Indexing & Abstracting
Full Text
Research ArticleDOI Number : 10.36811/ijbm.2020.110018Article Views : 19Article Downloads : 20
Study of Copolymerization Acrylamide with Methyl Methacrylate
Ameen Hadi Mohammed* and Susan Rasheed Jubair
Department of Chemistry, College of Science of Women, University of Baghdad, Baghdad, Iraq
*Corresponding Author: Ameen Hadi Mohammed, Department of Chemistry, College of Science of Women, University of Baghdad, 10071 Al Jadria, Baghdad, Iraq, Tel: +9647739824122; Email: ameenhadi80@yahoo.com
Article Information
Aritcle Type: Research Article
Citation: Ameen Hadi Mohammed, Susan Rasheed Jubair. 2020. Study of Copolymerization Acrylamide with Methyl Methacrylate. Int J Biol Med. 2: 01-09.
Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Copyright © 2020; Ameen Hadi Mohammed
Publication history:
Received date: 09 January, 2020Accepted date: 23 January, 2020
Published date: 24 January, 2020
Abstract
Copolymer of acrylamide (AM) with methyl methacrylate (MMA) was synthesized by free radical technique using dimethylsulfoxide (DMSO) as solvent and benzoyl peroxide (BPO) as initiator. The overall conversion was kept low (≤ 15% wt/wt) for all studies copolymer’s samples. The synthesized copolymers were characterized using fourier transform infrared spectroscopy (FT-IR), and their thermal properties were studied by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). The copolymers compositions were determined by elemental analysis. The monomer reactivity ratios have been calculated by linearization methods proposed by Kelen-Tudos and Fineman-Ross. The derived reactivity ratios (r1, r2) for (AM-co-MMA) are: (0.03, 0.593). The microstructure of copolymers and sequence distribution of monomers in the copolymers were calculated by statistical method based on the average reactivity ratios and found that these values are in agreement with the derived reactivity ratios. Copolymers of AM with MMA formed alternating copolymers.
Keywords: Acrylamide; Methyl methacrylate; Reactivity ratios; Sequence distribution
Introduction
The properties of polymers can be most effectively modified with the help of the technique of copolymerisation [1-4]. This technique is designed to manipulate the intra- and inter-molecular forces that are exerted amongst similar and dissimilar polymer segments, engendering broad variation in properties like temperature of glass transition, melting point, solubility, permeability, dyeability, adhesion, elasticity and chemical reactivity. The basic explorations of structure property correlations and the variety of commercial and biological applications all attest to the fact that copolymerisation is highly useful [5]. A copolymer composition equation relies greatly on reactivity ratios, which not only indicate the relative reactivity of pairs of monomers, but also outline the elements making up the copolymers. To understand how its utility has developed, it is first necessary to understand the copolymer composition itself. As emphasised above, the reactivity ratios are essential for copolymer composition and the manner in which it is distributed. The empirical data regarding copolymer composition and monomer feed mixtures must be mathematically processed before the monomer reactivity ratios can be determined. The reactivity of various comonomers can be calculated via a range of techniques. Furthermore, different analytical methods have been proposed to find out how much of a comonomer has been included in the copolymer [6]. New scientifically and commercially relevant materials can be obtained when two distinct monomers with various physical and or chemical attributes are incorporated in the same polymer molecule at different ratios. The monomer reactivity ratios of copolymerisation enable the determination of the relative reactivity of a monomer toward a specific polymer radical. A copolymers composition is a critical factor in the assessment of its uses. Controlling the polymer property parameters, for example, molecular weight averages, sequence distribution, and copolymer composition, which is a specific significance in the copolymerization forms [7]. To figure the rate of polymerization or polymer profitability and copolymer synthesis, monomer reactivity proportions must be known [8]. The technique which is utilized frequently recently for evaluating monomer reactivity ratios is to perform low conversion copolymerization at different starting monomer feed compositions. In this way, the copolymer composition is resolved for every reaction. Conventional techniques for evaluating monomer reactivity ratios depend on, first, changing the momentary copolymer composition equation into a frame that is straight in the parameters r1 and r2 and after that assessing the monomer reactivity ratios by graphical plotting or by the direct minimum squares technique. Linearization of the copolymer composition condition will contort the blunder dispersions related with the information [9-11]. The aim of this work is to copolymerize AM, a hydrophilic monomer with MMA, a hydrophobic monomer, and to study the best synthetic conditions and characterization of the copolymer. This study also determines the reactivity ratios of AM and MMA. From these parameters, a specific comonomer distribution is estimated.
