ref.: https://bit.ly/2Fzw6hB
158. Hong YH, Lee HS, Jung EY, et al. 2017. Photoprotective effects of topical ginseng leaf extract using Ultraflo L against UVB-induced skin damage in hairless mice. J Ginseng Res. 41: 456-462. Ref.: https://bit.ly/2KD9mS9
159. Bacha U, Nasir M, Iqbal S, et al. 2017. Nutraceutical, Anti-Inflammatory, and Immune Modulatory Effects of β-Glucan Isolated from Yeast. Biomed Res Int. Ref.: https://bit.ly/2YdCnH5
160. Jesenak M, Urbancek S, Majtan J, et al. 2016. β-Glucan-based cream (containing pleuran isolated from pleurotus ostreatus) in supportive treatment of mild-to-moderate atopic dermatitis. J Dermatolog Treat. 27: 351-354. Ref.: https://bit.ly/31YLjCL
161. Bashir KMI, Choi JS. Clinical and Physiological Perspectives of β-Glucans: The Past, Present, and Future. Int J Mol Sci. 18. Ref.: https://bit.ly/2JdvhMq
162. Wang Y, Viennet C, Jeudy A, et al. 2018. Assessment of the efficacy of a new complex antisensitive skin cream. J Cosmet Dermatol. 17: 101-107. Ref.: https://bit.ly/2NgeuO9
163. Choi FD, Sung CT, Juhasz ML, et al. 2019. Oral Collagen Supplementation: A Systematic Review of Dermatological Applications. J Drugs Dermatol. 18: 9-16. Ref.: https://bit.ly/1MDB5tJ
164. Maia Campos PMBG, Melo MO, Siqueira César FC. 2019. Topical application and oral supplementation of peptides in the improvement of skin viscoelasticity and density. J Cosmet Dermatol. Ref.: https://bit.ly/31TmsQJ
165. Proksch E, Segger D, Degwert J, et al. 2014. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 27: 47-55. Ref.: https://bit.ly/2kcjd22
166. Pyun HB, Kim M, Park J, et al. 2012. Effects of Collagen Tripeptide Supplement on Photoaging and Epidermal Skin Barrier in UVB-exposed Hairless Mice. Prev Nutr Food Sci.17: 245-253. Ref.: https://bit.ly/2XdpLmM
167. Kim DU, Chung HC, Choi J, et al. 2018. Oral Intake of Low-Molecular-Weight Collagen Peptide Improves Hydration, Elasticity, and Wrinkling in Human Skin: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 10. Ref.: https://bit.ly/2ZQt0O4
168. Czajka A, Kania EM, Genovese L, et al. 2018. Daily oral supplementation with collagen peptides combined with vitamins and other bioactive compounds improves skin elasticity and has a beneficial effect on joint and general wellbeing. Nutr Res. 57: 97-108. Ref.: https://bit.ly/2XzTw0y
169. Lee HJ, Jang HL, Ahn DK, et al. 2019. Orally administered collagen peptide protects against UVB-induced skin aging through the absorption of dipeptide forms, Gly-Pro and Pro-Hyp. Biosci Biotechnol Biochem. 83: 146-156. Ref.: https://bit.ly/2JfPzFo
170. Addor FAS, Cotta Vieira J, Abreu Melo CS. 2018. Improvement of dermal parameters in aged skin after oral use of a nutrient supplement. Clin Cosmet Investig Dermatol. 11: 195-201. Ref.: https://bit.ly/2YcVGAm
171. Zague V, do Amaral JB, Rezende Teixeira P, et al. 2018. Collagen peptides modulate the metabolism of extracellular matrix by human dermal fibroblasts derived from sun-protected and sun-exposed body sites. Cell Biol Int. 42: 95-104. Ref.: https://bit.ly/2IRJj7K
172. Song H, Meng M, Cheng X, et al. 2017. The effect of collagen hydrolysates from silver carp (Hypophthalmichthys molitrix) skin on UV-induced photoaging in mice: molecular weight affects skin repair. Food Funct. 8: 1538-1546. Ref.: https://rsc.li/2X2Su9b
173. Vollmer DL, West VA, Lephart ED. 2018. Enhancing Skin Health: By Oral Administration of Natural Compounds and Minerals with Implications to the Dermal Microbiome. Int J Mol Sci. 19. Ref.: https://bit.ly/2FBOeHD
