Indexing & Abstracting
Full Text
Case ReportDOI Number : 10.36811/ijcgh.2020.110003Article Views : 39Article Downloads : 37
Entamoeba Histolytica Cohabiting Colonic carcinoma -A rare case report
Mukesh Kalla1, Komal Kalla2, Menka Kapil3,*, Pankaj Shrimal4, Aman Manocha5, Alok Verma6 and Ganesh Agarwal7
1Chief Consultant Gastroenterologist, SR Kalla Memorial Gastro and General Hospital, Jaipur, India
2Cheif Consultant Histopathologist, SR Kalla Memorial Gastro and General Hospital, Jaipur, India
3Consultant Pathologist, SR Kalla Memorial Gastro and General Hospital, Jaipur, India
4Consultant Gastroenterologist, SR Kalla Memorial Gastro and General Hospital, Jaipur, India
5Consultant Radiologist, SR Kalla Memorial General Hospital, Jaipur, India
6Consultant Anesthesiologist, SR Kalla Memorial Gastro and General Hospital, Jaipur, India
7Gastrosurgeon, SR Kalla Memorial Gastro and General Hospital, Jaipur, India
*Corresponding Author: Dr. Menka Kapil, Consultant Pathologist, SR Kalla Memorial Gastro and General Hospital, India, Email: drmenkapath@yahoo.com
Article Information
Aritcle Type: Case Report
Citation: Mukesh Kalla, Komal Kalla, Menka Kapil, et al. 2020. Entamoeba Histolytica Cohabiting Colonic carcinoma -A rare case report. Int J Clin Gastro Hepato. 2: 27-32.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2020; Mukesh Kalla
Publication history:
Received date: 06 November, 2020Accepted date: 20 November, 2020
Published date: 23 November, 2020
Abstract
Endameba histolytic a of the gastrointestinal tract is common in developing countries. There are many cases where colonic amebiasis can mimic colonic carcinoma but amebiasis and coexisting carcinoma is exceedingly rare. We present a case of 43 years old male who presented with abdominal pain, generalized weakness and bleeding per rectum. Endoscopic examination showed superficial ulceration with edematous mucosa in proximal, transverse and ascending colon which was indistinguishable from ulceration and amebomas. Histopathological examination of tissue revealed the coexistence of Endameba trophozoites with adenocarcinoma of colon. The organism was demonstrated by Periodic Acid Schiff’s stain with engulfed red blood cell. Our report is to enhance the awareness that amebiasis is may not only mimic carcinoma but can also coexist with carcinoma. After extensive search of literature, our isolated case report might be under tenth of reported cases.
Keywords: Amoebiasis; Endameba histolytic; Carcinoma
Introduction
Amebiasis is caused by the infection of Endameba histolytic, it is an unicellular organism and is the pathogenic protozoan from the family Entamoebidae. It is one of the most common fatal parasitic in gestation across the world and is acquired by the ingestion of contaminated food and water. It is endemic in developing countries with poor sanitation. Endameba histolytic remains one of the leading parasite causing morbidity worldwide. Amoebiasis in humans may either be intestinal or parenteral. Intestinal amoebiasis which is the most common is manifested by diarrhea, abdominal pain, flatulence. In parenteral route amoeba enters through the blood from the intestine to other organs of the body such as the liver, lungs, brain, where it can form abscessed. The clinical symptoms of intestinal amoebiasis range from asymptomatic carrier to severe fulminant necrotizing colitis with bleeding and perforation. The clinical presentation and the endoscopic appearance of colonic amebiasis can mimic colonic carcinoma. Here we present a rare case of colonic amoebiasis with coexistence of mucinous adeno carcinoma of colon, diagnosed on histological examination.
