Indexing & Abstracting
Full Text
Review ArticleDOI Number : 10.36811/ijbm.2022.110025Article Views : 0Article Downloads : 0
Harmful impact of dimethoate and Chloropyrfios on the Hematological parameters in male rabbits
Osama H Aldeeb1, Bassam A Ibridan2, Fayrouz A Khaled3 and A Shah3
1Biomedical Science Department, Faculty of pharmacy, Omar Al-Mokhtar University, El -Beida-Libya
2Environmental Science, Faculty of Natural Resources and Environmental Science, Derna University, El -Qubba-Libya
3Chemistry Department, Faculty of Science, Omar El-Mokhtar University, El -Beyda-Libya
*Corresponding author: Fayrouz A Khaled, Chemistry Department, Faculty of Science, Omar El-Mokhtar University, El -Beyda-Libya; Email: fayalzobair@yahoo.com
Article Information
Aritcle Type: Review Article
Citation: Osama H Aldeeb, Bassam A Ibridan, Fayrouz A Khaled, et al. 2022. Harmful impact of dimethoate and Chloropyrfios on the Hematological parameters in male rabbits. Int J Biol Med. 4: 01-10.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2022; Osama H Aldeeb
Publication history:
Received date: 08 February, 2022Accepted date: 14 March, 2022
Published date: 18 March, 2022
Abstract
Dimethoate and chloropyrfios are two of widely used organophosphorus insecticides in agriculture. The irrational use of Dimethoata and chlyropyrfios in Libya play a crucial role in the occurrence of many diseases affecting plants, animals and the environment. The present work was conducted to investigate the alterations in hematological factors in male rabbits after orally administration a single dose of dimethoate (DM) by gavage at a dose of 43.2 mg/kg B.W/day (1/50 of DM) lethal dose and chloropyrfios by gavage at a dose of 33.3 mg/kg B.W/day (1/50 of CPF) lethal dose for 12-week. 15 Male Rabbits weighting (1.891-27.6 Kg)., were divided into 3 groups with 5 animals in each, first group served as control animals, they received 5 ml of corn oil, while animals in second group received Dimethoate, animals in third group received of chloropyrfios. Results indicated that treatment with Dimethoate and chloropyrfios caused significant (P<0.05) decrease in white blood cells (WBCs), haemoglobin (Hb), red blood cells (RBCs), zpacked cell volume (PCV) and platelets. While, increase MCV, MCH, MCVH compared to control. The erythrocytes sedimentation rate highly significant increase in the blood of Dimethoate and chloropyrfios treated rabbits as compared to control.
Keywords: Dimethoate; Chloropyrfios; Hematological parameters
Introduction
Insecticides known as chemicals manufactured and used to control insects like lice, ticks and used in veterinary medicine to control and eradicate mites and mange [1,2]. Chlorpyrifos a broad-spectrum insecticide belongs to organophosphorus compounds. It is used in public health, agricultural crops and in livestock to control different pests [3,4], chlorpyrifos eliminates and eradicates insects by direct exposure or in contact with treated surfaces. Chlorpyrifos classified as a moderate toxic compound within organophosphorus compounds in mice and rats and LD50 ranged between 50-500 mg/kg body weight [5], as well as have moderate toxicity in sheep and Guinea pigs [3]. Toxic effects of chlorpyrifos by affecting normal function of the insect nervous system [6] by irreversible inhibition of acetyl cholinesterase enzyme (AchE) that is responsible for the breakdown of acetylcholine (Ach) [7]. Dimethoate (DM) is an insecticide referred to as organophosphates, which is cholinesterase inhibitor. Cholinesterase is an enzyme that is essential for the proper working of the nervous systems of both humans and insects. Administration of DM to pregnant rats produced enzymatic changes associated with mild pathomorphological changes in liver and brain, [8]. Previous studies indicate that DM intoxication causes cellular injury and oxidation stress which leads to lipid peroxidation and free radical production [9]. Some studies have shown that acute and sub chronic exposure etestes of rats [10] and human erythrocytes [11].
Research Objectives: Determine the toxic effects of dimethoate and chloropyrfios on hematological parameters in adult male rabbits.
Materials and Methods
Tested compounds
In this study Dimethoate (DM) and chloropyrfios were used. Dimethoate and chloropyrfios (purity 400g/L) was purchased from B &W agrochemichals (China) all other chemicals used in the experiment were of analytical grade.