174. Kimoto-Nira H. New lactic acid bacteria for skin health via oral intake of heat-killed or live cells. Anim Sci J. 89: 835-842. Ref.: https://bit.ly/2ZPvkF0
175. Fabbrocini G, Bertona M, Picazo Ó, et al. 2016. Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signalling and improves adult acne. Benef Microbes. 7: 625-630. Ref.: https://bit.ly/2FBQi2l
176. Isawa K, Noma T, Yamamoto M et al. 2008. Verifying the ability of yogurt prepared with LB81 lactic acid bacteria to improve skin function. J Int Microbiol. 22: 1-5. Ref.: https://bit.ly/2X9oIo5
177. Mori N, Kano M, Masuoka N, et al. 2016. Effect of probiotic and prebiotic fermented milk on skin and intestinal conditions in healthy young female students. Biosci Microbiota Food Health. 35: 105-112. Ref.: https://bit.ly/2Jb9PYv
178. Spada F, Barnes TM, Greive KA. 2018. Skin hydration is significantly increased by a cream formulated to mimic the skin's own natural moisturizing systems. Clin Cosmet Investig Dermatol. 491-497. Ref.: https://bit.ly/2FvJFPh
179. Di Marzio L, Cinque B, Cupelli F, et al. 2008. Increase of skin-ceramide levels in aged subjects following a short-term topical application of bacterial sphingomyelinase from Streptococcus thermophilus. Int J Immunopathol Pharmacol. 21: 137-143. Ref.: https://bit.ly/2xg7plP
180. Mutanu Jungersted J, Hellgren LI, Høgh JK, et al. 2010. Ceramides and barrier function in healthy skin. Acta Derm Venereol. 90: 350-353. Ref.: https://bit.ly/2KF8w7q
181. Jensen JM, Förl M, Winoto-Morbach S, et al. 2005. Acid and neutral sphingomyelinase, ceramide synthase, and acid ceramidase activities in cutaneous aging. Exp Dermatol. 14: 609-618. Ref.: https://bit.ly/2X4KjZZ
182. Vozella V, Basit A, Piras F, et al. 2019. Elevated plasma ceramide levels in post-menopausal women: a cross-sectional study. Aging (Albany NY). 11: 73-88. Ref.: https://bit.ly/2X4iBwn
183. Draelos ZD, Raymond I. 2018. The Efficacy of a Ceramide-based Cream in Mild-to-moderate Atopic Dermatitis. J Clin Aesthet Dermatol. 11: 30-32. Ref.: https://bit.ly/2KIwZbN
184. Yazdanparast T, Nasrollahi SA, Firouzabadi LI, et al. 2018. A Phase II Trial to Assess the Safety and Efficacy of a Topical Repair Cream Containing Skin-identical Ceramide Complex in Patients with Contact Dermatitis. J Clin Aesthet Dermatol. 11: 40-44. Ref.: https://bit.ly/2FBhpL7
185. Zeichner JA, Del Rosso JQ. 2016. Multivesicular Emulsion Ceramide-containing Moisturizers: An Evaluation of Their Role in the Management of Common Skin Disorders. J Clin Aesthet Dermatol. 9: 26-32. Ref.: https://bit.ly/2X27unO
186. Zhang Q, Flach CR, Mendelsohn R, et al. 2015. Topically applied ceramide accumulates in skin glyphs. Clin Cosmet Investig Dermatol. 8: 329-337. Ref.: https://bit.ly/2Yp7f7T
187. Soma Y, Kashima M, Imaizumi A, et al. 2005. Moisturizing effects of topical nicotinamide on atopic dry skin. Int J Dermatol. 44: 197-202. Ref.: https://bit.ly/2Xa63IJ
188. Ashkani Esfahani S, Khoshneviszadeh M, Namazi MR, et al. 2015. Topical Nicotinamide Improves Tissue Regeneration in Excisional Full-Thickness Skin Wounds: A Stereological and Pathological Study. Trauma Mon. 20: 193. Ref.: https://bit.ly/2Xcvw4q
189. Shalita AR, Smith JG, Parish LC, et al. 1995. Topical nicotinamide compared with clindamycin gel in the treatment of inflammatory acne vulgaris. Int J Dermatol. 34: 434-437. Ref.: https://bit.ly/2X7P7h8