Experimental
Materials
The monomers, initiator, and solvents were obtained from Aldrich-oma chemical Co. The monomer AM was purified by recrystallization from methanol and dried in a vacuum. MMA was freed from the inhibitor by shaking with 10% W/V aqueous NaOH. After washing with water, it was vacuum distilled immediately prior to the copolymerization experiment. Initiator (benzoyl peroxide) was purified by twice recrystallizations from chloroform and refrigerated prior to use. Solvents were used as received.
Copolymerization
Copolymerization of (AM) with (MMA) was carried out using (10 ml) dimethylsulfoxide (DMSO) as solvent and (1×10-3 mol dm-3) (BPO) as initiator. In quick fit test glass tubes, the prescribed amount of monomers, initiator and solvent were charged, and then put in water bath at (80 °C). As shown in table 1, the feed ratio was varied in a series of copolymerization of (AM) with (MMA) (AM-co-MMA) whilst the total molar composition of the monomer mixture was maintained at (1 mol dm-3). Before starting the reaction, Nitrogen gas was bubbled through the mixture for 10 minutes in order to remove all Oxygen. Low conversion (<15%) of copolymers was obtained by controlling the time of copolymerization. Petroleum ether (b.p. 40-60°C) was used to precipitate the obtained copolymers. All the copolymers were filtered off, dissolved again in dimethylsulfoxide and precipitated in petroleum ether prior to constant weight in vacuum at 40°C. In order to determine the copolymer compositions, samples of the copolymer (0.2 mg) were checked by elemental analysis. Scheme 1 shows the reaction of copolymerization (AM) with (MMA).
Scheme 1: The process formation of AM-co-MMA from AM and MMA as monomers.
Characterization
Perken Elmer-1650 spectrometer was used to record FTIR spectra of the copolymers on KBr Pellets in the range 200-4000 cm-1. Intrinsic viscosity [?] was determined according to the Solomon Gotessman relationship [12] by using an Ostwald Viscometer with negligible kinetic energy correction. By following the variation of estimated nitrogen content arising from (AM) comonomers units, copolymer compositions were determined by elemental analysis. DSC-Mettler calorimetric system was employed to determine the glass transition temperature (Tg) whilst Perkin Elmer in a nitrogen atmosphere at a heating rate of 10 °C /min from 0 to 800 °C was used to study the thermal degradability of the copolymers.
Results and Discussion
The absorption bands which appear in the FTIR spectra of the copolymer Figure 1 (c) belong to the stretching vibration in different functional groups of corresponding homopolymers Figure 1 (a,b). The absorption bands of AM/MMA copolymer as follows: 3400 cm1 (amide N-H), 1670 cm-1 (amide C=0), 1750 cm-1 (ester C=0), 1450 cm-1 (methyl C-H), 1200 cm-1 (amide C-N), 1030 cm-1 (ester C-O), 2850 cm-1 (alkane C-H).
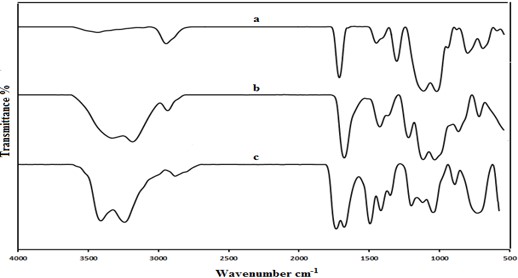
Figure 1: FT-IR of: a- Poly MMA, b- Poly AM, c- AM-co-MMA.
Copolymer Composition
It is very useful to study the monomer reactivity in the copolymer system because the composition of the copolymer depends mainly on the monomer feed composition. In AM/MMA copolymers, composition of the monomer in the copolymer was assessed by assurance N % in the copolymers and this proportion indirectly gave the mole fraction of AM in the copolymer. The monomer composition and the results of elemental analysis in addition to intrinsic viscosity values [?] for samples of five different compositions are listed in table 1. The values of [?] should be used in estimating qualitatively degree of polymerization. Figure 2 shows the plots of mole fraction of AM in the copolymer (F1) vs. that of mole fraction of MMA in the feed (f1).