Experimental animals
Mature male New Zealand White rabbits age of 6 months and initial weight of (1.891?27.6 Kg) were used. Animals were individually housed in cages and weighed weekly throughout 3-months experimental period. Fifteen mature male rabbits were randomly divided into three equal groups (each five rabbits) as follows group ? rabbits were used as control daily for 12 successive weeks. Group II rabbits were treated daily with Dimethoate (DM) by gavage at a dose of 43.2 mg/kg B.W/day (1/50 of DM) lethal dose [12]. Group III rabbits were treated daily with chloropyrfios by gavage at a dose of 33.3 mg/kg B.W/day (1/50 of CPF) lethal dose [13]. The doses of the dimethoate and chloropyrfios were calculated according to the animal’s body weight on the week before dosing. The tested doses of dimethoate and chloropyrfios were given daily for 12 weeks.
Blood Specimens
Blood samples were collected from the ear vein of all animals every other week throughout the 12-week experimental period. Blood samples were obtained in the morning before accesses to feed and water and placed immediately on ice. The blood samples were collected in tube containing heparin to obtain plasma.
Hematological Parameters
Blood samples were collected from the ear vein of all animals every week throughout the 12-week experimental period. Blood samples were obtained in the morning before accesses to feed and water. Values derived from complete blood count (CBC). All CBC tests were performed by automatic blood cell analyzer (XP-300 Automated Hematology Analyzer, Sysmex American, Inc [14]. CBC were performed on EDTA as anti-coagulated samples. Differential cell counts were performed manually using Dif-Quik-stained blood smears. Data were recorded according to the following categories: white blood cell (WBC); red blood cell (RBC); hemoglobin (HB); mean corpuscular volume (MCV); mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC).
Statistical analysis
Where applicable, statistical analysis was carried out in Minitab software (version17)/ statistical significance was assessed using ANOVA analysis with Tukey multiple comparison test after detection normal distribution to the data and appropriate P < 0.05 consider significant.
Result
Effect of dimethoate and chloropyrfios on hematological parameters
Table1 and Figure 1to 8 presents the hematological parameters of male rabbits treated with dimethoate and chloropyrfios. Results indicated that treatment with dimethoate and chloropyrfios decrease in WBCs, Hb, RBCs, PCV and platelets compared to control. On the other hand, caused a significant increase in MCV, MCH and MCHC comperd to control group. The haematological indices including Hb, WBC, MCV, MCH and MCHC were not altered significantly. However, PCV was significantly (P≤0.05) decreased (Table 1).
|
Table 1: Changes Complete blood counts Red blood cells (RBC), white blood cells (WBC), packed cell volume (PCV), platelets count (PLT), hemoglobin (Hb), mean cell volume (MCV; fi), mean cell hemoglobin (MCH; pg) and mean cell hemoglobin concentration (MCHC; g/dl) of male rabbits treated with dimethoate and chloropyrfios. |
|||
|
Parameter |
Experimental groups |
||
|
Control |
Dimethoate |
Chloropyrfios. |
|
|
RBC ´106 (µl) |
6.13±0.107a |
4.92±0.085a |
5.85±0.121b |
|
WBC ´103(µl) |
8.42 ±0.22b |
7.96±0.334b |
8.14±0.12b |
|
PCV´103(µl) |
40.36±0.420a |
38.83±0.531a |
32.35±0.426b |
|
PLT ´103(µl) |
311.45±13.369b |
218.8±8.378a |
258.03±8.493c |
|
Hb (g/dl) |
12.96±0.14a |
10.52±0.23a |
11.33±0.17b |
|
MCV (fi) |
69.73±0.94c |
70.24±0.58b |
75.48±0.92a |
|
MCH(pg) |
23.53±0.53a |
23.62±0.38a |
23.57±0.49a |
|
MCVH(g/dl) |
33.58±0.40b |
33.78±0.48b |
34.93±0.18a |
|
Values are expressed as means ± SE; n = 5 for each treatment group. Mean values within a row not sharing a common superscript letters (a, b, c, d) were significantly different, p<0.05. |
|||
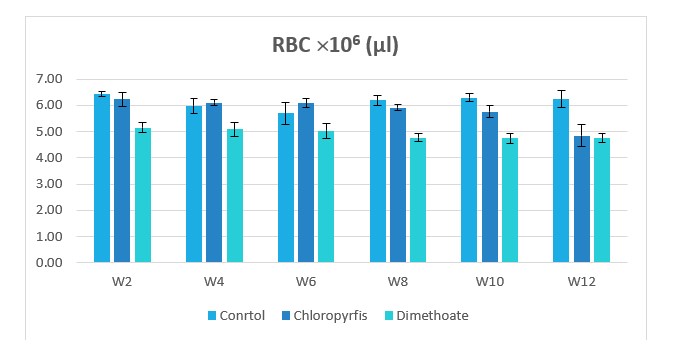
Figure 1: Change in RBC counts during treatment of male rabbits treated with dimethoate and chlyropyrfios.