190. Navarrete-Solís J, Castanedo-Cázares JP, Torres-Álvarez B, et al. 2011. A Double-Blind, Randomized Clinical Trial of Niacinamide 4% versus Hydroquinone 4% in the Treatment of Melasma. Dermatol Res Pract. Ref.: https://bit.ly/2RCLrCU
191. Gehring W. 2004. Nicotinic acid/niacinamide and the skin. J Cosmet Dermatol. 3: 88-93. Ref.: https://bit.ly/2YmG6T2
192. Levin J, Momin SB. 2010. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 3: 22-41. Ref.: https://bit.ly/2xhHavq
193. Oblong JE, Bissett DL, Ritter JL, et al. 2002. Effect of niacinamide on collagen synthesis and markers of keratinocyte differentiation. Presented at: The 60th Annual Meeting of the American Academy of Dermatology. Ref.: https://bit.ly/2XCCb7h
194. Kawada A, Konishi N, Momma T, et al. 2009. Evaluation of anti-wrinkle effects of a novel cosmetic containing retinol using the guideline of the Japan Cosmetic Industry Association. J Dermatol. 36: 583-586. Ref.: https://bit.ly/2xd8drG
195. Khodaeiani E, Fouladi RF, Amirnia M, et al. 2013. Topical 4% nicotinamide vs. 1% clindamycin in moderate inflammatory acne vulgaris. Int J Dermatol. 52: 999-1004. Ref.: https://bit.ly/2XHAJkg
196. Eren B, Tuncay Tanr?verdi S, Ayd?n Köse F, et al. 2019. Antioxidant properties evaluation of topical astaxanthin formulations as anti-aging products. J Cosmet Dermatol. 18: 242-250. Ref.: https://bit.ly/2XeCVjr
197. Davinelli S, Nielsen ME, Scapagnini G. 2018. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients. 10. Ref.: https://bit.ly/2NgD0yH
198. Eren B, Tuncay Tanr?verdi S, Ayd?n Köse F, et al. 2019. Antioxidant properties evaluation of topical astaxanthin formulations as anti-aging products. J Cosmet Dermatol. 18: 242-250. Ref.: https://bit.ly/2XDQwAu
199. Fang Q, Guo S, Zhou H, et al. 2017. Astaxanthin protects against early burn-wound progression in rats by attenuating oxidative stress-induced inflammation and mitochondria-related apoptosis. Sci Rep. 7: 440. Ref.: https://go.nature.com/2YirUdv
200. Davinelli S, Nielsen ME, Scapagnini G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients. 10. Ref.: https://bit.ly/2NgD0yH
201. Suganuma K, Nakajima H, Ohtsuki M, et al. 2010. Astaxanthin attenuates the UVA-induced up-regulation of matrix-metalloproteinase-1 and skin fibroblast elastase in human dermal fibroblasts. J Dermatol Sci. 58: 136-42. Ref.: https://bit.ly/2X9LhUT
202. Chou HY, Lee C, Pan JL, et al. 2016. Enriched Astaxanthin Extract from Haematococcus pluvialis Augments Growth Factor Secretions to Increase Cell Proliferation and Induces MMP1 Degradation to Enhance Collagen Production in Human Dermal Fibroblasts. Int J Mol Sci. 17. Ref.: https://bit.ly/2XerOXW
203. Meephansan J, Rungjang A, Yingmema W, et al. 2017. Effect of astaxanthin on cutaneous wound healing. Clin Cosmet Investig Dermatol. 10: 259-265. Ref.: https://bit.ly/2xgd2AF
204. Camera E, Mastrofrancesco A, Fabbri C, et al. 2009. Astaxanthin, canthaxanthin and beta-carotene differently affect UVA-induced oxidative damage and expression of oxidative stress-responsive enzymes. Exp Dermatol. 18: 22-31. Ref.: https://bit.ly/2ITzcPv
205. Chew BP, Mathison BD, Hayek MG, et al. 2011. Dietary astaxanthin enhances immune response in dogs. Vet Immunol Immunopathol. 140: 199-206. Ref.: https://bit.ly/2LoTd2g
206. Park JS, Mathison BD, Hayek MG, et al. 2011. Astaxanthin stimulates cell-mediated and humoral immune responses in cats. Vet Immunol Immunopathol. 144: 455-61. Ref.: https://bit.ly/2FDC1lU