Case Report
A 43 years old male who was admitted to our hospital with complaints of abdominal pain, generalized weakness, bleeding per rectum and malfeasance five days. He had similar past history of pain abdomen two years back for which he was hospitalized to some other hospital where he was investigated with endoscopic finding of thickening of colonic mucosa and had histopathological diagnosis of non specific colitis, he also had colonic abscess which was drained and showed inflammatory exudate on cytology smear. He had also taken antitubercular treatment for two months previously but when he showed no improvement in his general well being and again developed severe abdominal pain. He was referred to our hospital, where we investigated him thoroughly. His complete blood investigation showed hemoglobin-6.7gm%, WBC-6.7thousand/comm., platelets- 5,00,000/comm., MCV -72 fl. Peripheral blood picture was microcytic hypochromic with no schistocytes or fragmented cells which exclude hemolytic anemia. WBC and Platelets were normal. No hem parasites were seen. No immature cells were seen and thus ruled out any hematological malignancy. Biochemical investigations were SGOT- 10.6 u/l, SGPT-14.0 u/l, protein- 6.4 g/dl, albumin- 3.3 g/dl, globulin- 3.1 g/dl, alkaline phosphates- 97.1 u/i, bilirubin- 0.21 mg/dl, direct bilirubin- 0.08 mg/dl, iron- 10.2 ug/dl, serum vitamin B12- 61.14 pg/ml, folic acid - 18.18 ng/ml. The endoscopic findings were superficial ulceration with edematous mucosa in proximal transverse colon and ascending colon. The ulcerated lesions were found to be atypical of crowns disease with differential diagnosis of tuberculosis, carcinoma and lymphoma. Multiple endoscopic biopsies were done and submitted for histopathology.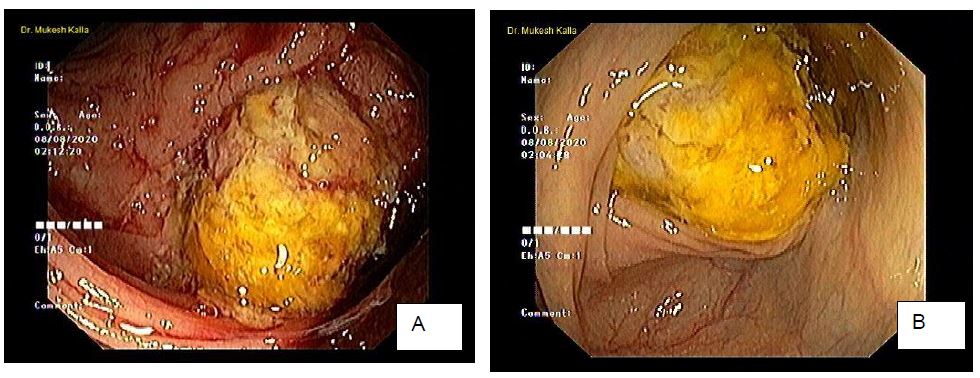
Figure 1: Colonoscopy. A, B; Endoscopic view of ulcerated and edematous mucosa.
USG guided FNAC was performed, two types of material were aspirated, one aspirate was thick, purulent and other was mucinous. On evaluation of cytology, smears showed mucinous material with poor cellularity, few clusters of cells were identified that showed nuclear pleomorphism, atypical, altered N:C ratio and coarse chromatin, which is highly suggestive of Malignant Lesion. The second aspirate showed sheets of neutrophils and necrotic material. There by correlation with radiology and biopsy was advised. Colonic biopsy showed adenomatous lesion with presence of moderate to severe dysplasia, as the biopsy was superficial, it was not possible to comment on invasion. Pools of cumin favored the diagnosis of mucinous lesion with superadded infection of Endameba Histolytica. Thuswe asked for repeat biopsy, correlation with radiological finding and serum markers.
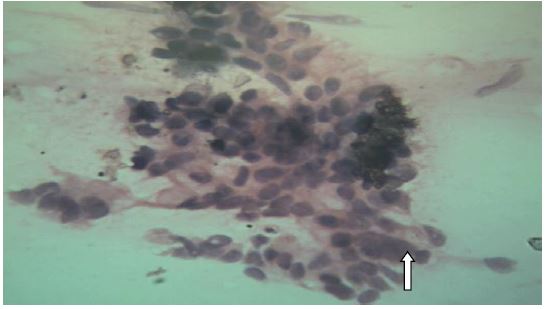
Figure 2: Fine needle aspirate cytology shows clusters of cells with high N:C ratio, pleomorphism, nuclear atypia shown by arrow.
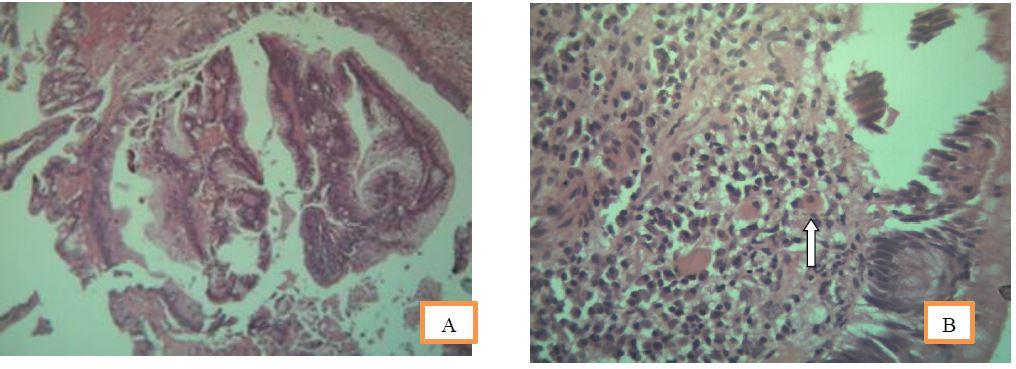
Figure 3 A: Histomorphology of endoscopic biopsy showing adenomatous changes with dysplasia.