In AM/MMA system, AM forms alternate copolymer with MMA (Figure 2). Here, the presence of carbonyl and amide groups for each AM and MMA monomers units gave rise to a significant attraction of free electron in the double bond and generate a positive charge in the growing polymer chain and stabilization of the corresponding macroradical. Since both monomers are electron rich, it forms the bond easily with electron deficient species, thus they easily involved in polymerization.
|
Table 1: Feed and copolymer compositions, conversion, nitrogen analysis and intrinsic viscosity values [?] of AM/MMA copolymer. |
|||||
|
Sample Code |
f1 (feed) |
Conversion% |
N% |
F1 (copolymer) |
[?](dL/g) |
|
AM/MAA-1 AM/MAA-2 AM/MAA-3 AM/MAA-4 AM/MAA-5 |
0.10 0.25 0.50 0.75 0.90 |
12.3 11.5 13.7 10.9 12.8 |
1.91 3.81 4.51 6.93 12.3 |
0.128 0.254 0.296 0.437 0.691 |
0.52 0.71 0.78 1.06 1.74 |
|
(a) f1 is the mole fraction of monomer-1 (AM) in the initial feed; f2 = 1- f1 (b) F1 is the mole fraction of monomer-1 (AM) in the copolymer; F2 = 1- F1 |
|||||
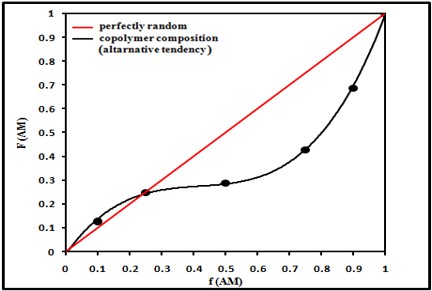
Figure 2: Variation of copolymer composition F1 (AM) with feed composition f1 (AM) for AM/MMA copolymer.
Reactivity Ratio
The sort of copolymer framed can be best comprehended from the information of reactivity ratios of the copolymers. The most widely recognized scientific model of copolymerization depends on finding the connection between the composition of the monomer feed and the composition of copolymers in which the monomer reactivity ratios are the parameters to be resolved [13]. In our work two procedures have been used for the best fitting of (r1& r2) pair from a set of [M1], [M2], d [M1] and d [M2] pair, using linearization methods representing by Kelen-Tudos and Fineman-Ross. The references [14,15] of these methods should be consulted for more details about the mathematical processes. The values are showed in figure 3 and figure 4, and represented in table 2.
|
Table 2: Kelen-Tudos and Fineman-Ross parameters of AM/MMA copolymer. |
|||||
|
Sample Code |
f1 (feed) |
G |
X |
η |
ζ |
|
AM/MAA-1 AM/MAA-2 AM/MAA-3 AM/MAA-4 AM/MAA-5 |
0.10 0.25 0.50 0.75 0.90 |
-7.702 -1.996 -0.588 -0.081 0.142 |
11.53 3.012 0.412 0.084 0.028 |
-0.638 -0.551 -0.600 -0.124 0.238 |
0.952 0.840 0.420 0.128 0.047 |
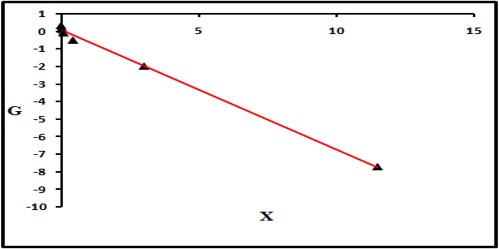
Figure 3: Fineman-Ross plot of AM/MMA copolymer.