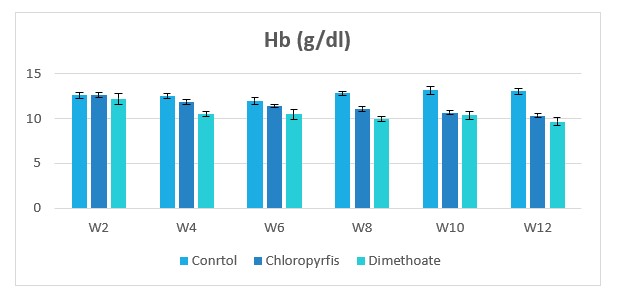
Figure 2: Change in hemoglobin (Hb) during treatment of male rabbits treated with dimethoate and chlyropyrfios.
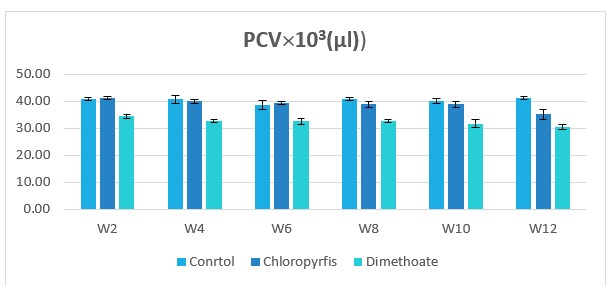
Figure 3: Change in PCV counts during treatment of male rabbits treated with dimethoate and chlyropyrfios.
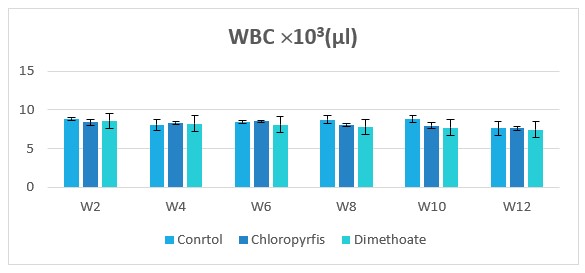
Figure 4: Change in WBC counts during treatment of male rabbits treated with dimethoate and chlyropyrfios.
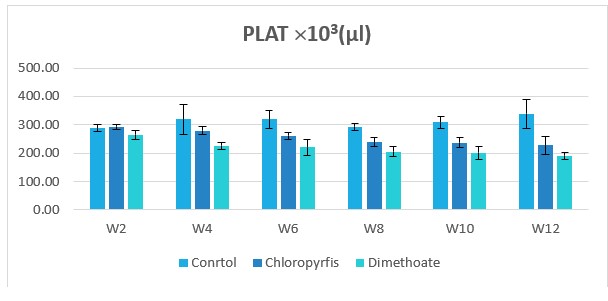
Figure 5: Change in PLAT counts during treatment of male rabbits treated with dimethoate and chlyropyrfios.
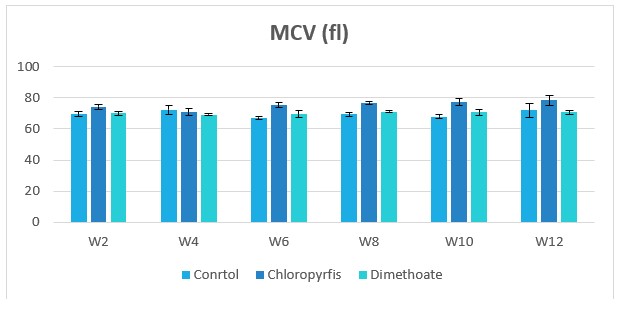
Figure 6: Change in MCV during treatment of male rabbits treated with dimethoate and chlyropyrfios.
