Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ijcgh.2019.110002Article Views : 1468Article Downloads : 28
A new, economical, and easy protocol to culture 3D mouse hepatoid and cholangoid
Xiaolun Sun* and Hong Wang
Center of Excellence for Poultry Science, University of Arkansas, Fayetteville, USA
*Corresponding author: Xiaolun Sun, Center of Excellence for Poultry Science, 1260 W Maple St. O-409, Fayetteville, AR 72701, Phone: 479-575-232; Fax: 479-575-7139; Email: xiaoluns@uark.edu
Article Information
Aritcle Type: Research Article
Citation: Xiaolun Sun, Hong Wang. 2019. A new, economical, and easy protocol to culture 3D mouse hepatoid and cholangoid. Int J Clin Gastro Hepato. 1: 07-11.
Copyright:This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2019; Xiaolun Sun
Publication history:
Received date: 14 February, 2019Accepted date: 26 February, 2019
Published date: 28 February, 2019
Abstract:
Despite the high efficiency of self-regeneration in liver, liver stem cell organoid culture is way behind other cell types such as intestinal organoid (Enteroid). The contributing factor is the lack of effi¬cient technique to isolate liver cells and to culture liver stem cell organoid. Here we reported a new, eco¬nomical, and simple method to generate 3D hepatocyte organoid (Hepatoid) and cholangiocyte organoid (Cholangoid). We have isolated liver cells using ice-cold 3mM EDTA PBS medium. The isolated cells were cultured on 50% Conditional Medium. After 1 or 4 days post isolation and culture, Cholangoid and Hepatoid were readily to be detected under light microscope and they continued to grow well after several passages. This method could be used to isolate and culture human liver organoids. This feasible protocol will greatly facilitate more research on liver function and diseases using liver cell organoids.
Keywords: Organoid; Stem cell; 3D culture; Liver
Introduction
Liver is comprised of 80% hepatocytes in volume and the rest cells are sinusoidal endothelial cells, Kupffer cells, and hepatic stellate cells, cholangiocytes (bile duct epithelium), and intrahepatic lym¬phocytes [1]. Hepatocytes are responsible for many critical biological processes including protein synthesis, sugar metabolism, detoxification, as well as synthesis and secretion of cholesterol, bile acids, and phospholipids. Because of hepatocyte essential role in liver function, freshly isolating primary hepatocytes is important to research various aspects of liver health and diseases including metabolic dis¬ease, toxicology, oncology, and parasitology [2]. The current golden standard method for isolating hepatocytes is so-call “two-step perfusion” method [3]. In this method, water bath, heating table, perfusion pump, and collagenase I or IV are required. The operation is time consuming, complicated, and expensive.
Liver possesses incredible regenerative capacity where it recovers from two third partial hepa¬tectomy in mice [4]. Recently, primary stem cell and 3D organoid culture become more and more popular to be used for biomedical research. Despite its easy regeneration, organ¬oid culture using liver cells is still at infantile stage. Recently, Hu and colleagues reported a method to culture hepatocyte 3D organoid from mouse and human liver with expensive reagents and complicated procedure [5]. Here we reported a new, economy, and simple method to isolate liver cells and successfully generate 3D hepatocyte organoid (hepatoid) and cholangiocyte organoid (Cholangoid). This feasible protocol will greatly facilitate more research on liver function and diseases using liver organoid.
Method and Materials
Reagents and dispensable used: cold PBS
1. Cold shaking buffer (3mM EDTA and 2% Sorbitol in PBS)
2. 15 and 50 ml tubes
3. 70 μm strainers
4. Matrigel, put on ice to pre-thawed for 2-3 hours or overnight at refrigerator.
5. 50% conditional medium from L-WRN cells was prepared according to protocol reported by Miyoshi et al. [6].
6. The 50% conditional medium was supplemented with 1x antibiotics, 10 μm Y27632 (ROCK inhibitor), and 10 μm SB431542 (inhibitor of the TGF-β/Activin/NODAL).