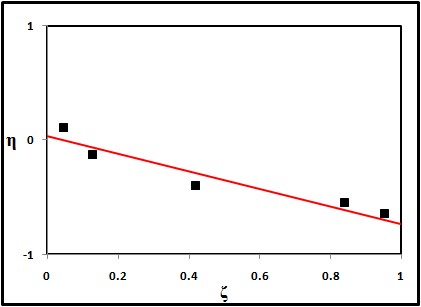
Figure 4: Kelen-Tudos plot of AM/MMA copolymer.
|
Table 3: Monomer reactivity ratios values for AM/MMA copolymer. |
|
Procedure r1 r2 |
|
Kelen-Tudos 0.032 0.575 Fineman-Ross 0.029 0.612 Average values 0.030 0.593 |
Table 3 shows the values of reactivity ratios by different methods, the values (r1, r2) from the different methods are very close. It is easy to observe that (AM/MMA) copolymer has the r1r2 values less than unity demonstrating the alternation behavior of the monomers. The alternative behavior for the two monomers (AM and MMA) could be explained in terms of increasing the stabilization of their radicals by the carbonyl groups resonance. On the other hand, the value of r2 is slightly greater than r1 which lead to a greater incorporation of MMA units compared to AM units. In this case, the double bond of MMA appears to have slightly more positive charge due to the presence of carbonyl ester bond. The charge density generated on carbonyl carbon atom would favor a significant electron attraction in MA radicals, which creates a slightly more positive charge on the double bond. A similar behavior was observed in our earlier case [16] wherein acrylamide was copolymerized with 3-(Trimethoxysilyl) Propyl Methacrylate, which contained similar carbonyl ester bond attached to the double bond.
When the reactivity ratios of the two monomers are less than unity, the synthesized copolymer shows an alternating behavior. Each monomer prefers to react with other monomer more than itself [17]. The possibility of an azeotropic composition increases in case of r1, r2 are both > 1 and < 1. For (AM/MMA) copolymer system, this condition is fulfilled since r1 and r2 < 1. Figure 2 (copolymer composition curve) proves this fact, in which a value of 0.25 for f1 (az.) could be clearly observed. The azeotropic feed composition f1 (az.) can be expressed by the following equation:
The results of reactivity ratios were then utilized for microstructural figuring. The microstructure of the copolymers is required to be critical in deciding the arrangement properties which the copolymer shows [18]. Igarashi,s [19,20] procedures are used to calculate the fraction of M1-M1, M2-M2 and M1-M2 units in the copolymers, the data are listed in table 4.
where: m1 (AM) and m2 (MMA)- the mole fractions in the copolymer, ?1-1, ?2-2, and ?1-2 - the mole fractions of 1-1, 2-2, and 1-2 sequences, respectively, r1 and r2 - the reactivity ratios.
The following equations were then used to calculate the probabilities of finding the sequence and the average length sequences of AM and MMA units [21,22]; the data are listed in Table 4.
????11=????1[????]/(????1[????]+[????]) (5)
????22=????2[????]/(????2[????]+[????]) (6)
????12=[????]/(????1[????]+[????]) (7)
????21=[????]/(????2[????]+[????]) (8)
????1=1/????12 (9)
????2=2/????21 (10)
Where: A (AM) and B (MMA) are the mole fractions in the feed. In these equations, P11, P22, P12 and P21- the probability of a AM or MMA unit to be followed by AM or MMA unit.
For (AM/MMA) copolymer system, [M1]-[M1], [M1]-[M2], P11 and P21 increase as (AM) increases in the feed, whilst [M2]-[M2], P22 and P12 increase as (MMA) increases. At the same time, the mean sequence length of AM, μ1, varied from 1.003 to 1.270. For these copolymer compositions, values of μ2 were between 6.369 and 1.066, respectively. From these results, (AM) have a tendency to react with other monomer (MMA) more than it selves in the growing chain, whilst (MMA) have a tendency to react with it selves more than other monomer (AM) to form alternation copolymer and block (MMA) units distributes in the growing chain. These results are in agreement with the values of rAM (0.03) and rMMA (0.593).