B: Endameba histolytic a shown as arrow in H and E stain showing engulfed red blood cell.
On CT of abdomen large lobulated heterogeneous enhancing necrotic mass seen in caecum and proximal ascending colon completely filling and expanding lumen. The mass had necrotic and cystic areas also stranding is seen to adjacent fat with few enlarged parricidal and mesenteric lymph nodes. Interiorly the mass abutting anterior abdominal wall with mild extension of fluid component into parity. Superiorly mass is involving proximal transverse colon with irregular asymmetrical wall thickening and loss of fat planes. His serum CEA was 1074 ng/ml which was too high and, CA 19.9 was normal. Polymerase chain reaction for tuberculosis was negative.
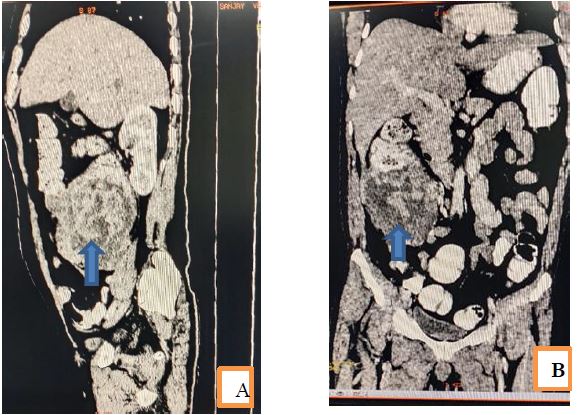
Figure 4 A and B: saggital and coronal sections of CT scan from abdomen show large mass lesion represented as arrow in caecum and proximal ascending colon with expansion of lumen, periceal expansion of mass into anterior abdominal wall and infiltration of adjacent reductant loop of proximal transverse colon. Patient underwent right extended hemicolectomy with ileotransverse anatomizes. Tissue was submitted for histopathology. On grossing a large circumferential, mucinous grey white growth measuring 10x10x8 centimeters was identified. Microscopic finding of respected specimen showed features of mucinous adenocarcinoma infiltrating up to the serosa. Also, amoebic trophozoites were seen which were positive with PAS stain.
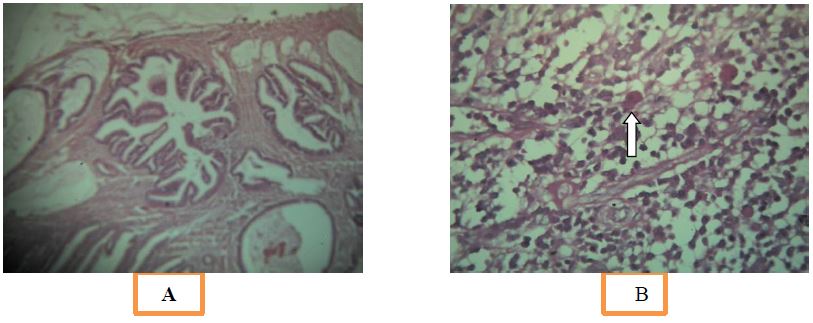
Figure 5A: Microscopy of respected specimen showing features of adenocarcinoma,
B: PAS positive Endameba Histolytica trophozoite shown as arrow.
Post operatively patient recovered on metronidazole and other supportive therapy. On sixth post operative day patient had severe peritonitis and abdominal distension, for which relook laparotomy was performed and diversion ileostomy was done. Subsequently patient did well and wasreferred to oncologist.