Liver and its cell collection:
1. Immediately after 8-50-week-old BL6 Il10-/- mice were sacrificed, the abdominal cavity was opened.
2. The gastric system was moved to the right and the visceral vena cava was exposed.
3. A 22G syringe filled with 6 ml shaking buffer was inserted into the vena cava through the right atrium. Fix the needle with a string tiring the needle and the blood vessel.
4. After making a small cut in the visceral vena cava, the shaking buffer was injected into the liver.
5. The liver was then resected and put into ice-cold PBS.
6. The liver was cut into small pieces and pipetted into 15 ml conical tube.
7. The tube was rocked in 4 °C cold room for 5 min.
8. After settling on ice, the supernatant was decanted (careful not to discard tissue!).
9. Then 5 ml shaking buffer was added into 15ml tube and rocked in cold room for 30 minutes.
10. After settling, the supernatant was discarded, 5 ml PBS was then added.
11. The tube was then shaken gently by hand for 2 minutes to release hepatocytes.
12. To check the release of hepatocyte, 10 μl of resuspended tissue (right after shaking) onto a slide and check for hepatocyte release.
13. The supernatant was then filtered through 70 μm strainer.
14. The tube was then centrifuged at 150 g for 10 min at 4 °C and the cells were resuspended in 50% conditional medium on ice.
15. The cells were then mixed at 1:1 with Matrigel on ice and pipet multiple times till homogeneous mixture.
16. 50 μl of the cell mixture were pipetted into the middle of a 24-well plate.
17. The plate was then put into 37 °C incubator for 15-20 min to harden the Matrigel.
18. 500 μl 50% conditional medium was then slowly added into the well.
19. The cells were checked daily and pictures were taken using Nikon ST2 microscope. The medium was replaced with fresh 50% conditional medium every 3 days.
20. To pass the organoids, put the plate on ice for 30-45 minutes to thaw Matrigel.
21. Pipet up and down with P-1000 to assist on breaking up Matrigel.
22. Transfer to a 1.7mL tube and pass through needle (26 ½ to 30 ½ -G).
23. Follow procedures 14 to 19.
Results and Discussion
Successfully culturing hepatocyte organoid from mouse and human liver tissues was first reported in November 2018 [5]. In the report, the liver cells were isolated using two-step perfusion and a total of 11 growth factors and inhibitors were used, hence the method was expensive and difficult to operate. Here, we showed a new, inexpensive, and simple protocol to isolate liver cells and to success¬fully culture 3D hepatocyte organoid (Hepatoid) and cholangiocyte organoid (Cholangoid).
To isolate mouse liver cells, we adopted a protocol for isolating and culturing mouse intestinal organoid (Enteroid). Our protocol was based on using EDTA to release cells and on sitting tissue/cells on ice to increase isolated cell viability. In addition, as showed in the method section, our procedure to isolate liver cells was easy to perform. In contrast, the old “two-step perfusion” method requires anesthesia, complicated surgery procedures, and perfusion pump [3]. Hence, our protocol has obvious advantage over the “two-step perfusion” on liver cell collection.
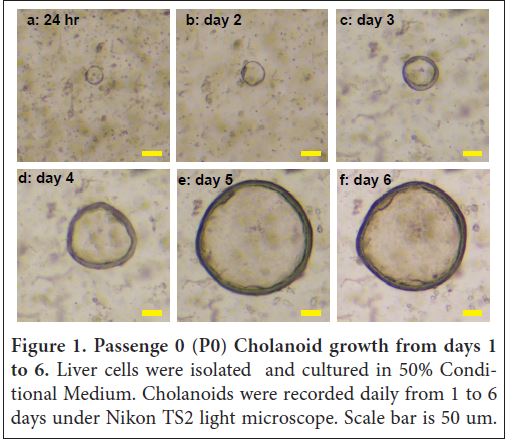
Furthermore, our protocol is the ease to make liver organoid culture medium. We used L-WRN cells to generate 50% conditional medium [6]. The liver cell grew very well in the medium. Interestingly, the medium supported the growth of both Hepatoid and Cholangoid. Specifically, at 24 hours (1 day) post isolation and culture, ring/circle-shape organoids with size of 50 μm was detected in the Matrigel under light microscope (Figure 1a) and the center of the ring was void of cells, resembling a duct. According to Hu and colleagues’ report, these cells comprised of chol¬angiocyte (bile duct epithelial cell) organ¬oids (Cholangoid). At days 2, 3, 4, 5, and 6, the Cholangoid siz increased to 65, 100, 175, 300, and 325 μm without obvious cell budding (Figure 1b-f). At this time, we decided it is time to separate the Cholangoid with other cells and transferred the individual Cholangoid without disrupt¬ing the ring/tube to new wells. After four time passages, the Cholangoid continued to grow well. Interestingly, some Cholangoids developed into Hepatoids after several day culture.