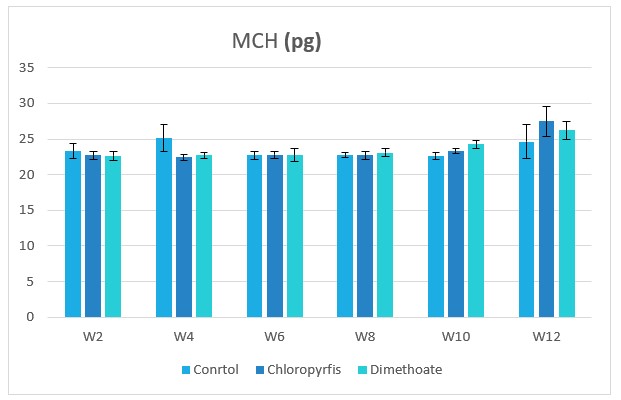
Figure 7: Change in MCH during treatment of male rabbits treated with dimethoate and chlyropyrfios.

Figure 8: Change in MCHC during treatment of male rabbits treated with dimethoate and chlyropyrfios.
Discussion
Table 1 and Figures 1 to 8 represent the hematological parameters of male rabbits treated with DM and chlyropyrofis. Pesticides exposure poses a serious risk to all domestic animals, to the environment and the public health [15]. The results of this study showed that, the hematological parameters RBC and Hb were significantly decreased in DM treated rabbits when the erythrocytes sedimentation rate was highly significant increased as compared to control. The effect of organophosphorus pesticides on the Hb of several workers has been studied by [16,17]. The decrease in the Hb along with the decrease in the RBC might be due to the effect of pesticides on blood forming organ (bone marrow and liver), and inhibition of many steps of heme biosynthesis in rabbits, as the result of pesticides exposure [17].
The poisoning by pesticide residues leads to the development of anemia due to interference of Hb biosynthesis and shortening of the life span of circulating erythrocytes [18,19]. The increase of E.S.R. indicates to inflammation caused by organophosphorus pesticides [20]. Our finding is in agreement with [19], that showed that pesticides decrease RBC and Hb levels, and [20], who noticed the reduce of R.B.C., Hb, and increase in erythrocytes sedimentation rate in rabbit’s exposure to orally dose of 10mg/kg. Of the organophosphorus pesticide Methidathion.
In this study significant decrease alterations were observed in Hb concentration in rabbits which were intoxicated. However, one study has observed decrease in Hb concentration due to a direct effect on bone marrow as reported in rabbits [21]. The discrepancy might be attributed to short period of study with intact compensatory mechanisms. Significantly decreased PCV might be due to malabsorption of nutrients or the hyperactivity of the animal [22]. The present observation was in accordance with previous workers [22], who observed no effect on the basophil values following DM administration in mice. However, decrease in TLC and lymphocyte values following DM administration has been reported in Oncorhynchus mykiss and mice [23].
The above mentioned effects of organophosphorus pesticides could be due to their ability to form free radicals [24,25], his fact may ensure the hypothesis of the ability of organophosphorus pesticides to form free radicals, which have been implicated as playing a role in the etiology of many alterations [26].
The low hematological parameters of PCV, Hb, and RBC show that chronic CPF administration causes anemia. This agreed with earlier ?ndings [27,28]. [27] attributed the anemia to the ability of CPF to reduce serum iron concentration, thereby compromising the synthesis of Hb. The anemia may also be related to interference with Hb synthesis and shortening of RBC lifespan [17]. We have earlier shown that chronic CPF exposure causes increased erythrocyte fragility, partly due to increase dlipoperoxidation of the erythrocyte membranes [28]. The increased lipoperoxidation in the CPF group, re?ected by signi?cant MDA concentration, may have caused increased vulnerability of the RBC to destruction, but may directly destroy the erythrocytes thereby leading to anemia. MDA is a major oxidation product to per oxidized poly unsaturated fatty acids (PUFAs), and increased MDA content is an important indicator of lipid peroxidation [29]. The RBC is susceptible to lipoperoxidative changes because of its direct association with molecular oxygen, high content of metal ions catalyzing oxidative reactions, and availability of high amount of PUFAs, which are susceptible to lipid peroxidation. Inability to repair membrane damage and regenerate due to lack of nucleus and poor antioxidant enzymes composition of the plasma medium in which they are bathed [30] are some of the other factors responsible for the increased vulnerability of RBC to lip peroxidation. Therefore, CPF-induced oxidative damage to the erythrocyte membrane may have contributed to the anemia recorded in the CPF group. This is because the process of lipid peroxidation impairs the functions and homeostasis of the erythrocyte membranes through decrease in hydrophobic characteristics of bilayer membrane, and altering the a?nity and interaction of proteins and lipids [31]. ROS can equally a?ect the proteins resulting in modi?cation of enzymes activity, and damage to the membrane transport proteins may produce disturbed cellular ionic homeostasis, leading to alterations in intracellular calcium and potassium that triggers a series of changes in the cell [32].