Discussion
Endameba histolytic a is a major cause of diarrhea in the developing countries. Every year it causes 40-50 million symptomatic infections worldwide, and approximately 40,000 to 100,000 people die each year from the disease. It is the second leading cause of death among parasitic diseases worldwide [1,2]. Amoeba reside on a diet of intestinal bacteria and partially digested host food. They are also capable of tissue invasion and dissemination, most commonly to the liver followed by lungs. The majority of the patients approximately 90% remain asymptomatic [3]. Clinical symptoms range from, abdominal cramps, diarrhea, right lower quadrant tenderness and bloody diarrhoea. Symptomatic patients can have simple acute proctocolitis to severe potentially fatal acute fulminant colitis. Morphologically presence of red blood cells in the cytoplasm of the trophozoites is indicative of tissue invasion by virulent Endameba histolytic parasites, and in these cases, the patient generally presents with acute amoebic dysentery. Seroprevalence studies have shown that developing invasive or disseminated amoebiasis increases in immune suppressed patients [4]. Risk of fulminant colitis also increases in patients on chemotherapy or corticosteroid treatment [5]. There are several case reports of colonic amebiasis which can be confused with a neoplastic process and leads to misdiagnosis but amoebic infection co-existing with colon cancer is exceedingly rare. On extensive search of literature, till date, we found only eight isolated cases of E. histolytic coexisting with malignancies, including lung adenocarcinoma, cervical squalors carcinoma, perinea carcinoma, gastric carcinoma, rectal carcinoma and one case each of sigmoid and colonic adenocarcinoma [6-11]. Our case might be under tenth of the cases published so far. In our case endoscopy had finding of ulcerative mucosa with well circumscribed margin, the infestation was identified on biopsy as an incidental finding. The trophozoites were seen microscopically between necrotic slough, granulation tissue, chronic inflammatory cells and around the tumors. The trophozoites were oval-shaped with a thin cell membrane and single nucleus with prominent nuclear membrane. The cytoplasm was vacuolated which might be confused with macrophages. The PAS stain was performed showed cytoplasm of the trophozoites red and made them stand out in tissue sections. One of the limitation of our study was that the species identification either with antigenic or molecular tests could not be performed. However, based on prevalence of amoebiasis in our country, and infiltration of the parasites between the malignant glands, E. histolytic was considered the most likely organism. As our country is endemic for amoebiasis and we are working in high volume tertiary care referral gastroenterology centre, we found only one cases of co- existent amoebic infestation in colonic malignancies over the last fifteen years. In the literature also, such cases are extremely rare. An explanation for the rarity of this co-existence may be that microscopically, the trophozoites of E. histolytic may be misinterpreted as shed epithelial tumors cells, cell debris or macrophages. A PAS stain is helpful in such cases, as it stains the organisms red, in contrast to tumors cells or macrophages. Belly et al studied in their in vitro experiments, that mucins secreted by colonic adenocarcinoma cell line LS174T, were effective in inhibiting amoebic adherence to mammalian tissue culture cells. We also observed that some type of mucins produced in vivo by colonic adenocarcinoma, may inhibit E. histolytic and this may also account for their infrequent co-existence. Amoebiasis can potentially be a life-threatening complications, it must be treated promptly, especially in colon carcinoma cases where the patients are operated and may develop post-operative complications. They may receive chemotherapy and radiotherapy, and hence become immuno Endameba histolytic a is a major cause of diarrhea in the developing countries. Every year it causes 40-50 million symptomatic infections worldwide, and approximately 40,000 to 100,000 people die each year from the disease. It is the second leading cause of death among parasitic diseases worldwide [1,2]. Amoeba reside on a diet of intestinal bacteria and partially digested host food. They are also capable of tissue invasion and dissemination, most commonly to the liver followed by lungs. The majority of the patients approximately 90% remain asymptomatic [3]. Clinical symptoms range from, abdominal cramps, diarrhea, right lower quadrant tenderness and bloody diarrhoea. Symptomatic patients can have simple acute proctocolitis to severe potentially fatal acute fulminant colitis. Morphologically presence of red blood cells in the cytoplasm of the trophozoites is indicative of tissue invasion by virulent Endameba histolytic parasites, and in these cases, the patient generally presents with acute amoebic dysentery. Seroprevalence studies have shown that developing invasive or disseminated amoebiasis increases in immune suppressed patients [4]. Risk of fulminant colitis also increases in patients on chemotherapy or corticosteroid treatment [5]. There are several case reports of colonic amebiasis which can be confused with a neoplastic process and leads to misdiagnosis but amoebic infection co-existing