|
Table 4: Statistical data of AM/MMA copolymer. |
|
Sample Blockness Alternation Sequence probability Sequence length code (mol%) (mol%) M1-M1 M2-M2 M1-M2 P11 P22 P12 P21 μ1 μ2 |
|
AM/MMA-1 0.10 74.39 25.51 0.003 0.842 0.996 0.157 1.003 6.369 AM/MMA-2 0.30 49.51 50.19 0.009 0.639 0.990 0.360 1.010 2.777 AM/MMA-3 0.40 41.25 58.35 0.029 0.371 0.970 0.628 1.031 1.592 AM/MMA-4 2.18 14.71 83.11 0.082 0.164 0.917 0.835 1.094 1.197 AM/MMA-5 38.3 0.510 61.17 0.212 0.061 0.787 0.938 1.270 1.066 |
Thermal properties
For (AM and MMA) homopolymers, Tg value observed around 155°C and 90°C respectively whereas AM/MMA-1 copolymer showed the Tg around 110°C. It is found that by increasing the amount of AM content in the copolymers result in increased Tg, this may be due to the presence of rigid amide group in the backbone in AM side chain. TGA results are presented in Figure 5. The AM/MMA copolymer is more stable than the homopolymer of MMA with 10% weight loss at about 350°C of AM/MMA-1 which is higher than 100°C of Poly (MMA). This result could be attributed to the presence of methyl group in the backbone in MMA side chain which significantly lowers the Tg value and the thermal stability of AM/MMA copolymer. Values of Tg and data of TGA are given in table 5.
|
Table 5: Thermal data of AM/MMA copolymers. |
|
Samples Tg(°C) T10%(°C) T50%(°C) Residual at 800 °C (wt %) |
|
PAM 155 370 490 35 AM-co-MMA-1 90 100 475 28 PMMA 110 350 420 8 |
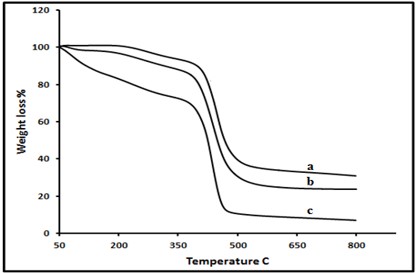
Figure 5: TGA thermogram of: (a) Poly AM, (b) AM-co-MMA-1, (c) Poly MMA.
Conclusions
The copolymer AM/MMA was successfully synthesized. The structure of synthesized copolymer was confirmed by FT-IR technique. Elemental analysis test was employed to determine the copolymer compositions. Then, the results of N% were used to calculate the reactivity ratios by different methods: Kelen-Tudos and Fineman-Ross methods and a good agreement was observed between the two procedures. The copolymer tends to be alternative (r1=0.03, r2=0.593). The results of sequence distribution of monomers and microstructure show a good agreement with the obtained reactivity ratios. DSC and TGA analysis were used to study the thermal properties of the (AM and MMA) homopolymers and copolymer.
References
1. Gavrilov AA, Chertovich AV. 2017. Copolymerization of Partly Incompatible Monomers: An Insight from Computer Simulations. Macromolecules. 50: 4677-4685. Ref.: https://bit.ly/2RhkuWR