207. Santocono M, Zurria M, Berrettini M, et al. 2006. Influence of astaxanthin, zeaxanthin and lutein on DNA damage and repair in UVA-irradiated cells. J Photochem Photobiol B. 85: 205-215. Ref.: https://bit.ly/2XBIh7L
208. Camera E, Mastrofrancesco A, Fabbri C, et al. 2009. Astaxanthin, canthaxanthin and beta-carotene differently affect UVA-induced oxidative damage and expression of oxidative stress-responsive enzymes. Exp Dermatol. 18: 222-231. Ref.: https://bit.ly/2ITzcPv
209. Tominaga K, Hongo N, Fujishita M, et al. 2017. Protective effects of astaxanthin on skin deterioration. J Clin Biochem Nutr. 61: 33-39. Ref.: https://bit.ly/2NfNVIS
210. Kim SH, Kim H. 2018. Inhibitory Effect of Astaxanthin on Oxidative Stress-Induced Mitochondrial Dysfunction-A Mini-Review. Nutrients. 10. Ref.: https://bit.ly/31R6C90
211. Hornsby PJ. 2007. Telomerase and the aging process. Exp Gerontol. 42: 575-581. Ref.: https://bit.ly/2XeHL05
212. Anti-Aging Skin Care Benefits of Colostrum. Ref.: https://bit.ly/31XgmyB
213. Cabrera ÁJ. 2015. Zinc, aging, and immunosenescence: an overview. Pathobiol Aging Age Relat Dis. 5: 92. Ref.: https://bit.ly/2Xazgyu
214. Ogawa Y, Kinoshita M, Shimada S, et al. 2018. Zinc and Skin Disorders. Nutrients. 10. Ref.: https://bit.ly/2Xf3eq1
215. Gupta M, Mahajan VK, Mehta KS, et al. 2014. Zinc therapy in dermatology: a review. Dermatol Res Pract. Ref.: https://bit.ly/2jrQO7V
216. Cai Z, Zhang J, Li H. 2018. Selenium, aging and aging-related diseases. Aging Clin Exp Res. Ref.: https://bit.ly/2ZQRzuj
217. Favrot C, Beal D, Blouin E, et al. 2018. Age-Dependent Protective Effect of Selenium against UVA Irradiation in Primary Human Keratinocytes and the Associated DNA Repair Signature. Oxid Med Cell Longev. Ref.: https://bit.ly/2xk5E7o
218. Jobeili L, Rousselle P, Béal D, et al. 2017. Selenium preserves keratinocyte stemness and delays senescence by maintaining epidermal adhesion. Aging (Albany NY). 9: 2302-2315. Ref.: https://bit.ly/2IUSHao
219. Wang N, Tan HY, Li S, et al. 2017. Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant. Oxid Med Cell Longev. Ref.: https://bit.ly/2EYxyLv
220. Palm MD, Woodhall KE, Butterwick KJ, et al. 2010. Cosmetic use of poly-l-lactic acid: a retrospective study of 130 patients. Dermatol Surg. 36: 161-70. Ref.: https://bit.ly/2X7PLeL
221. Jabbar A, Arruda S, Sadick N. 2017. Off Face Usage of Poly-L-Lactic Acid for Body Rejuvenation. J Drugs Dermatol. 16: 489-494. Ref.: https://bit.ly/2Yseony
222. Sickles CK, Gross GP. 2019. Poly-L-Lactic Acid. In: StatPearls. Treasure Island. Ref.: https://bit.ly/2Jcapp2
223. Kim CM, Kim BY, Hye Suh D, et al. 2019. The efficacy of powdered polydioxanone in terms of collagen production compared with poly-L-lactic acid in a murine model. J Cosmet Dermatol. Ref.: https://bit.ly/2FDyjc7
224. de Jager MW, Gooris GS, Dolbnya IP, et al. 2004. Novel lipid mixtures based on synthetic ceramides reproduce the unique stratum corneum lipid organization. J Lipid Res. 923-932. Ref.: https://bit.ly/2XCDoMP
225. Kim M, Park HJ. 2016. Molecular Mechanisms of Skin Aging and Rejuvenation. Intech Open. Ref.: https://bit.ly/2JnHAam
226. Goenka S. 2017. Monica Belluci’s Makeup, Beauty And Fitness Secrets Revealed. STYLECRAZE. Ref.: https://bit.ly/2JnIUdk
227. Monica Bellucci Beauty Secrets. Ref.: https://bit.ly/2S5wjhS