with colon cancer is exceedingly rare. On extensive search of literature, till date, we found only eight isolated cases of E. histolytic coexisting with malignancies, including lung adenocarcinoma, cervical squalors carcinoma, perinea carcinoma, gastric carcinoma, rectal carcinoma and one case each of sigmoid and colonic adenocarcinoma [6-11]. Our case might be under tenth of the cases published so far. In our case endoscopy had finding of ulcerative mucosa with well circumscribed margin, the infestation was identified on biopsy as an incidental finding. The trophozoites were seen microscopically between necrotic slough, granulation tissue, chronic inflammatory cells and around the tumors. The trophozoites were oval-shaped with a thin cell membrane and single nucleus with prominent nuclear membrane. The cytoplasm was vacuolated which might be confused with macrophages. The PAS stain was performed showed cytoplasm of the trophozoites red and made them stand out in tissue sections. One of the limitation of our study was that the species identification either with antigenic or molecular tests could not be performed. However, based on prevalence of amoebiasis in our country, and infiltration of the parasites between the malignant glands, E. histolytic was considered the most likely organism. As our country is endemic for amoebiasis and we are working in high volume tertiary care referral gastroenterology centre, we found only one cases of co- existent amoebic infestation in colonic malignancies over the last fifteen years. In the literature also, such cases are extremely rare. An explanation for the rarity of this co-existence may be that microscopically, the trophozoites of E. histolytic may be misinterpreted as shed epithelial tumors cells, cell debris or macrophages. A PAS stain is helpful in such cases, as it stains the organisms red, in contrast to tumors cells or macrophages. Belly et al studied in their in vitro experiments, that mucins secreted by colonic adenocarcinoma cell line LS174T, were effective in inhibiting amoebic adherence to mammalian tissue culture cells. We also observed that some type of mucins produced in vivo by colonic adenocarcinoma, may inhibit E. histolytic and this may also account for their infrequent co-existence. Amoebiasis can potentially be a life-threatening complications, it must be treated promptly, especially in colon carcinoma cases where the patients are operated and may develop post-operative complications. They may receive chemotherapy and radiotherapy, and hence become immuno
Conclusion
To conclude, gastroenterologist and pathologists need to be aware of the possibility of co-existing Endameba histolytic with colonic malignancies. Endoscopic findings must be correlated with histopathology of tissue to provide timely and correct diagnosis of patient. As in our case since last two years the patient was wandering for the exact diagnosis and took antitubercular treatment for no reason. This is also one of the serious issue of economic and healthcare burden in developing countries.
Consent
Written informed consent for publication of this case along with images was taken from patient.
References
1. Stanley SL. 2003. Amoebiasis. Lancet. 361: 1025-1034. Ref.: https://pubmed.ncbi.nlm.nih.gov/12660071/
2. Walsh JA. 1986. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev Infect Dis. 8: 228-238. Ref.: https://pubmed.ncbi.nlm.nih.gov/2871619/
3. Gathiram V, Jackson TF. 1987. A longitudinal study of asymptomatic carriers of pathogenic zymodemes of Entamoeba histolytica. S Afr Med J. 72: 669-672. Ref.: https://pubmed.ncbi.nlm.nih.gov/2891197/
4. Stark D, Barratt JL, van Hal S, et al. 2009. Clinical significance of enteric protozoa in the immunosuppressed human population. Clinical Microbiology Reviews. 22: 634-650. Ref.: https://pubmed.ncbi.nlm.nih.gov/19822892/
5. Evening T, Weiss LM. 2006. The immunology of parasite infections in immunocompromised hosts. Parasite Immunology. 28: 549-565. Ref.: https://pubmed.ncbi.nlm.nih.gov/17042927/
6. Mhlanga BR, Lanoie LO, Norris HJ, et al. 1992. Amebiasis complicating carcinomas: a diagnostic dilemma. The American Journal of Tropical Medicine and Hygiene. 46: 759-764. Ref.: https://pubmed.ncbi.nlm.nih.gov/1621901/
7. Arroyo G, Elgueta R. 1989. Squamous cell carcinoma associated with amoebic cervicitis. Report of a case. Acta Cytologica. 33: 301-304. Ref.: https://pubmed.ncbi.nlm.nih.gov/2728784/
8. Zhu H, Min X, Li S, et al. 2014. Amebic lung abscess with coexisting lung adenocarcinoma: a unusual case of amebiasis. International Journal of Clinical and Experimental Pathology. 7: 8251-8254. Ref.: https://pubmed.ncbi.nlm.nih.gov/25550881/
9. Shigeo Higami, Eiji Nomura, Masashi Yamazaki, et al. 2015. The first case of huge amebic intra-abdominal tumor with asymptomatic amebic colitis Surg Case Rep. 1: 48. Ref.: https://pubmed.ncbi.nlm.nih.gov/26366345/
10. Grosse A. 2016. Diagnosis of colonic amebiasis and coexisting signet-ring cell carcinoma in intestinal biopsy. World Journal of Gastroenterology. 22: 8234-8241. Ref.: https://pubmed.ncbi.nlm.nih.gov/27688666/
11. PalaviGoel, Ruchita Tyagi, Gursheen Kaur, et al. 2018. Entamoeba histolytica: A surprising coexistence with adenocarcinoma - Never brush aside brushings for biopsy J Lab Physicians. Apr-Jun. 10: 251-254. Ref.: https://pubmed.ncbi.nlm.nih.gov/29692598/




