2. Ambade AV. 2017. Controlled radical polymerization. InMetal-Catalyzed Polymerization. CRC Press. 161-177.
3. Erol I, Sen O, Dedelioglu A, et al. 2009. Synthesis and characterization of novel fluorine?containing methacrylate copolymers: Reactivity ratios, thermal properties, and antimicrobial activity. J Appl Polym Sci. 114: 3351-3359. Ref.: https://bit.ly/2GffziP
4. Beckingham BS, Sanoja GE, Lynd NA. 2015. Simple and Accurate Determination of Reactivity Ratios Using a Nonterminal Model of Chain Copolymerization. Macromolecules. 48: 6922-6930. Ref.: https://bit.ly/37k6z8d
5. Mohammed AH, Ahmad MB, Ibrahim NA, et al. 2018. Effect of crosslinking concentration on properties of 3-(trimethoxysilyl) propyl methacrylate/N-vinyl pyrrolidone gels. Chem Cent J. 12: 15. Ref.: https://bit.ly/30JeTMf
6. Riahinezhad M, Kazemi N, McManus N, et al. 2014. Effect of ionic strength on the reactivity ratios of acrylamide/acrylic acid (sodium acrylate) copolymerization. J Appl Polym Sci. 131: 20. Ref.: https://bit.ly/2upyG7c
7. El-Newehy MH, Al-Deyab SS, Al-Hazmi AM. 2010. Reactivity ratios for organotin copolymer systems. Molecules. 15: 2749-2758. Ref.: https://bit.ly/2NScGJf
8. Habibi A, Vasheghani?Farahani E, Semsarzadeh MA, et al. 2003. Monomer reactivity ratios for lauryl methacrylate-isobutyl methacrylate in bulk free radical copolymerization. Polym Int. 52: 1434-1443. Ref.: https://bit.ly/37mR2nZ
9. Zubov A, Naeem O, Hauger SO, et al. 2017. Bringing the On?Line Control and Optimization of Semibatch Emulsion Copolymerization to the Pilot Plant. React Eng. 11: 1700014. Ref.: https://bit.ly/2GgBJ4m
10. Manski CF, Tamer E. 2002. Inference on regressions with interval data on a regressor or outcome. Econometrica. 70: 519-546. Ref.: https://bit.ly/2tHecXu
11. Dubé MA, Saldívar?Guerra E, Zapata?González I. 2013. Copolymerization (2013) Handbook of Polymer Synthesis, Characterization, and Processing. 25: 105-125. Ref.: https://bit.ly/2GgBR3L
12. Nachman M, Kwiatkowski K. 2013. The effect of thermal annealing on the abrasion resistance of a segmented block copolymer urethane elastomers. Wear. 306: 113-118. Ref.: https://bit.ly/30M2mr9
13. Acikses A, Taskan G, Barim G. 2018. A Study on Copolymer Systems of Styrene with Diethanolamine Side Group and Methyl Methacrylate. J Chem. 2018. Ref.: https://bit.ly/36lq7Yp
14. Kelen T, Tüdöus F, Turcsányi B, et al. 1977. Analysis of the linear methods for determining copolymerization reactivity ratios. IV. A comprehensive and critical reexamination of carbocationic copolymerization data. J Polym Sci a Polym Chem. 15: 3047-3074.
15. Fineman M, Ross SD. 1950. Linear method for determining monomer reactivity ratios in copolymerization. J Polym Sci A. 5: 259-262. Ref.: https://bit.ly/2TNgH5m
16. Mohammed AH, Ahmad MB, Ibrahim NA, et al. 2016. Synthesis and monomer reactivity ratios of acrylamide with 3-(trimethoxysilyl) propyl methacrylate and tris (methoxyethoxy) vinylsilane copolymers. Polimery. 61: 758-765. Ref.: https://bit.ly/2RkkRjn
17. Anastasaki A, Nikolaou V, Nurumbetov G, et al. 2015. Cu (0)-mediated living radical polymerization: a versatile tool for materials synthesis. Chem Rev. 116: 835-877. Ref.: https://bit.ly/2vfAMah
18. Craciun G, Ighigeanu D, Manaila E, et al. 2015. Synthesis and characterization of poly (acrylamide-co-acrylic acid) flocculant obtained by electron beam irradiation. Mater Res. 18: 984-993. Ref.: https://bit.ly/38AYDzF
19. Igarashi S. 1963. Representation of composition and blockiness of the copolymer by a triangular coordinate system. J Polym Sci B. 1: 359-363. Ref.: https://bit.ly/36lXABS
20. Vijaykumar S, Prasannkumar S, Sherigara BS, et al. 2009. Copolymerization of N-vinyl pyrrolidone with functionalized vinyl monomers: Synthesis, characterization and reactivity relationships. Macromol Res. 17: 1003-1009. Ref.: https://bit.ly/36gcM3v
21. Parambil AM, Puttaiahgowda YM, Shankarappa P. 2012. Copolymerization of N-Vinyl pyrrolidone with methyl methacrylate by Ti (III)-DMG redox initiator. Turk J Chem. 36: 397-409. Ref.: https://bit.ly/2vgI6T1
22. Mohammed AH, Bin Ahmad M, Shameli K. 2015. Copolymerization of tris (methoxyethoxy) vinyl silane with N-vinyl pyrrolidone: synthesis, characterization, and reactivity relationships. International Journal of Polymer Science. Ref.: https://bit.ly/3avcskV




