228. Zahr AS, Kononov T, Sensing W, et al. 2019. An open-label, single-site study to evaluate the tolerability, safety, and efficacy of using a novel facial moisturizer for preparation and accelerated healing pre and post a single full-face radiofrequency microneedling treatment. J Cosmet Dermatol.18: 94-106. Ref.: https://bit.ly/2G1YlWK
229. Portugal-Cohen M, Oron M, Cohen D, Ma'or Z. 2017. Antipollution skin protection - a new paradigm and its demonstration on two active compounds. Clin Cosmet Investig Dermatol.10:185-193. Ref.: https://bit.ly/2JuoApw
230. Mistry N. 2017. Guidelines for Formulating Anti-Pollution Products. Cosmetics. 4:57. Ref.: https://bit.ly/2XNsbIz
231. Fernández JR, Webb C, Rouzard K, et al. 2018. SIG-1273 protects skin against urban air pollution and when formulated in AgeIQ™ Night Cream anti-aging benefits clinically demonstrated. J Cosmet Dermatol. Ref.: https://bit.ly/2XRP6Cu
232. Addor FAS. 2019. Topical effects of SCA(®) (Cryptomphalus aspersa secretion) associated with regenerative and antioxidant ingredients on aged skin: evaluation by confocal and clinical microscopy. Clin Cosmet Investig Dermatol. 12:133-140. Ref.: https://bit.ly/2YEjugU
233. Narda M, Bauza G, Valderas P, et al. 2018. Protective effects of a novel facial cream against environmental pollution: in vivo and in vitro assessment. Clin Cosmet Investig Dermatol. 11: 571-578. Ref.: https://bit.ly/2YCMLbH
234. Giacomelli L, Togni S, Meneghin M, et al. 2018. In vivo validation of the multicomponent powder (Vitachelox(®)) against the deposition of polluting ions. Clin Cosmet Investig Dermatol. 11: 109-113. Ref.: https://bit.ly/2XRK4WI
235. Borda LJ, Wong LL, Tosti A. 2019. Bioidentical hormone therapy in menopause: relevance in dermatology. Dermatol Online J. Ref.: https://bit.ly/2XzHJeJ
236. Piérard GE, Humbert P, Berardesca E, et al. Revisiting the cutaneous impact of oral hormone replacement therapy. Biomed Res Int. Ref.: https://bit.ly/2LaBzzW
237. Samaras N, Papadopoulou MA, Samaras D, et al. 2014. Off-label use of hormones as an antiaging strategy: a review. Clin Interv Aging. 9:1175-86. Ref.: https://bit.ly/2G6h3MS
238. Vinogradova Y, Coupland C, Hippisley-Cox J. 2019. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. Ref.: https://bit.ly/2XQBpnb
239. Jospe N, Orlowski CC, Furlanetto RW. 1995. Comparison of transdermal and oral estrogen therapy in girls with Turner's syndrome. J Pediatr Endocrinol Metab. Ref.: https://bit.ly/2XBkIbk
240. Warren MP, Shu AR, Dominguez JE. 2000. Menopause and Hormone Replacement. Ref.: https://bit.ly/2XQCbR7
241. Sassarini J, Lumsden MA. 2015. Oestrogen replacement in postmenopausal women, Age and Ageing. 44: 551-558. Ref.: https://bit.ly/2G3pHeK
242. Botelho MA, Queiroz DB, Barros G, et al. 2014. Nanostructured transdermal hormone replacement therapy for relieving menopausal symptoms: a confocal Raman spectroscopy study. Clinics (Sao Paulo). 69: 75-82. Ref.: https://bit.ly/2XYpdAO
243. Kopper NW, Gudeman J, Thompson DJ. 2009. Transdermal hormone therapy in postmenopausal women: a review of metabolic effects and drug delivery technologies. Drug Des Devel Ther. 2:193-202. Ref.: https://bit.ly/2G1WkcY
244. Abdi F, Darooneh T, Ghorbani M, et al. 2017. Transdermal hormone replacement therapy with nanostructured medicines. Ginekol Pol. 88:103-108. Ref.: https://bit.ly/2XwfVrG
245. Posição da FEBRASGO sobre o Tratamento de "Modulação Hormonal" para o Antienvelhecimento. Febrasgo, 05 October 2018.
246. Huizen J. 2019. Which skin conditions are linked to type 2 diabetes? Medical News Today. Ref.: https://bit.ly/2LLlDDB




