Conclusion
A pervasively-used chlorpyrifos and dimethoate helps in minimizing the damages caused by pests, but its exposure could be harmful for animal as well as human population. The present investigation provides an insight of chlorpyrifos and dimethoate induced toxicity onhematological parameters.
References
1. Sharma A, Mishra M, Shukla AK. 2012. Organochlorine pesticide, endosulfan induced cellular and organismal response in Drosophila melanogaster. Journal of hazardous materials. 221: 275-287. Ref.: https://pubmed.ncbi.nlm.nih.gov/22579458/ DOI: https://doi.org/10.1016/j.jhazmat.2012.04.045
2. El-Shenawy NS. 2010. Effects of insecticides fenitrothion, endosulfan and abamectin on antioxidant parameters of isolated rat hepatocytes. Toxicology in vitro. 24: 1148-1157. Ref.: https://pubmed.ncbi.nlm.nih.gov/20214973/ DOI: https://doi.org/10.1016/j.tiv.2010.03.001
3. Ishii S, Bell JNB, Marshall FM. 2007. Phytotoxic risk assessment of ambient air pollution on agricultural crops in Selangor State, Malaysia. Environmental Pollution. 150: 267-279. Ref.: https://pubmed.ncbi.nlm.nih.gov/17379364/ DOI: https://doi.org/10.1016/j.envpol.2007.01.012
4. Watts M. 2012. Chlorpyrifos as a possible global POP. Pesticide Action Network North America, Oakland, CA.
5. Pope C, Karanth S, Liu J. 2005. Pharmacology and toxicology of cholinesterase inhibitors: uses and misuses of a common mechanism of action. Environmental toxicology and pharmacology. 19: 433-446. Ref.: https://pubmed.ncbi.nlm.nih.gov/21783509/ DOI: https://doi.org/10.1016/j.etap.2004.12.048
6. Karanth S, Pope C. 2000. Carboxylesterase and A-esterase activities during maturation and aging: relationship to the toxicity of chlorpyrifos and parathion in rats. Toxicological sciences. 58: 282-289. Ref.: https://pubmed.ncbi.nlm.nih.gov/11099640/ DOI: https://doi.org/10.1093/toxsci/58.2.282
7. Al-Zubaidy MH, Amin WM. 2019. Cholinesterase inhibition in chicks treated with manganese chloride. Iraqi Journal of Veterinary Sciences. 32: 37-42.
8. Srivastava MK, Raizada RB. 1996. Development effect of technical dimethoate in rats: maternal and fetal toxicity evaluation. Indian journal of experimental biology. 34: 329-333. Ref.: https://pubmed.ncbi.nlm.nih.gov/8698421/
9. Sharma Y, Bashir S, Irshad Nag. 2005. Effect of acute DM administration on antioxidant status of liver and brain of rats following subchronic exposure, Toxicology. 215: 173-181. Ref.: https://pubmed.ncbi.nlm.nih.gov/16112789/ DOI: https://doi.org/10.1016/j.tox.2005.06.029
10. Saafi EB, Louedi M, Elfeki A. 2011. Protective effect of date palm fruit extract (Phoenix dactylifera L.) on dimethoate induced-oxidative stress in rat liver. Experimental and Toxicologic Pathology. 63: 433-441. Ref.: https://pubmed.ncbi.nlm.nih.gov/20359872/ DOI: https://doi.org/10.1016/j.etp.2010.03.002
11. Gargouri BBR, Mansour BF, Abdallah A. 2011. Protective effect of quercetin against oxidative stress caused by DM in human peripheral blood lymphocytes. Lipids Health Dis. 10: 149-149. Ref.: https://pubmed.ncbi.nlm.nih.gov/21861917/ DOI: https://doi.org/10.1186/1476-511x-10-149
12. Massoud MA, Fayad R, El-Fadel M. 2010. Drivers, barriers and incentives to implementing environmental management systems in the food industry: A case of Lebanon. Journal of cleaner production. 18: 200-209.
13. Manna S, Bhattacharyya D, Mandal TK. 2004. Repeated dose toxicity of alfa-cypermethrin in rats. Journal of veterinary science. 5: 241-245. Ref.: https://pubmed.ncbi.nlm.nih.gov/15365239/
14. Turgeon M. 2012. Clinical Hematology Theory and Procedures, Fundamentals of Hematological Analysis. Philadelphia Lippincott Williams & Wilkins.a Wolters Kluwer business. 456-580.