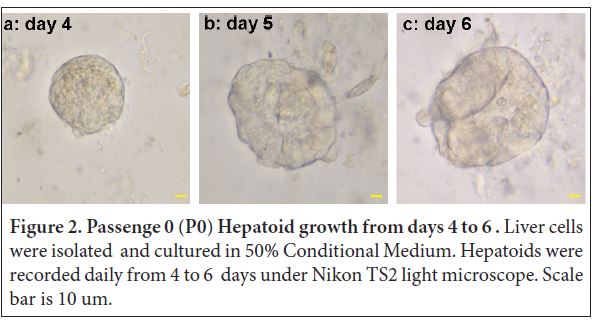
For Hepatoid development, at day 4, ball-shape Hepatoids with diameter of 50 μm were read¬ily detected with cell budding on the edge (Figure 2a). At day 5, the Hepatoid size increased to 80 and it was more visible for more grape/ball-shape buddings at the surface (Figure 2b). Interestingly, the total number of Hepatoid (34) at day 5 was twice of Cholangoid (17). At day 6, Hepatoid size increased to 88 μm and grape/ball shape buddings at the sur¬face were more evident (Figure 2c). At this time, we decided it is time to separate the Hepatoid with other cells and we then transferred the individual Hepatoid without disrupting the ball to new wells. After four-time passages, the Hepatoids continued to grow well. We plan to nanoinject bacteria (e.g. Campylobacter jejuni, Clos¬tridium perfringens) or cytokine (e.g. TNFα) into the Hepatoids and host responses (e.g. inflammation, apoptosis) will be assessed.
In summary, we have developed a new, economical, and simple procedure to isolate and culture mouse Hepatoid and Cholangoid. The culture media for human hepatoid reported by Hu and colleagues is the same formula to culture mouse hepatoid [5]. Hence, this method could be adopted to isolate and culture human Hepatoid and Cholangoid. Our finding would promote the liver research using liver organoids.
Acknowledgement
The authors would like to thank Dr. Jillian Pope at University of Florida at Gainesville, FL. The method of isolating liver cells was adopted from her protocol of isolating intestinal epithelial cells.
Author Contribution: X.S. and H.W. wrote the manuscript.
Grant Support
This research was supported by Arkansas Biosciences Institute, USDA National In-stitute of Food and Agriculture (NIFA) Hatch project 1012366, and USDA NIFA Hatch/Multi State project 1018699 grants to X. Sun. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
References
- Kmiec Z. 2001. Cooperation of liver cells in health and disease. Advances in anatomy, embryology, and cell biology 161. III-XIII. 1-151.[Ref.]
- Wallace K, Fairhall EA, Charlton KA, et al. 2010. AR42J-B-13 cell: an expandable progenitor to generate an unlimited supply of functional hepatocytes. Toxicology. 278: 277-287. [Ref.]
- Severgnini M, Sherman J, Sehgal A, et al. 2012. A rapid two-step method for isolation of functional primary mouse hepatocytes: cell characterization and asialoglycoprotein receptor based assay development. Cytotech-nology. 64: 187-195. [Ref.]
- Mitchell C, Willenbring H. 2014. Addendum: A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nature protocols 9. [Ref.]
- Hu H, Gehart H, Artegiani B, et al. 2018. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell. 175: 1591-1606. [Ref.]
- Miyoshi H, Stappenbeck TS. 2013. In vitro expansion and genetic modification of gastrointesti¬nal stem cells in spheroid culture. Nature protocols. 8: 2471-2482. [Ref.]




