15. Oheme WF, Mannala S. 2001. Pesticide Use in Veterinary Medicine: Handbook of Pesticide Toxicology.
16. Bhatnagar JA. 1980. Effect of pesticide stress among pesticide factory workers in Delhi. 74: 380.
17. Ray G. 1992. Pollution and health. Wiley Eastern Ltd. New-Delhi. 45: 18.
18. Betrosian A, Balla M, Kafiri G. 1995. Multiple systems organ failure from organophosphate poisoning. Journal of Toxicology: Clinical Toxicology. 33: 257-260. Ref.: https://pubmed.ncbi.nlm.nih.gov/7760452/ DOI: https://doi.org/10.3109/15563659509017994
19. Jyotsna AP, Arun JP, Sanjay P. 2003. Biochemical effects of various pesticides on sprayers of Grape garden. Indian J Biochem. 18: 16-22. Ref.: https://pubmed.ncbi.nlm.nih.gov/23105387/ DOI: https://doi.org/10.1007/bf02867362
20. Elias MA, Saif MA. 2009. The protection effect of vitamins A, C, and E, against the potential toxicity of Methidathion on blood factors in male rabbits. Yem J Biol Sci. 5: 133-136.
21. Salih EM. 2010. Toxic effect of dimethoate and diazinon on the biochemical and hematological parameters in male rabbits. Jordan J Biol Sci. 3: 77-82.
22. Khogali FA, Sheikh JB, Rahman SA. 2015. Histopathological and hematological effects of dimethoate 40EC on some organs of albino mice. J. King Saud Univ. 18: 73-87.
23. Dogan D, Can C. 2011. Hematological, biochemical, and behavioral responses of Oncorhynchus mykiss to dimethoate. Fish Physiology and Biochemistry. 37: 951-958. Ref.: https://pubmed.ncbi.nlm.nih.gov/21547568/ DOI: https://doi.org/10.1007/s10695-011-9492-1
24. Hazarika A, Sarkar SN, Hajare S. 2003. Influence of malathion pretreatment on the toxicity of anilofos in male rats: a biochemical interaction study. Toxicology. 185: 1-8. Ref.: https://pubmed.ncbi.nlm.nih.gov/12505439/ DOI: https://doi.org/10.1016/s0300-483x(02)00574-7
25. Vidyasagar J, Karunakar N, Reddy MS. 2004. Oxidative stress and antioxidant status in acute organophosphorous insecticide poisoning. Indian journal of pharmacology. 36: 76.
26. Halliwell B. 1989. The chemistry of oxygen radicals and other derived species. Free radicals in biology and medicine.
27. Goel A, Dani V, Dhawan DK. 2006. Role of zinc in mitigating the toxic effects of chlorpyrifos on hematological alterations and electron microscopic observations in rat blood. Biometals. 19: 483-492. Ref.: https://pubmed.ncbi.nlm.nih.gov/16937254/ DOI: https://doi.org/10.1007/s10534-005-5148-x
28. Ambali SF, Onukak C, Idris SB. 2010. Vitamin C attenuates short-term hematological and biochemical alterations induced by acute chlorpyrifos exposure in Wistar rats. Journal of Medicine and Medical Sciences. 1: 465-477.
29. Celik I, Suzek H. 2009. Effects of subacute exposure of dichlorvos at sublethal dosages on erythrocyte and tissue antioxidant defense systems and lipid peroxidation in rats. Ecotoxicology and environmental safety. 72: 905-908. Ref.: https://pubmed.ncbi.nlm.nih.gov/18539328/ DOI: https://doi.org/10.1016/j.ecoenv.2008.04.007
30. Marklund SL, Holme E, Hellner L. 1982. Superoxide dismutase in extracellular fluids. Clinica chimica acta. 126: 41-51. Ref.: https://pubmed.ncbi.nlm.nih.gov/7172448/ DOI: https://doi.org/10.1016/0009-8981(82)90360-6
31. Dargel R. 1992. Lipid peroxidation—a common pathogenetic mechanism? Experimental and toxicologic. 44: 169-181. Ref.: https://pubmed.ncbi.nlm.nih.gov/1392519/ DOI: https://doi.org/10.1016/s0940-2993(11)80202-2
32. Kerr LD, Inoue JI, Verma IM. 1992. Signal transduction: the nuclear target. Current opinion in cell biology. 4: 496-501. Ref.: https://pubmed.ncbi.nlm.nih.gov/1497922/ DOI: https://doi.org/10.1016/0955-0674(92)90017-7




















